Abstract
Vascular endothelial growth factor (VEGF) and erythropoietin (EPO) have profound effects on the endothelium and endothelial progenitor cells (EPCs), which originate from the bone marrow and differentiate into endothelial cells. Both EPO and VEGF have demonstrated an ability to increase the number and performance properties of EPCs. EPC behavior is highly dependent on nitric oxide (NO), and both VEGF and EPO can stimulate intracellular NO. EPO can bind to the homodimeric EPO receptor (EPO-R) and the heterodimeric receptor, EPO-R and the common β receptor (βC-R). Although VEGF has several receptors, VEGF-R2 appears most critical to EPC function. We demonstrate that EPO induction of NO is dependent on the βC-R and VEGF-R2, that VEGF induction of NO is dependent on the expression of the βC-R, and that the βC-R and VEGF-R2 interact. This is the first definitive functional and structural evidence of an interaction between the 2 receptors and has implications for the side effects of EPO.
Introduction
In the embryonic blood island, a central hematopoietic stem cell is surrounded by hematopoietic and endothelial precursors. This physical association between endothelial precursors and hematopoietic stem cells as well as the realization that these cells share antigenic determinants suggested that these cells are derived from a common precursor, the hemangioblast. Because hematopoietic stem cells isolated from the peripheral circulation were able to provide sustained hematopoietic function,1 it was reasonable to speculate that circulating cells may also function as endothelial progenitor cells (EPCs). Asahara et al demonstrated that cells isolated on the basis of the expression of the hematopoietic stem cell marker CD34 not only express CD31, Flk-1, and E-selectin after 7 days in culture, but also express endothelial nitric oxide synthase (eNOS), produce nitric oxide (NO), and home to areas of angiogenesis in vivo.2 Other investigators have demonstrated that the number of circulating CD34+ cells and potentially their ability to direct endothelial repair are increased by treatment with 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors3,4 as well as with erythropoietin (EPO).5-7 Interestingly, EPO5 not only affects CD34+ cell number but also has been shown to affect EPC activity by increasing chemotaxis and enhancing their ability to form tubes, respectively.
The action of EPO on EPCs has been suggested to be secondary to the EPO-induced phosphorylation of Akt,5 which results in an increase in eNOS activity. Dysregulated NO generation can adversely affect EPC behavior. In hematopoietic stem cells/EPCs, NO production is involved in maturation and differentiation.8,9 eNOS is essential for the functional activity of hematopoietic stem and progenitor cells. Inactivation of the eNOS gene has been shown to impair angiogenesis and wound healing.8 eNOS knockout mice have reduced hematopoietic recovery, increased mortality after myelosuppression, decreased EPC number, and reduced angiogenesis.10 The eNOS knockouts also have reduced matrix metalloproteinase-9 activity within the bone marrow that may underlie their EPC mobilization defect.11 In addition to regulating matrix metalloproteinase-9, NO modulates proliferation, differentiation, and apoptosis in a variety of cell types.9 Aicher et al postulated that endothelial derived NO interferes with cell cycle progression and/or differentiation of stem cells in the bone marrow; NO in the bone marrow may act in a paracrine manner as it is generated mainly by vascular cells of the bone marrow.10
There are thought to be 2 receptors for EPO. One is the homodimeric EPO receptor (EPO-R) that is responsible for erythropoiesis.12 The second is the heterodimeric receptor that consists of the EPO-R and the common β receptor (βC-R). One mechanism for the decrease in erythropoiesis in persons with kidney disease is that the high levels of urea lead to carbamylation of EPO, which has diminished biologic activity because it does not activate the homodimeric EPO-R.13 However, it was soon recognized that the tissue-protective effects of EPO in models of ischemic, traumatic, and inflammatory injuries14 were not lost by carbamylation.15 Further studies have demonstrated that heterodimeric EPO-R/βC-R is required for mediation of the tissue-protective effects of EPO.16 Because EPO has previously been shown to affect EPCs, we investigated whether NO production was mediated via the homodimeric EPO-R or the heterodimeric EPO-R/βC-R.
Methods
Tissue culture and reagents
All tissue culture reagents were obtained from Invitrogen and MediaTech. All other reagents were obtained from Sigma-Aldrich unless otherwise indicated. rhEPO was kindly provided by Amgen.
Carbamylation of EPO
cEPO was synthesized from rhEPO similar to a previously described method.13 Briefly, EPO (∼ 1 mg/mL) was mixed with an equal volume of 1M sodium borate (pH ∼ 8.8), and recrystallized KOCN was added to a final concentration of 1M. The mixture was incubated at 37°C for 24 hours before being dialyzed first against Milli-Q water and then against 20mM sodium citrate in 0.1M NaCl, pH 6.0. After dialysis, the samples were concentrated in a Centricon (Amicon) concentrator and the protein content determined by Bicinchoninic acid (Pierce Chemical). The completeness of the carbamylation was determined by the inability of the cEPO, in contrast to EPO, to induce hemoglobin in mouse embryonic stem cells differentiated to generate cells of the hematopoietic lineage (results not shown).
Isolation of CD34+ cells
The study protocol was approved by the Institutional Review Board at the University of Florida, and written informed consent was obtained from each volunteer in accordance with the Declaration of Helsinki. Blood was collected from healthy controls by routine venipuncture into CPT tubes with heparin (BD Biosciences). After centrifugation at room temperature in a swinging bucket rotor for 20 minutes at 1500g, the mononuclear cells were diluted with phosphate-buffered saline (PBS) supplemented with 2mM ethylenediaminetetraacetic acid (PBSE). The cells were centrifuged for 15 minutes at 300g, and the cell pellet washed; this procedure was repeated once. The 3.3 × 107 cell peripheral blood mononuclear cells (PBMCs) were resuspended in 100 μL of PBSE to which 33 μL of FcR blocking reagent (Miltenyi Biotec) and 33 μL of magnetic microbeads conjugated with an anti-CD34 antibody were added. After incubation for 30 minutes at 4°C, the cells were diluted in 10× volume of PBSE supplemented with 0.1% bovine serum albumin. The CD34+ cells were positively selected using the automated magnetic selection autoMACS (Miltenyi Biotec). The selected cells were confirmed to be CD34+ cells by costaining with phycoerythrin-conjugated anti-CD34 (Miltenyi Biotec) and fluorescein isothiocyanate (FITC)–conjugated anti-CD45.
Cell culture
CD34+ EPCs were prepared for assays as follows. CD34+ EPCs were isolated as in “Isolation of CD34+ cells,” and plated and cultured on fibronectin-coated dishes in HPGM Medium (Lonza Baltimore) supplemented with 10% fetal bovine serum and the following cytokines: stem cell factor (25 ng/mL), thrombopoietin (50 ng/mL), and granulocyte-macrophage colony-stimulating factor (GM-CSF; 25 ng/mL) from R&D Systems. Cells were cultured in 50 mU/mL of EPO or cEPO as indicated.
Isolation and culture of EPC (colony assay)
EPC colonies were cultured from PBMCs isolated from 8 mL of peripheral blood using CPT tubes with heparin (BD Biosciences) as in “Isolation of CD34+ cells.” The PBMCs were plated on fibronectin-coated 6-well dishes and incubated at 37°C in humidified 5% CO2. After 2 days, the nonadherent cells were replated into 12-well dishes at a concentration of 106 cells per well. At day 5, cells were supplemented with fresh EGM-2 medium (Lonza Switzerland) and changed every third day. After at least 7 days, we observed the appearance of colonies. When colonies became well established, cells were used for further experiments. The endothelial phenotype of the cells was confirmed by staining with fluorescently labeled acetylated-LDL (Biomedical Technologies) and FITC-conjugated Ulex europaeus agglutinin-1 as well as by detecting an expression of mRNA for eNOS and live imaging of NO production.
CD34+ EPC transfection
EPCs were transfected with shRNA-vector for human VEGF-R2 using the pSM2c template obtained from Opened Biosystems and Lipofectamine2000 (Invitrogen) following the protocol of the manufacturer in serum-free basal endothelial basal medium. After a 3-hour incubation, the medium was supplemented with 10% fetal bovine serum.
Detection of NO produced by EPCs
EPCs were cultured on a 35-mm dish with a glass-bottom insert (MatTek). Bioavailable NO was determined as previously described.17 Briefly, EPCs were incubated with 5μM 4-amino-5-methylamino-2′,7′-difluorofluorescein (DAF-FM) diacetate (Invitrogen) for 30 minutes at 37°C in the dark. Excess extracellular probe was removed by washing in Hanks balanced salt solution followed by incubation for 10 minutes at room temperature to allow for probe deesterification. DAF-FM fluorescence increases by approximately 160-fold when it reacts with NO. Green fluorescence was measured using an inverted microscope Axiovert 200 (Carl Zeiss) equipped with CCD camera and image acquisition/analysis software AxioVision (Version 4.5). Images were acquired every 1 minute for at least 20 minutes, and fluorescence intensity was measured in 20 to 30 cells per field in at least 6 fields per experiment. Alternatively, imaging was performed using a confocal microscope at excitation and emission maxima of 495 and 515 nm, respectively. Intensity of fluorescence was quantified using LSM 510 (Version 3.0 SP3) software for the Carl Zeiss Laser Scanning Microscope.
Angiogenesis assay
To show the functional activity of the EPCs, we used a tube formation (angiogenesis) assay. Briefly, EPCs obtained from healthy control subjects were mock-transfected or transfected with mock shRNA or shRNA VEGFR2 24 hours before being coplated with human umbilical vein endothelial cells (HUVECs) at the 1:1 ratio on a 48-well tissue culture plate precoated with 250 μL of Cultrex Basement Membrane Extract from Trevigen in 100 μL endothelial basal medium-2 with or without treatment(s). After 12 hours of incubation in a 5% CO2 humidified atmosphere at 37°C, the 3-dimensional structures formed by the cells in this matrix were examined using an inverted phase-contrast microscope. Tube-like structures were determined by a measuring the sum of the lengths of all tubules per field using the image analysis software AxioVision, Version 4.5. Four randomly selected low-power fields were examined for each sample.
cGMP measurement
EPCs were washed with PBS and kept in serum-free media for 3 hours, at which time EPO at a concentration of 50 mU/mL was added to the cells. After 15, 30, and 90 minutes, the medium was aspirated and 0.1M HCl was added to lyse the cells. After 20 minutes, the cells were scraped and the cell lysates were centrifuged at 1000g for 10 minutes and the supernatants were used for the measurement of 3′:5′-cyclic monophosphate (cGMP) by immunoassay (Cayman Chemical).
RNA isolation
RNA was extracted using TRIzol reagent (Invitrogen). Cells were lysed on the culture dish with TRI Reagent, collected into RNA-free tubes followed by chloroform separation, and then centrifuged at 12 000g for 15 minutes at 4°C. The colorless upper phase was transferred into fresh tubes followed by RNA precipitation with isopropanol, centrifugation at 12 000g for 10 minutes at 4°C, and washing RNA with 75% ethanol.
RT-PCR
Expression of eNOS, EPO-R, and βC-R along with the β-actin gene as control was detected by reverse-transcribed polymerase chain reaction (RT-PCR) using the SuperScript One-Step RT-PCR kit with Platinum Taq (Invitrogen). We performed primer design using Vector NTI Advance and the online tool OligoPerfect Designer (both from Invitrogen). Reverse transcription was performed at 50°C for 30 minutes. The amplification step consisted of 28 cycles (leading to linear range of amplification, as shown in preliminary experiments): denaturation at 94°C for 40 seconds, annealing for 1 minute at 55°C, and extension of primers for 1 minute at 72°C. The products were then held at 72°C for 5 minutes for DNA extensions to occur. Amplified fragments of EPO-R, βC-R, and β-actin cDNA were separated by electrophoresis on 1% agarose gel, and the products were visualized by staining with 0.5 μg/mL ethidium bromide. The identity and specificity of the PCR products were confirmed by sequencing.
Immunofluorescence
After fixing the EPC cells with 4.0% paraformaldehyde in PBS for 15 minutes at room temperature, the cells were washed with PBS for 5 minutes 3 times and permeabilized by incubating with 0.1% Triton X-100 in PBS for 15 minutes at room temperature. The cells were then incubated in blocking solution (10% goat serum, 1% bovine serum albumin in PBS) for 60 minutes at room temperature before being exposed to rabbit VEGF-R2 Antibody (Cell Signaling Technology) at a 1:100 dilution, mouse β subunit antibody (Santa Cruz Biotechnology) at a 1:100 dilution, or isotype control antibodies all diluted in blocking solution. After overnight incubation at 4°C, the cells were rinsed 3 times with PBS and incubated with secondary antibody, either AlexaFluor 488 goat anti–mouse or AlexaFluor 594 goat anti–rabbit (Invitrogen) for 60 minutes at room temperature. The cells were then rinsed and imaged using an Olympus IX81-DSU Spinning Disk Confocal microscope equipped with CCD camera and image acquisition/analysis software SlideBook (Version 4.1).
Analysis of colocalization
βC-R/VEGF-R2 colocalization analysis was performed using the Delta Vision Core Imaging System equipped with the software softWoRx 3.7.0 analysis tools to visualize and quantitate data (Applied Precision).
Pull-down assay
αVEGF-R2 rabbit IgG (10 μg/mL), αβC-R mouse IgG2b (2 μg/mL), or a mouse IgG2b (20 μg/mL) was added to a fixed quantity of cell extract. After pull-down with protein A agarose, the immunoprecipitates were resolved by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to polyvinylidene difluoride (PVDF) membrane as previously described.18 The blots were then probed with the αVEGF-R2 antibody and after stripping, with αEPO-R antibody (Santa Cruz Biotechnology). After being washed in 50mM Tris-buffered saline/0.2% Tween 20 (pH 7.4), the blot was overlaid with horseradish peroxidase–conjugated secondary antibody and the immunoreactive polypeptides were detected by enhanced chemiluminescence (Pierce Chemical). The chemiluminescence was measured digitally using a Fluorchem Imaging System and software (Alpha Innotech).
Immunoblot analysis
Phosphorylated and total Akt and eNOS were detected by immunoblot analysis with phosphorylation state-specific antibodies for p-Akt (Ser473), total Akt, p-eNOS (Ser1177), and total eNOS obtained from Cell Signaling Technology. EPCs were rinsed with ice-cold PBS, lysed with ice-cold buffer containing 50mM Tris-HCl (pH 7.6), 120mM NaCl, 1% Nonidet P-40, 10% glycerol, 1mM phenylmethylsulfonyl fluoride, 2mM sodium orthovanadate, 10mM sodium pyrophosphate, 40 μg/mL leupeptin, 5 μg/mL aprotinin, 1 μg/mL pepstatin, 100mM NaF, 1mM ethylenediaminetetraacetic acid, and 1mM ethyleneglycoltetraacetic acid, and scraped into microcentrifuge tubes. After lysis on ice for 30 minutes, extracts were centrifuged for 10 minutes at 14 000g at 4°C. The protein concentration of the supernatants was measured, and 20 μg protein samples of cell lysate were mixed (1:1) with Laemmli sample buffer and incubated at 95°C for 5 minutes. Proteins were resolved by SDS-PAGE, followed by electroblotting onto PVDF membrane. Membranes were blocked in 10mM Tris (pH 7.5), 100mM NaCl, and 0.1% Tween 20 containing 5% nonfat dry milk, followed by incubation with primary antibodies (total Akt, total eNOS and p-Akt, p-eNOS at a 1:1000 dilution). Membranes were washed 3 times and incubated with the appropriate horseradish peroxidase–conjugated secondary antibody. The immunocomplexes were visualized by chemiluminescence with the Phototope Western blot detection system (Cell Signaling Technology). The images were digitalized using the AlphaEase FluorChem digital imaging system (Alpha Innotech).
Image acquisition and preparation
All microscopy was performed using an Axiovert 200 inverted microscope (Carl Zeiss), unless otherwise indicated. For image acquisition and analysis of fluorescence intensity of DAF-FM diacetate and for live imaging of NO in Hanks' Balanced Salt Solution (HBSS) without Calcium, Magnesium, Phenol Red, we used the LD Achroplan 40×/0.60 Corr objective (Carl Zeiss), the AxioCam MRm charge-coupled device camera (CCD; Carl Zeiss), FITC filter (excitation 480/30 nm, emission 535/40 nm), and AxioVision (v.4.5) image acquisition and analysis software. All optical filters were obtained from Chroma Technologies. The imaging of EPC colonies was done in the same fashion except with an A-Plan ×10 objective (Carl Zeiss), and the AxioCam MRm CCD camera was used. Immunofluorescence was performed using an Olympus IX81-DSU Spinning Disk Confocal microscope, UPLSAPO 60×/1.20 W NA water immersion objective with DAPI (4′,6-diamidino-2-phenylindole) imaging medium equipped with a Hamamatsu ORCA-ER C4742-80-12AG Monochrome CCD camera (1344 × 1024 resolution) and the image acquisition/analysis module of SlideBook 4.1 software (Intelligent Imaging Innovations). We used Adobe Photoshop CS3 for subsequent image editing and assembling of the figures.
Statistical analysis
Statistical analysis was carried out using Student t test and the Mann-Whitney rank sum test.
Results
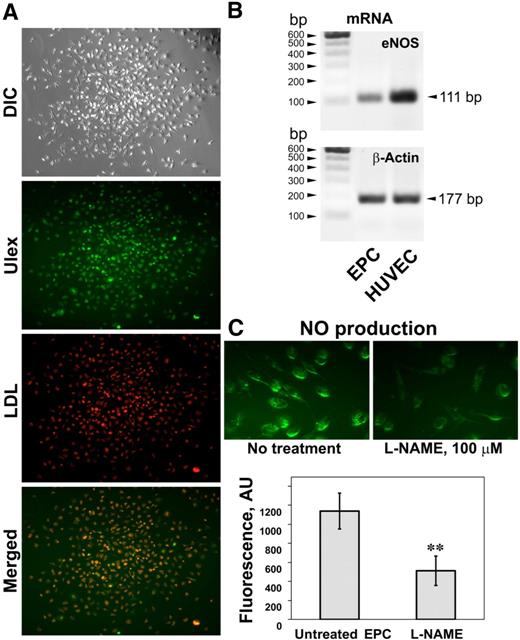
The presence of EPCs within the population of PBMCs can be detected by monitoring for markers of the endothelial phenotype as well as by eNOS NO production, a crucial component of endothelial cell function. Our protocol for culturing EPCs while selecting against mature endothelial cells is a combination of previously published protocols19,20 and yielded multiple distinct colonies after 2 to 3 weeks of culture (Figure 1A). The majority of the cells in these colonies were positive for endothelial markers: incorporation of the acetylated low-density lipoprotein and binding of FITC-Ulex-lectin (Figure 1A). In addition, these cells expressed eNOS mRNA at the levels lower but comparable with HUVECs (Figure 1B). We were also able to detect NO production in these cells using the NO-specific fluorescence probe DAF-FM diacetate (Figure 1C). L-NAME, a nitric oxide synthase inhibitor that is relatively specific to neuronal NOS and eNOS isoform, substantially attenuated fluorescence intensity (Figure 1C), confirming that the probe specifically responded to NO produced by nitric oxide synthase. In addition, there was a linear relationship with the DAF-FM fluorescence of media and the concentration of diethylenetriamine, an NO donor, added to the media, which encompassed a broad range of concentrations, including the physiologic range of NO concentrations (results not shown). The compound 1400W, a potent and selective iNOS inhibitor, was without effect on the DAF-FM diacetate fluorescence (data not shown), suggesting that NO production in these cells can be attributed predominantly to eNOS. In addition, similar to the protocol of Case et al,21 a large percentage of these cells was CD45−CD34+/VEGF-R2+ as determined by flow cytometry (data not shown). VEGF-R2 expression was additionally confirmed by immunofluorescence (Figure 6). Taken together, these data demonstrate that the population of cells isolated by our approach has some characteristic phenotypic and functional properties of endothelial cells and might be related to the cells recently described as “late endothelial progenitor cells.”22
Functional characterization of EPCs isolated from PBMCs. (A) A typical EPC colony formed after culturing PBMCs on fibronectin in Endocult medium followed by EGM-2 medium and appearance after staining cells with the endothelial markers DiI-acetylated LDL and FITC-Ulex-lectin. Both endothelial markers are colocalized in the majority of cells. (B) Expression of the eNOS mRNA in the isolated colony-forming cells and HUVECs. (C) NO production in the cells within the colonies monitored by live imaging of NO with the NO-specific fluorescent probe DAF-FM diacetate (top panel). Fluorescence of NO-specific probe was sensitive to L-NAME, an inhibitor of nitric oxide synthase. Values are means ± SD (n = 3). **P < .01 compared with untreated cells.
Functional characterization of EPCs isolated from PBMCs. (A) A typical EPC colony formed after culturing PBMCs on fibronectin in Endocult medium followed by EGM-2 medium and appearance after staining cells with the endothelial markers DiI-acetylated LDL and FITC-Ulex-lectin. Both endothelial markers are colocalized in the majority of cells. (B) Expression of the eNOS mRNA in the isolated colony-forming cells and HUVECs. (C) NO production in the cells within the colonies monitored by live imaging of NO with the NO-specific fluorescent probe DAF-FM diacetate (top panel). Fluorescence of NO-specific probe was sensitive to L-NAME, an inhibitor of nitric oxide synthase. Values are means ± SD (n = 3). **P < .01 compared with untreated cells.
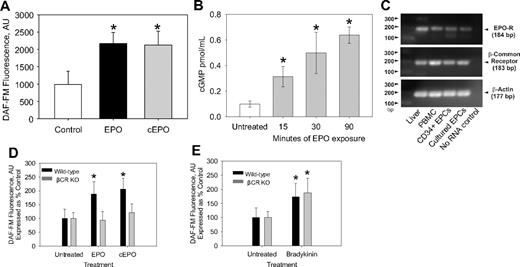
To test the hypothesis that EPO can regulate NO production via a nonhematopoietic signaling pathway, we carbamylated EPO and compared the effects of EPO and carbamylated EPO (cEPO) on NO production. Our cEPO, similar to previous reports,15,23 does not induce erythropoiesis (data not shown). Treatment of EPC with both EPO and cEPO stimulated significant increases in NO production (Figure 2A), suggesting that binding to the heterodimeric EPO-R/βC-R may be required for EPO induction of NO. Because NO is well known to lead to an increase in guanylate cyclase activity leading to an increase in cGMP,24-26 the effect of exposing EPCs to NO on cGMP levels was determined. Exposing EPCs to EPO rapidly, within 15 minutes, led to a significant increase in cGMP levels, thus confirming the ability of EPO to induce NO bioavailability (Figure 2B).
The effect of EPO and cEPO on NO production in EPCs. (A) CD34+ EPCs were incubated with 50 mU/mL EPO, 50 mU/mL cEPO, or vehicle (Control) for 3 hours before measuring bioavailable NO with DAF-FM diacetate. Shown are the representative results of 10 independent experiments (n ≥ 10). *P < .001 compared with control. (B) EPCs were exposed to EPO ( ) for the indicated time before cell lysis and determination of cGMP intracellular concentration. Shown is the average of 3 different experiments. *P < .026 compared with untreated. (C) RNA was isolated from CD34+ EPCs, human EPC colony-forming units, PBMCs, and human liver. After RT-PCR was performed, the PCR products were analyzed on a 1% agarose EtBr gels. The representative results of 2 experiments are shown. (D) Bone marrow cells isolated from wild-type C57/BL6 mice and βC-R knockout mice were grown under conditions that promoted EPC growth. After 1 week, the cells were trypsinized, replated for 24 hours, and pretreated with EPO or cEPO for 3 hours before measurement of bioavailable NO by DAF-FM. Shown are the representative results of 2 independent experiments as mean ± SD (n ≥ 20). *P < .001 compared with control. (E) Bone marrow–derived mice EPC colonies as in panel C were treated with (5μM) bradykinin. Shown is the representative result of 2 different experiments as mean ± SD (n ≥ 15). *P < .001 compared with control.
) for the indicated time before cell lysis and determination of cGMP intracellular concentration. Shown is the average of 3 different experiments. *P < .026 compared with untreated. (C) RNA was isolated from CD34+ EPCs, human EPC colony-forming units, PBMCs, and human liver. After RT-PCR was performed, the PCR products were analyzed on a 1% agarose EtBr gels. The representative results of 2 experiments are shown. (D) Bone marrow cells isolated from wild-type C57/BL6 mice and βC-R knockout mice were grown under conditions that promoted EPC growth. After 1 week, the cells were trypsinized, replated for 24 hours, and pretreated with EPO or cEPO for 3 hours before measurement of bioavailable NO by DAF-FM. Shown are the representative results of 2 independent experiments as mean ± SD (n ≥ 20). *P < .001 compared with control. (E) Bone marrow–derived mice EPC colonies as in panel C were treated with (5μM) bradykinin. Shown is the representative result of 2 different experiments as mean ± SD (n ≥ 15). *P < .001 compared with control.
The effect of EPO and cEPO on NO production in EPCs. (A) CD34+ EPCs were incubated with 50 mU/mL EPO, 50 mU/mL cEPO, or vehicle (Control) for 3 hours before measuring bioavailable NO with DAF-FM diacetate. Shown are the representative results of 10 independent experiments (n ≥ 10). *P < .001 compared with control. (B) EPCs were exposed to EPO ( ) for the indicated time before cell lysis and determination of cGMP intracellular concentration. Shown is the average of 3 different experiments. *P < .026 compared with untreated. (C) RNA was isolated from CD34+ EPCs, human EPC colony-forming units, PBMCs, and human liver. After RT-PCR was performed, the PCR products were analyzed on a 1% agarose EtBr gels. The representative results of 2 experiments are shown. (D) Bone marrow cells isolated from wild-type C57/BL6 mice and βC-R knockout mice were grown under conditions that promoted EPC growth. After 1 week, the cells were trypsinized, replated for 24 hours, and pretreated with EPO or cEPO for 3 hours before measurement of bioavailable NO by DAF-FM. Shown are the representative results of 2 independent experiments as mean ± SD (n ≥ 20). *P < .001 compared with control. (E) Bone marrow–derived mice EPC colonies as in panel C were treated with (5μM) bradykinin. Shown is the representative result of 2 different experiments as mean ± SD (n ≥ 15). *P < .001 compared with control.
) for the indicated time before cell lysis and determination of cGMP intracellular concentration. Shown is the average of 3 different experiments. *P < .026 compared with untreated. (C) RNA was isolated from CD34+ EPCs, human EPC colony-forming units, PBMCs, and human liver. After RT-PCR was performed, the PCR products were analyzed on a 1% agarose EtBr gels. The representative results of 2 experiments are shown. (D) Bone marrow cells isolated from wild-type C57/BL6 mice and βC-R knockout mice were grown under conditions that promoted EPC growth. After 1 week, the cells were trypsinized, replated for 24 hours, and pretreated with EPO or cEPO for 3 hours before measurement of bioavailable NO by DAF-FM. Shown are the representative results of 2 independent experiments as mean ± SD (n ≥ 20). *P < .001 compared with control. (E) Bone marrow–derived mice EPC colonies as in panel C were treated with (5μM) bradykinin. Shown is the representative result of 2 different experiments as mean ± SD (n ≥ 15). *P < .001 compared with control.
Previously, it was unknown as to whether CD34+ cells express the 2 EPO receptors. We performed RT-PCR on RNA isolated from CD34+ EPCs as well as on the EPC colonies and determined that both cell types express the βC-R and the EPO-R (Figure 2C). Another approach to confirm the dependence on the βC-R for EPO and cEPO-mediated NO stimulation was to isolate EPC colonies from βC-R knockout mice. EPC colonies isolated from the βC-R knockout mice had no enhancement of NO in response to EPO or cEPO (Figure 2D) but exhibited an increase in NO on exposure to bradykinin (Figure 2E). Thus, the colonies were capable of increasing NO levels through the bradykinin pathway.
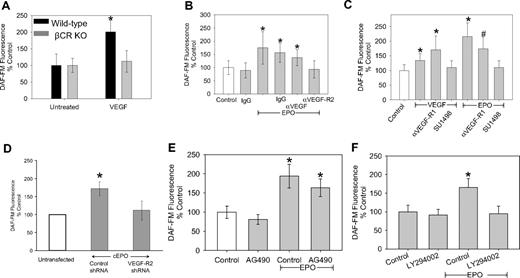
Although it was not unexpected, considering our cEPO results, βC-R knockout mice had no enhancement of NO in response to EPO or cEPO; the surprising result was that the bone marrow cells isolated from the βC-R knockout mice had no stimulation of NO in response to VEGF (Figure 3A). To investigate this further, we examined the effect of blocking VEGF, VEGF-R2, and VEGF-R1 on EPO-mediated increases in NO. EPO stimulation of NO was completely blocked by an antibody against VEGF-R2 (Figure 3B) as well as blocking VEGF-R2 signaling with SU1498, a specific inhibitor of VEGF-R2 tyrosine kinase (Figure 3C). Blocking VEGF-R1 had only a small effect on NO production (Figure 3C). Consistent with this, PIGF, a VEGF-R1-selective VEGF homologue, did not induce NO in CD34+ EPCs (data not shown). A recent report demonstrated that EPO-induced angiogenesis was via the induction of VEGF in neural progenitor cells27 ; however, we found that an anti-VEGF antibody added to the human CD34+ EPCs had minimal effect on NO induction (Figure 3B), even though the concentration of anti-VEGF antibody was able to block stimulation of NO of exogenously added VEGF (data not shown). Thus, EPO stimulation of NO was strictly dependent on the VEGF-R2 receptor. Because VEGF added to bone marrow cells isolated from the βC-R knockout mice was not able to lead to an induction of NO (Figure 3A), NO stimulation via VEGF-R2 seemed to be dependent on the βC-R as well, suggesting a cross-talk communication between VEGF-R2 and the βC-R.
The NO-stimulatory actions of EPO require VEGF-R2 and its downstream signaling. (A) Bone marrow cells isolated from wild-type C57/BL6 mice and βC-R knockout mice were grown for 1 week under conditions that promoted EPC growth. Then the cells were trypsinized and allowed to plate for 24 hours before treatment with 100 ng/mL VEGF for 3 hours before measurement of bioavailable NO by DAF-FM. Shown are the representative results of 2 independent experiments as mean ± SD (n ≥ 20). *P < .01. (B) CD34+ EPCs were isolated from healthy controls and incubated with isotype IgG, 50 mU/mL EPO alone or in combination with 0.01μg of anti-VEGF, anti-VEGF-R2, or isotype IgG 3 hours before measuring bioavailable NO with DAF-FM. Shown are the representative results of 3 independent experiments as mean ± SD (n ≥ 23). *P < .001 compared with untreated. (C) CD34+ EPCs were isolated from healthy controls and incubated with VEGF (100 ng/mL) and/or EPO (50 mU/mL) alone and in combination with anti-VEGF-R1 (1 μg/mL) or SU1498 (5μM). Shown are the representative results of 2 independent experiments as mean ± SD (n ≥ 15). *P < .001 compared with control. #P < .001 compared with control. (D) CD34+ EPCs were isolated from healthy controls and after 24 hours transfected with control shRNA or VEGF-R2 shRNA. After another 24 hours, the cells were treated with 50 mU/mL cEPO, as indicated, and DAF-FM fluorescence was determined. Shown are the representative results of 2 independent experiments as mean ± SD (n ≥ 10). *P < .001 compared with untreated. (E) CD34+ EPCs were isolated from healthy controls and incubated with AG490 (10μM) alone and in combination with EPO (50 mU/mL). Shown are the representative results of 3 different experiments as mean ± SD (n ≥ 32). *P < .001 compared with control. (F) CD34+ EPCs were isolated from healthy controls and incubated with LY294002 (5μM) alone and in combination with EPO (50 mU/mL). Shown is a representative of 4 independent experiments as mean ± SD (n ≥ 37). *P < .001 compared with control, no EPO.
The NO-stimulatory actions of EPO require VEGF-R2 and its downstream signaling. (A) Bone marrow cells isolated from wild-type C57/BL6 mice and βC-R knockout mice were grown for 1 week under conditions that promoted EPC growth. Then the cells were trypsinized and allowed to plate for 24 hours before treatment with 100 ng/mL VEGF for 3 hours before measurement of bioavailable NO by DAF-FM. Shown are the representative results of 2 independent experiments as mean ± SD (n ≥ 20). *P < .01. (B) CD34+ EPCs were isolated from healthy controls and incubated with isotype IgG, 50 mU/mL EPO alone or in combination with 0.01μg of anti-VEGF, anti-VEGF-R2, or isotype IgG 3 hours before measuring bioavailable NO with DAF-FM. Shown are the representative results of 3 independent experiments as mean ± SD (n ≥ 23). *P < .001 compared with untreated. (C) CD34+ EPCs were isolated from healthy controls and incubated with VEGF (100 ng/mL) and/or EPO (50 mU/mL) alone and in combination with anti-VEGF-R1 (1 μg/mL) or SU1498 (5μM). Shown are the representative results of 2 independent experiments as mean ± SD (n ≥ 15). *P < .001 compared with control. #P < .001 compared with control. (D) CD34+ EPCs were isolated from healthy controls and after 24 hours transfected with control shRNA or VEGF-R2 shRNA. After another 24 hours, the cells were treated with 50 mU/mL cEPO, as indicated, and DAF-FM fluorescence was determined. Shown are the representative results of 2 independent experiments as mean ± SD (n ≥ 10). *P < .001 compared with untreated. (E) CD34+ EPCs were isolated from healthy controls and incubated with AG490 (10μM) alone and in combination with EPO (50 mU/mL). Shown are the representative results of 3 different experiments as mean ± SD (n ≥ 32). *P < .001 compared with control. (F) CD34+ EPCs were isolated from healthy controls and incubated with LY294002 (5μM) alone and in combination with EPO (50 mU/mL). Shown is a representative of 4 independent experiments as mean ± SD (n ≥ 37). *P < .001 compared with control, no EPO.
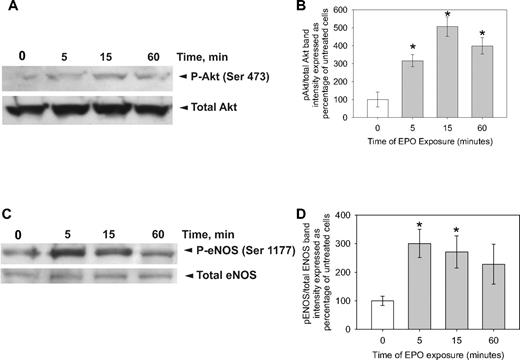
To further investigate the dependence of βC-R on VEGF-R2, we used shRNA to knock down VEGF-R2 expression. In EPCs transfected with shRNA against VEGF-R2, cEPO was unable to induce NO (Figure 3D). These results confirmed a βC-R dependence on VEGF-R2. Approaching the issue from the standpoint of downstream signaling, EPO exerts its effects by inducing homodimerization of 2 EPO-R molecules, initiating the Janus kinase (JAK)/signal transducer and activator of transcription (STAT) signal transduction cascade that regulates cell proliferation and differentiation,28 whereas the VEGF effect is mediated by the VEGF-R2/IRS-1/phosphatidylinositol 3-kinase (PI3-K)/Akt axis to regulate eNOS phosphorylation on Ser1177 in conjunction with the ERK signaling pathway.29 Consistent with a role of VEGF-R2 in the EPO-NO pathway, tyrphostin AG-490, a potent inhibitor of the JAK-2 tyrosine kinase, did not block EPO stimulation of NO, whereas LY294002, a specific Akt inhibitor, did (Figure 3E-F). The inhibitor studies were also confirmed by immunoblot. Cell lysates of EPCs treated with EPO for 5, 15, and 60 minutes were probed for Akt or eNOS by immunoblot. Within 5 minutes of EPO treatment, there was a significant increase in Akt phosphorylated at serine 473 and Akt-dependent eNOS phosphorylation at serine 1177 (Figure 4).
EPO leads to phosphorylation of Akt and eNOS. CD34+ EPCs were treated with EPO (50 mU/mL) for 0, 5, 15, or 60 minutes as indicated before lysing the cells, running an SDS-PAGE, and transferring to PVDF. (A) The membranes were probed with an antibody against Akt phosphorylated at serine 473 or total Akt. Shown is one of 2 experiments. (B) Mean ± SD of the densitometric analysis of the bands from 2 different experiments. All data are normalized to the average ratio of phosphorylated Akt over total Akt in the 0 minute of exposure lane. *P < .001 compared with the 0 minutes of exposure. (C) The membranes were probed with an antibody against eNOS phosphorylated at serine 1177 or total eNOS. Shown is 1 of 2 experiments. (D) Mean ± SD of the densitometric analysis of the bands from 2 different experiments. All data are normalized to the average ratio of phosphorylated eNOS over total eNOS in the 0 minute of exposure lane. *P < .003 compared with 0 minutes of exposure.
EPO leads to phosphorylation of Akt and eNOS. CD34+ EPCs were treated with EPO (50 mU/mL) for 0, 5, 15, or 60 minutes as indicated before lysing the cells, running an SDS-PAGE, and transferring to PVDF. (A) The membranes were probed with an antibody against Akt phosphorylated at serine 473 or total Akt. Shown is one of 2 experiments. (B) Mean ± SD of the densitometric analysis of the bands from 2 different experiments. All data are normalized to the average ratio of phosphorylated Akt over total Akt in the 0 minute of exposure lane. *P < .001 compared with the 0 minutes of exposure. (C) The membranes were probed with an antibody against eNOS phosphorylated at serine 1177 or total eNOS. Shown is 1 of 2 experiments. (D) Mean ± SD of the densitometric analysis of the bands from 2 different experiments. All data are normalized to the average ratio of phosphorylated eNOS over total eNOS in the 0 minute of exposure lane. *P < .003 compared with 0 minutes of exposure.
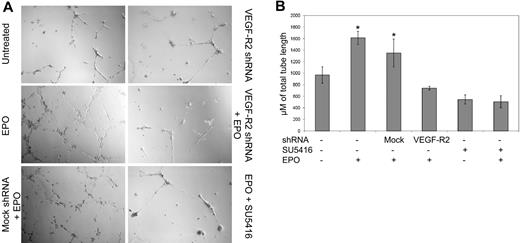
To examine whether the dependence on VEGF-R2 on EPO-mediated actions was limited to NO stimulation or extended to other downstream effects, we examined tube formation. Several previous studies have demonstrated that EPO leads to an increase in tube formation of endothelial cells30 and EPCs.5,31 We trypsinized cultured EPC colonies that have been transfected with a control shRNA or transfected with the VEGF-R2 shRNA and plated onto Matrigel-plated dishes in the presence or absence of EPO. EPO caused a statistical increase in tube formation compared with the untreated, control shRNA transfected cells (Figure 5). However, if the cells were transfected with the VEGF-R2 shRNA, EPO did not induce any increase tube formation above background. SU5416, a specific VEGF-R2 tyrosine kinase inhibitor, also blocked the ability for EPO to increase tube formation (Figure 5B).
EPO-induced tube formation is dependent on VEGF-R2. EPCs obtained from healthy control subjects were left untreated or transfected with mock shRNA or shRNA specific for VEGFR2 24 hours before plating with HUVECs on a precoated tissue culture plate and adding EPO 50 mU/mL or EPO 50 mU/mL + SU5416 1μM as indicated. (A) After 12 hours, an inverted phase-contrast microscope was used to visualize 4 randomly selected low-powered fields. (B) Tube length (mean ± SD) is shown, and the shRNA transfected and treatment with EPO or SU5416 is indicated, representative of 2 different experiments. *P < .001 compared with untreated cells.
EPO-induced tube formation is dependent on VEGF-R2. EPCs obtained from healthy control subjects were left untreated or transfected with mock shRNA or shRNA specific for VEGFR2 24 hours before plating with HUVECs on a precoated tissue culture plate and adding EPO 50 mU/mL or EPO 50 mU/mL + SU5416 1μM as indicated. (A) After 12 hours, an inverted phase-contrast microscope was used to visualize 4 randomly selected low-powered fields. (B) Tube length (mean ± SD) is shown, and the shRNA transfected and treatment with EPO or SU5416 is indicated, representative of 2 different experiments. *P < .001 compared with untreated cells.
Next, we examined the colocalization of VEGF-R2 and βC-R by immunofluorescent staining (Figure 6). At baseline, both VEGF-R2 and βC-R have a perinuclear localization. With EPO stimulation, the distribution of both VEGF-R2 and the βC-R takes on a much more diffuse cell membrane appearance. In addition, with VEGF and EPO stimulation, the distribution of both VEGF-R2 and βC-R localizes to the periphery. Importantly, under all conditions, VEGFR and βC-R remain colocalized. Software analysis of the double immunofluorescence of VEGF-R2 and βC-R confirmed the true localization of βC-R and VEGF-R2 (Figure 6).
VEGF-R2 interacts with the βC-R. (A) CD34+ EPCs were treated with EPO (50 mU/mL) or VEGF (100 ng/mL) or mock-treated for 3 hours before fixation. Cells were stained with VEGF-R2 and βC-R antibodies before probing with secondary antibodies and imaging by confocal microscopy. (B) Spatial analysis of localization of βC-R and VEGF-R2 demonstrates colocalization.
VEGF-R2 interacts with the βC-R. (A) CD34+ EPCs were treated with EPO (50 mU/mL) or VEGF (100 ng/mL) or mock-treated for 3 hours before fixation. Cells were stained with VEGF-R2 and βC-R antibodies before probing with secondary antibodies and imaging by confocal microscopy. (B) Spatial analysis of localization of βC-R and VEGF-R2 demonstrates colocalization.
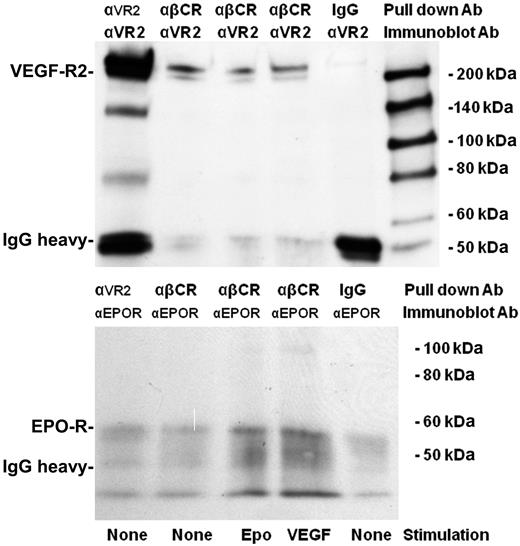
To examine whether VEGF-R2 and βC-R were in a stable complex, we performed a coimmunoprecipitation (Figure 7). An α-βC-R antibody was used to immunoprecipitate βC-R from CD34+ EPC cell lysates before immunoblotting with an α-VEGF-R2 antibody. A band of the correct molecular weight for VEGF-R2 was identified in the βC-R immunoprecipitate (Figure 6 top panel). It does not appear that treatment with either VEGF or EPO alters the amount of VEGF-R2 that associates with the βC-R. In addition, reprobing βC-R immunoprecipitates with EPO-R antibody revealed the bands of the expected molecular weight (Figure 7 bottom panel), demonstrating that the EPO-R interacts with βC-R. Interestingly, neither the immunofluoresence (Figure 6) nor coimmunoprecipitation (Figure 7) suggested that treatment with either EPO or VEGF affects the amount of EPO-R bound to the βC-R/VEGF-R2 signaling complex.
Coimmunoprecipitation demonstrating interactions between βC-R with VEGF-R2 and EPO-R. CD34+ EPCs were treated with EPO (50 mU/mL) or VEGF (100 ng/mL) or mock-treated for 3 hours before lysing the cells and immunoprecipitating with an αVEGF-R2 rabbit IgG (10 μg/mL), an αβC-R mouse IgG2b (2 μg/mL), or a mouse IgG2b (20 μg/mL). The immunoprecipitated products were separated by SDS-PAGE, transferred to nitrocellulose, and probed with an αVEGF-R2 antibody, followed by membrane stripping and reprobing with EPO-R antibody.
Coimmunoprecipitation demonstrating interactions between βC-R with VEGF-R2 and EPO-R. CD34+ EPCs were treated with EPO (50 mU/mL) or VEGF (100 ng/mL) or mock-treated for 3 hours before lysing the cells and immunoprecipitating with an αVEGF-R2 rabbit IgG (10 μg/mL), an αβC-R mouse IgG2b (2 μg/mL), or a mouse IgG2b (20 μg/mL). The immunoprecipitated products were separated by SDS-PAGE, transferred to nitrocellulose, and probed with an αVEGF-R2 antibody, followed by membrane stripping and reprobing with EPO-R antibody.
Discussion
EPCs originate from the bone marrow and differentiate into endothelial cells. EPCs are thought to be important in processes, such as vasculogenesis and endothelial repair. Several studies have indirectly addressed the issue of the presence of EPCs in the circulation and their role in postnatal vasculogenesis.2,32 Importantly, the number of circulating EPCs and potentially their ability to direct endothelial repair are increased by treatment with 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors3,4,33 as well as with EPO.5-7
The EPO-R belongs to the cytokine receptor superfamily whose members include receptors for growth hormone, prolactin, G-CSF, GM-CSF, thrombopoietin, oncostatin M, and several interleukins.34 EPO exerts its effects by inducing homodimerization of 2 EPO-R molecules, initiating the JAK/STAT signal transduction cascade that regulates cell proliferation and differentiation.28 Activated JAK2 phosphorylates several intracellular proteins, including the EPO-R itself and STAT1, STAT3, or STAT5 resulting in downstream signal transduction.35,36 The phosphorylated EPO-R acts as a docking site for various intracellular proteins containing Src (tyrosine kinase) homology 2 domains. It has previously been reported that the EPO-R has a physical37 and functional38 interaction with the common β receptor (βC-R) subunit, also known as CD131. The βC-R is a signal-transducing component of GM-CSF, interleukin-3 (IL-3), and the IL-5 receptors.39 The βC-R is not thought to be involved in erythropoiesis because the βC-R knockout mouse exhibits normal hematopoiesis.40
The tissue-protective effects of EPO have been demonstrated in models of ischemic, traumatic, and inflammatory injuries14 and are not lost by carbamylation.15 Interaction of the EPO-R with the βC-R is necessary for the tissue-protective effects of EPO.16 However, the role of the βC-R is controversial. Um et al demonstrated that, using a differentiated human neuroblastoma cell line, SH-SY5Y, knock-down of the EPO-R using shRNA resulted in loss of the antiapoptotic effect of EPO.41 In addition, the authors were not able to detect the βC-R on the differentiated SH-SY5Y cells.
Previously, EPO has been shown to increase EPC mobilization,42,43 proliferation,43 and differentiation.43 EPO treatment is associated with an increase in the adhesive and proliferative properties of circulating EPCs in patients with congestive heart failure in a PI3-K–dependent fashion.44 In animal models, the ability of EPO to induce EPC mobilization is associated with its ability to decrease doxorubicin-induced myopathy45 and infarct size,46 improve cardiac function, and induce neovascularization in chronic heart failure models.47 This has prompted a study of the ability of EPO to improve left ventricular function after a myocardial infarction.48
The action of EPO on EPCs has been suggested to be secondary to the EPO-induced phosphorylation of Akt,5 which results in an increase in eNOS activity. However, this was previously assumed to be secondary to EPO activation of the homodimeric EPO receptor activating JAK2 leading to PI3-K activation.49,50 We have used 2 different approaches to demonstrate that the action of EPO on EPCs is mediated via the heterodimeric EPO-R/βC-R (Figure 8). First, we have demonstrated that cEPO, a form of EPO that does not activate the homodimeric EPO-R, is able to mediate an increase in bioavailable NO and an increase in migration of EPCs. Second, EPC colonies isolated from βC-R knockout mice did not increase their level of bioavailable NO in response to NO treatment. These findings are consistent with EPO acting through the heterodimeric EPO-R/βC-R to mediate its effect on EPCs.
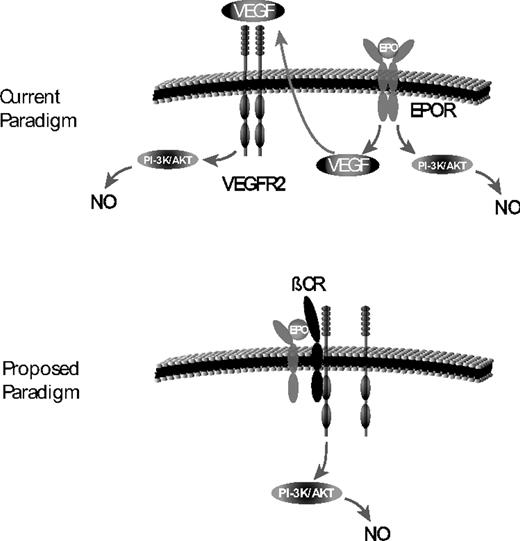
New paradigm for EPO-induced NO. Currently, it is thought that EPO induction of NO is secondary to downstream signaling of the homodimeric EPO receptor via Akt. In addition, it is thought that EPO stimulation of the EPO receptor can lead to an increase in VEGF that can bind to VEGF-R2 also leading to NO expression. We propose that it is the heterodimeric EPO receptor (composed of the βC-R and EPO receptor) that is responsible for NO stimulation. In addition, the βC-R forms a complex with VEGF-R2, and this interaction is critical for stimulation of NO not only by the heterodimeric EPO receptor but also for VEGF-R2.
New paradigm for EPO-induced NO. Currently, it is thought that EPO induction of NO is secondary to downstream signaling of the homodimeric EPO receptor via Akt. In addition, it is thought that EPO stimulation of the EPO receptor can lead to an increase in VEGF that can bind to VEGF-R2 also leading to NO expression. We propose that it is the heterodimeric EPO receptor (composed of the βC-R and EPO receptor) that is responsible for NO stimulation. In addition, the βC-R forms a complex with VEGF-R2, and this interaction is critical for stimulation of NO not only by the heterodimeric EPO receptor but also for VEGF-R2.
Perhaps the more significant aspect of our work is the demonstration that EPO requires the VEGF-R2 for its actions. If we block VEGF-R2 signaling using SU1498, a relatively specific VEGF-R2 tyrosine kinase inhibitor, or by knocking down VEGF-R2 with shRNA, EPO cannot mediate its effects on EPCs. This was true whether we examined an increase in bioavailable NO concentrations, cGMP, or the ability of EPCs to form tubes. One, perhaps trivial, explanation for the requirement of VEGF-R2 is that EPO leads to an induction of VEGF that then binds and stimulates VEGF-R2. Although this cannot be entirely ruled out, it is crucial to note that adding anti-VEGF antibody to the media did not demonstrably diminish the ability of EPO to stimulate NO, even though the addition of anti-VEGF to the media inhibited the ability of VEGF to induce NO. In addition, arguing against this simplistic explanation is that VEGF induction of NO in EPCs derived from mouse bone marrow required the presence of the βC-R. Thus, all of these findings suggested a novel interaction for the βC-R and VEGF-R2. This interaction was further confirmed by pull-down assays and by colocalization by immunostaining.
Previous studies have demonstrated that all effects of EPO-induced target genes in erythroid progenitor cells were blocked by LY294002.50 In this paper, the authors demonstrated that EPO treatment led to the induction of hyperphosphorylation of retinoblastoma protein as well as up-regulation of cyclin D3, E, and A. Interestingly, VEGF also is known to induce hyperphosphorylation of retinoblastoma protein, cyclin E, and cyclin A51 and cyclin D3.52 Our results that demonstrate that EPO stimulation of the heterodimeric EPO-R/βC-R signals through VEGF-R2 is consistent with all of these findings.
Our results are also consistent with a recent report demonstrating that activation of either GM-CSFR or VEGF-R2 was shown to determine the migration of both receptor elements (VEGF-R2 and the common β-chain of the GM-CSFR) to lipid rafts.53 In addition, there was a recent demonstration that erythropoietin activates nitric oxide synthase in murine erythroctyes via the PI3-K/Akt pathway.54
The results presented suggest a novel interaction between VEGF-R2 and the βC-R that is necessary for VEGF or EPO to lead to an induction of NO. Clinical evidence supporting this cross-stimulation comes from studies of EPO in cancer patients. Recent studies suggest that EPO treatment leads to increased mortality in patients with metastatic breast cancer.55 Interaction of the βC-R with VEGF-R2 may explain this clinical finding because stimulation of VEGF-R256 and its induction of NO57 are critical in angiogenesis, an essential process in disease metastasis. Our finding may also explain the eosinophilia in immature infants treated with EPO58 because IL-5, a chemokine that shares the βC-R, primes neutrophils and stimulates their recruitment. Indeed, the other chemokines that share the βC-R (IL-3 and GM-CSF) can stimulate eosinophilic chemotaxis.59 Thus, the novel interaction between VEGF-R2 and βC-R may explain several clinical observations and side effects of commonly used treatments.
Erythropoietin is the most widely prescribed cytokine in the world, and it has numerous clinical benefits. This work provides further important insights in the mechanism of action of the EPO and EPO-βC-VEGF receptors, which in our opinion might be helpful in avoiding or correcting side effects of the erythropoietin therapies.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Applied Precision (Issaquah, WA) for access to the Delta Vision Core Imaging System and technical help. M.S.S. thanks Dr Richard Johnson and Dr Bruce Kone for many helpful discussions and for critically reading the manuscript.
This work was supported by University of Florida Division of Nephrology Gatorade Funds.
Authorship
Contribution: L.S. isolated EPCs, did the live imaging experiments, transfections, IP, and immunoblots, and wrote the first draft of the manuscript; Y.P.D. assisted in the animal experiments and study design; E.B. assisted with the experiments; Z.Z. performed the experiment to determine that the carbamylated EPO was devoid of erythropoiesis activity; A.S. initiated the studies on erythropoietin; J. Brennan did background research and proofread the manuscript; S.I.Z. developed and oversaw the DAF-FM measurements; Y.S. helped with design of signaling pathway experiments and immunohistochemistry; J. Bungert confirmed that the carbamylated EPO is void of erythropoiesis activity and helped with study design; and M.S.S. was involved in the planning of all experiments, finalized the manuscript, and designed the figure layout.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Mark S. Segal, PO Box 100224, Gainesville, FL 32610-0267; e-mail: segalms@medicine.ufl.edu.