Abstract
Our recent study demonstrated that a novel proteasome inhibitor NPI-0052 is distinct from bortezomib (Velcade) and, importantly, triggers apoptosis in multiple myeloma (MM) cells resistant to bortezomib. Here we demonstrate that combining NPI-0052 and lenalidomide (Revlimid) induces synergistic anti-MM activity in vitro using MM-cell lines or patient MM cells. NPI-0052 plus lenalidomide-induced apoptosis is associated with (1) activation of caspase-8, caspase-9, caspase-12, caspase-3, and poly(ADP) ribose polymerase; (2) activation of BH-3 protein BIM; (3) translocation of BIM to endoplasmic reticulum; (4) inhibition of migration of MM cells and angiogenesis; and (5) suppression of chymotrypsin-like, caspase-like, and trypsin-like proteasome activities. Importantly, blockade of BIM using siRNA significantly abrogates NPI-0052 plus lenalidomide-induced apoptosis. Furthermore, studies using biochemical inhibitors of caspase-8 versus caspase-9 demonstrate that NPI-0052 plus lenalidomide-triggered apoptosis is primarily dependent on caspase-8 signaling. In animal tumor model studies, low-dose combination of NPI-0052 and lenalidomide is well tolerated, significantly inhibits tumor growth, and prolongs survival. Taken together, our study provides the preclinical rationale for clinical protocols evaluating lenalidomide together with NPI-0052 to improve patient outcome in MM.
Introduction
Defects in the ubiquitin-proteasome signaling pathway are linked to the pathogenesis of various human diseases1 because it regulates normal cellular processes, including cell cycle, transcription, DNA replication, and apoptosis via proteolysis of regulatory proteins. Targeting proteasomes therefore offers great promise as a novel therapeutic strategy. Bortezomib (Velcade) is the first in class proteasome inhibitor, approved by the Food and Drug Administration for the treatment of relapsed, relapsed/refractory, and newly diagnosed multiple myeloma (MM).1-5 Even though bortezomib therapy is a major advance,3,4 it has been associated with possible off-target toxicities and the development of drug resistance.6,7 Our recent study demonstrated that a novel proteasome inhibitor NPI-00528 is distinct from bortezomib, and importantly, triggers apoptosis in MM cells resistant to bortezomib therapies.9 These preclinical data provided the basis for the ongoing phase 1 clinical trial of NPI-0052 in relapsed/refractory MM patients.
Besides the development of new proteasome inhibitors, combination approaches have also shown promise in reducing toxicities and overcoming drug resistance associated with bortezomib. For example, a phase 1/2 clinical trial of bortezomib with the immunomodulatory agent lenalidomide (Revlimid) and low-dose dexamethasone demonstrated safety and remarkable efficacy in relapsed refractory and newly diagnosed MM patients.10,11 The clinical trial was based on preclinical studies showing that lenalidomide triggered growth arrest or apoptosis in drug-resistant MM cells. The mechanism mediating lenalidomide activity includes caspase-8 activation, down-regulation of cIAP-2 and FLICE inhibitory protein, blockade of angiogenesis, reduced adhesion of MM cells to stromal cells, inhibition of cytokines (vascular endothelial growth factor [VEGF], interleukin-6 [IL-6]), and attenuation of NF-κB activity.12-16 In addition, lenalidomide has been shown to stimulate host anti-MM natural killer–cell immunity.17 The observation that the combination of bortezomib with lenalidomide can overcome clinical bortezomib resistance, coupled with our findings that NPI-0052 is a potent proteasome inhibitor, suggests that combining NPI-0052 with lenalidomide may trigger synergistic anti-MM activity.
In the present study, we characterized the effects of NPI-0052 and lenalidomide combinations against MM-cell lines and primary patient MM cells resistant to conventional and novel therapies. Both in vitro and in an in vivo MM xenograft model, combined NPI-0052 and lenalidomide inhibits growth of MM cells and overcomes drug resistance, setting the stage for potential clinical trials of combination therapy to improve patient outcome in MM.
Methods
Cell culture
MM.1S (dexamethasone [Dex]–sensitive), MM.1R (Dex-resistant), RPMI 8226, doxorubicin (Dox)–resistant (Dox-40), U266, KMS12PE, and INA-6 (IL-6–dependent) human MM-cell lines were cultured in complete medium (RPMI 1640 media supplemented with 10% fetal bovine serum, 100 units/mL penicillin, 100 μg/mL streptomycin, and 2mM l-glutamine). Tumor cells from MM patients were purified (> 95% purity) by CD138+ selection using the Auto MACS magnetic cell sorter (Miltenyi Biotec). Informed consent was obtained from all patients in accordance with the Helsinki protocol. Peripheral blood mononuclear cells (PBMCs) from normal healthy donors were maintained in complete culture medium. The drug sources are as follows: NPI-0052 from Nereus Pharmaceuticals, and lenalidomide (discarded patient drug) and Dex from Calbiochem.
Cell viability, proliferation, and apoptosis assays
Cell viability was assessed by 3-(4,5-dimethylthiozol-2-yl)-2,5-diphenyltetrazolium bromide (MTT; Chemicon International), as previously described.12 Percentage cell death in control versus untreated cells was obtained using Trypan blue exclusion assay. Apoptosis was quantified using annexin V/propidium iodide staining assay kit, as per the manufacturer's instructions (R&D Systems) and analysis on a FACSCalibur (BD Biosciences). Cell proliferation was assessed by the nonradioactive WST-1 colorimetric assay, as per the manufacturer's instructions (BioVision).
In vitro migration and capillary-like tube structure formation assays
Migration was assessed by Transwell Insert Assays (Chemicon), as previously described.18 Angiogenesis was determined in vitro by Matrigel capillary-like tube structure formation assay.19 For endothelial tube formation assay, human vascular endothelial cells (HUVECs) were purchased from Clonetics and maintained in endothelial cell growth medium-2 (EGM2 MV SingleQuots; Clonetics) supplemented with 5% fetal bovine serum. After 3 passages, HUVEC viability was measured using Trypan blue exclusion assay, and less than 10% of cell death was observed with single or combined agents.
Western blotting and protein quantification
Immunoblot analysis was performed using antibodies to caspase-8, caspase-9, caspase-3 (Cell Signaling), poly(ADP) ribose polymerase (PARP), BIM, Hsp-27, Hsp-70, Hsp-90, Bcl-6, actin, or tubulin (BD Biosciences PharMingen). Blots were then developed by enhanced chemiluminescence (GE Healthcare). Densitometry of protein bands was acquired using an AlphaImager EC gel documentation system (Alpha Innotec), and bands were analyzed using the spot densitometry analysis tool (Alpha Ease FC software, Version 4.1.0). Relative BIM and caspase-12 levels were obtained by dividing total values of each protein by the corresponding actin value.
Preparation of ER fractions
Cell fractionation was performed as previously described.20 Briefly, MM.1S cells were disrupted with a Dounce homogenizer in 10mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid/KOH (pH 7.6), 10mM KCl, 1mM MgCl2, 1mM dithiothreitol containing aprotinin (Sigma-Aldrich), 0.5mM phenylmethlylsulfonyl fluoride, and complete protease inhibitor mixture. Immediately after homogenization, sucrose was added to 250mM; nuclei and unbroken cells were removed by centrifugation at 3000g for 3 minutes; and the heavy membrane (mitochondria-rich) fraction was sedimented by centrifugation at 9000g for 20 minutes. The supernatant was split into the S-100 (cytosol) and the light membrane (endoplasmic reticulum [ER]) fractions by centrifugation at 100 000g for 60 minutes.
Tranfection assays
The BIM knockdown experiment was performed using siRNA BIM (kindly provided by Anthony Letai, Dana-Farber Cancer Institute, Boston, MA).21 MM.1S cells were transfected with siRNA BIM or scrambled siRNA (Dharmacon) using the cell line Nucleofactor Kit V solution (Amaxa Biosystems/Lonza), as per the manufacturer's instructions. Cells were pretreated with lenalidomide for 24 hours, and then NPI-0052 was added for an additional 24 hours, followed by analysis for apoptosis by annexin V/propidium iodide staining.
In vitro proteasome activity assays were performed using fluorogenic peptide substrates, as previously described.19
Human plasmacytoma xenograft model
All animal studies were approved by the Dana-Farber Cancer Institute Institutional Animal Care and Use Committee. The xenograft tumor model was performed as previously described.9,19,22 CB-17 SCID-mice (n = 30; Charles River Laboratories) were subcutaneously inoculated with 5.0 × 106 MM.1S cells in 100 μL of serum-free RPMI 1640 medium. When tumors were measurable (∼ 150 mm3) approximately 3 weeks after MM-cell injection, mice were treated orally with vehicle alone, NPI-0052 (0.15 mg/kg), lenalidomide (2.5 mg/kg), lenalidomide (5.0 mg/kg), NPI-0052 (0.15 mg/kg) plus lenalidomide (2.5 mg/kg), or NPI-0052 (0.15 mg/kg) plus lenalidomide (5.0 mg/kg) for 24 days on a twice-weekly schedule for NPI-0052 and 4 consecutive days weekly for lenalidomide. Statistical significance of differences observed in NPI-0052-, lenalidomide-, or NPI-0052 plus lenalidomide–treated mice was determined using a Student t test.
In situ detection of apoptosis, IHC determination of proliferation, and assessment of microvessel density D
Apoptotic cells in resected mice tumors were identified by immunohistochemical (IHC) staining for caspase-3 activation and hematoxylin and eosin staining.19 Microvessel density, reflective of tumor angiogenesis, was quantified by IHC staining of factor VIII. VEGFR1/FLT-1 and YB-1 expression was examined by IHC staining with specific VEGFR1 and YB-1 antibodies (Abs; Abcam), as previously described.19,23
Statistical analysis
Statistical significance of differences observed in drug-treated versus control cultures was determined using the Student t test. The minimal level of significance was P less than .05. The Jonckheere-Terpstra trend test was used to measure viability of lymphocytes and cell lines. Statistical significance in animal studies was determined using a Student t test. Tumor volume and survival of mice were measured using the GraphPad PRISM (GraphPad Software). Isobologram analysis24 was performed using the CalcuSyn software program (Biosoft). A combination index value less than 1.0 indicates synergism and a value more than 1.0 indicates antagonism.
Results
Combined low doses of NPI-0052 and lenalidomide trigger synergistic anti-MM activity
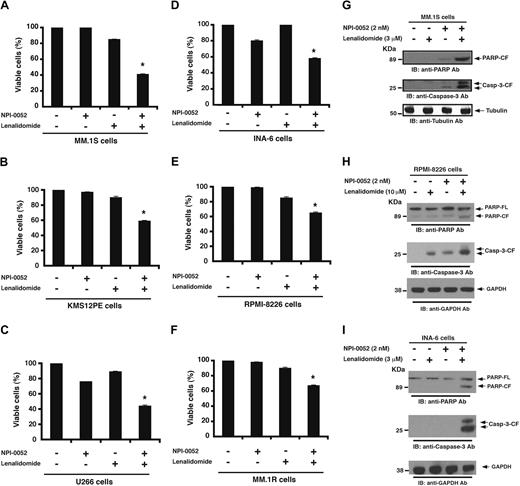
Human MM-cell lines (MM.1S, INA-6, RPMI-8226, MM.1R, KMS12PE, and U266) were pretreated with lenalidomide for 24 hours; NPI-0052 was then added for an additional 24 hours, followed by assessment for cell viability using MTT assays. For these studies, we used NPI-0052 and lenalidomide at concentrations lower than their maximal cytotoxic concentration for each cell line. A significant decrease in viability of all cell lines was observed in response to treatment with combined low doses of NPI-0052 and lenalidomide compared with either agent alone (P < .05; n = 3; Figure 1A-F). Shown in Figure 1 are representative results from minimally toxic and maximally additive concentrations of each agent. For example, treatment of MM.1S cells with low doses of NPI-0052 (2nM) and lenalidomide (3μM) triggers a 59% decrease in viability, whereas only minimal cell killing was observed using either of these agents alone at these low concentrations (Figure 1A). Isobologram analysis confirmed synergistic24 anti-MM activity of agents, with a combination index less than 1.0 in all MM-cell lines tested. These data demonstrate synergistic anti-MM activity of NPI-0052 plus lenalidomide.
Combination of low doses of NPI-0052 and lenalidomide induces synergistic MM-cell death. (A-F) MM-cell lines were pretreated with or without lenalidomide for 24 hours, and then NPI-0052 was added for an additional 24 hours, followed by assessment for cell viability using MTT assays. The experiments with single agents and the respective combinations were carried out simultaneously. The concentrations of drugs, either alone or in combination, were as follows: for MM.1S, KMS12PE, and INA-6 cells: 2nM NPI-0052, 3μM lenalidomide, or NPI-0052 (2nM) plus lenalidomide (3μM); for RPMI-8226 cells: 2nM NPI-0052, 10μM lenalidomide, or NPI-0052 (2nM) plus lenalidomide (10μM); for U266 cells: 5nM NPI-0052, 10μM lenalidomide, or NPI-0052 (5nM) plus lenalidomide (10μM); and for MM.1R cells: 3nM NPI-0052, 5μM lenalidomide, or NPI-0052 (3nM) plus lenalidomide (5μM). *P < .05 for all cell lines. (G-I) MM.1S, RPMI-8226, or INA-6 cells were pretreated with or without lenalidomide for 24 hours, and then NPI-0052 was added for an additional 24 hours and harvested, and whole cell lysates were subjected to immunoblot analysis with anti-PARP, anticaspase-3, antitubulin, or anti-GAPDH Abs. FL indicates full length; and CF, cleaved fragment. Blots shown are representative of 3 independent experiments.
Combination of low doses of NPI-0052 and lenalidomide induces synergistic MM-cell death. (A-F) MM-cell lines were pretreated with or without lenalidomide for 24 hours, and then NPI-0052 was added for an additional 24 hours, followed by assessment for cell viability using MTT assays. The experiments with single agents and the respective combinations were carried out simultaneously. The concentrations of drugs, either alone or in combination, were as follows: for MM.1S, KMS12PE, and INA-6 cells: 2nM NPI-0052, 3μM lenalidomide, or NPI-0052 (2nM) plus lenalidomide (3μM); for RPMI-8226 cells: 2nM NPI-0052, 10μM lenalidomide, or NPI-0052 (2nM) plus lenalidomide (10μM); for U266 cells: 5nM NPI-0052, 10μM lenalidomide, or NPI-0052 (5nM) plus lenalidomide (10μM); and for MM.1R cells: 3nM NPI-0052, 5μM lenalidomide, or NPI-0052 (3nM) plus lenalidomide (5μM). *P < .05 for all cell lines. (G-I) MM.1S, RPMI-8226, or INA-6 cells were pretreated with or without lenalidomide for 24 hours, and then NPI-0052 was added for an additional 24 hours and harvested, and whole cell lysates were subjected to immunoblot analysis with anti-PARP, anticaspase-3, antitubulin, or anti-GAPDH Abs. FL indicates full length; and CF, cleaved fragment. Blots shown are representative of 3 independent experiments.
We next examined whether the NPI-0052 plus lenalidomide–induced decrease in viability resulted from apoptosis. Treatment of MM.1S, RPMI-8226, and INA-6 cells with combined low doses of NPI-0052 plus lenalidomide triggered a marked increase in proteolytic cleavage of PARP,25 a signature event during apoptosis (Figure 1G-I). Similarly, the combination of NPI-0052 and lenalidomide induced cleavage of caspase-3, an upstream activator of PARP.26 Moreover, the apoptotic effect of NPI-0052 plus lenalidomide was evidenced by a significant increase in the annexin V+/propidium iodide− apoptotic cell population in all cell lines examined (P < .05, n = 3; supplemental Figure 1A-C, available on the Blood website [see the Supplemental Materials link at the top of the online article]). Cell-cycle analysis showed that the low-dose combination of lenalidomide and NPI-0052 triggered a marked increase in sub-G0/G1 population, followed by a significant decrease in G2/M and S phase. No change in G1 phase was observed at that time point. Longer periods of exposure to combined drug regimen further enhanced cell death (sub-G0/G1 phase; data not shown).
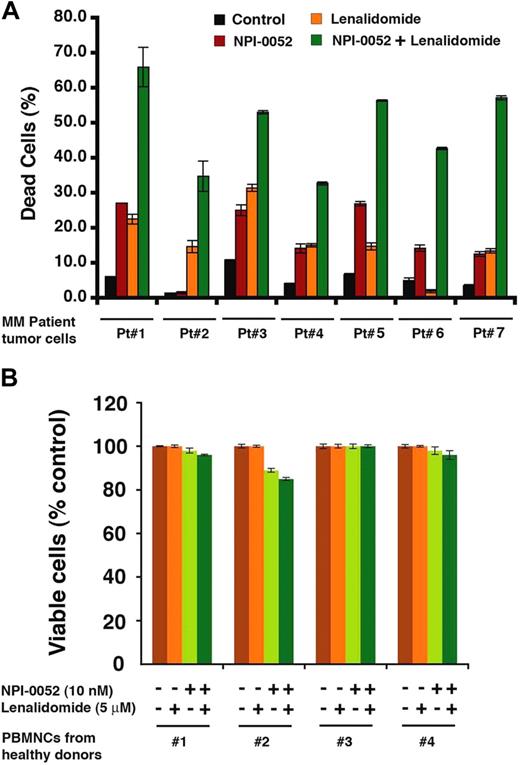
To determine whether NPI-0052 plus lenalidomide similarly affected purified patient MM cells, tumor cells from 7 MM patients relapsing after multiple previous therapies, including lenalidomide (patient 1), bortezomib (patients 2, 3, and 5), Dex/melphalan (patient 4), and lenalidomide/bortezomib (patients 6 and 7) were treated with NPI-0052 plus lenalidomide, or both. A significant increase in cell death of all patient MM cells was noted after combination therapy (P < .05 for all patients; Figure 2A). Importantly, the combination of NPI-0052 (2nM) plus lenalidomide (3μM) did not trigger a significant decrease in the viability of normal PBMCs (P = .25 from Jonckheere-Terpstra trend test; Figure 2B). Patients were considered refractory to specific therapy when disease progressed on therapy or relapsed within 2 months of stopping therapy. Of note, 3 of 7 patients studied were refractory to bortezomib therapy, 1 was refractory to lenalidomide, 1 was resistant to Dex and melphalan, and 2 were refractory to lenalidomide and bortezomib therapies.
Combined NPI-0052 and lenalidomide trigger antitumor activity in MM patient cells. (A) Purified patient MM cells (CD138+) were pretreated with lenalidomide for 24 hours; NPI-0052 was then added for an additional 24 hours, followed by cell death analysis using Trypan blue exclusion assay. Data are mean ± SD of triplicate samples (P < .05 for all patient samples). (B) PBMCs from healthy donors were treated (as in panel A) with indicated concentrations of NPI-0052, lenalidomide, or NPI-0052 plus lenalidomide, and then analyzed for viability using MTT assay. Data are mean ± SD (n = 3; P = .25 from Jonckheere-Terpstra test for trend).
Combined NPI-0052 and lenalidomide trigger antitumor activity in MM patient cells. (A) Purified patient MM cells (CD138+) were pretreated with lenalidomide for 24 hours; NPI-0052 was then added for an additional 24 hours, followed by cell death analysis using Trypan blue exclusion assay. Data are mean ± SD of triplicate samples (P < .05 for all patient samples). (B) PBMCs from healthy donors were treated (as in panel A) with indicated concentrations of NPI-0052, lenalidomide, or NPI-0052 plus lenalidomide, and then analyzed for viability using MTT assay. Data are mean ± SD (n = 3; P = .25 from Jonckheere-Terpstra test for trend).
Combined low doses of NPI-0052 and lenalidomide block migration of MM cells and angiogenesis and overcome the cytoprotective effects of the MM bone marrow microenvironment
We and others have shown that migration and angiogenesis play an important role in the progression of MM.18,27 The effect of NPI-0052 plus lenalidomide on these events was therefore next examined using Transwell insert systems and in vitro tubule formation assays. Serum alone markedly increased MM.1S cell migration; importantly, NPI-0052 (2nM) plus lenalidomide (3μM) significantly inhibited serum-dependent MM.1S cell migration, as reflected by a decrease in the number of crystal violet–stained cells (Figure 3A left panel). These results were further confirmed by quantification of cell migration in response to treatment with serum (18 × 104 migrated cells) versus NPI-0052 plus lenalidomide (3 × 104 migrated cells; P < .05, n = 3). These cells were more than 90% viable before and after performing the migration assay, excluding the possibility that drug-induced inhibition of migration is the result of cell death.
Combined low doses of NPI-0052 and lenalidomide block migration and tubule formation. (A) For migration assay, MM.1S cells were pretreated with lenalidomide for 12 hours, and then NPI-0052 was added for an additional 6 hours; the cells were more than 90% viable at this time point. The cells were washed and cultured in serum-free medium. After 2 hours of incubation, cells (viability > 90%) were plated on a fibronectin-coated polycarbonate membrane in the upper chamber of Transwell inserts and exposed for 4 hours to serum-containing medium in the lower chamber. Cells migrating to the bottom face of the membrane were fixed with 90% ethanol and stained with crystal violet (original magnification, 10×/0.25 numeric aperture [NA] oil). A total of 3 randomly selected fields were examined for cells that had migrated from top to bottom chambers. (Left panel) Image is representative of 2 experiments with similar results. (Right panel) The bar graph represents quantification of migrated cells. Data are mean ± SD (n = 2; P < .05 for control vs NPI-0052 plus lenalidomide–treated cells). (B) HUVECs were cultured in the presence or absence of combined low doses of NPI-0052 plus lenalidomide for 48 hours, and then assessed for in vitro angiogenesis using Matrigel capillary-like tube structure formation assays (original magnification, 4×/0.10 NA oil, media: EBM-2). (Left panel) Image is representative from 3 experiments with similar results. The in vitro angiogenesis is reflected by capillary tube branch formation (dark brown). (Right panel) The bar graph represents quantification of capillary-like tube structure formation in response to indicated agents: Branch points in several random view fields/well were counted, values were averaged, and statistically significant differences were measured using Student t test. (C-D) MM.1S and RPMI-8226 cells were cultured for 48 hours in BMSC-coated or noncoated wells with control media, NPI-0052, lenalidomide, or NPI-0052 plus lenalidomide. Cell proliferation was assessed by the nonradioactive WST-1 colorimetric assay. Data are mean ± SD of 2 independent experiments. Error bars represent SD.
Combined low doses of NPI-0052 and lenalidomide block migration and tubule formation. (A) For migration assay, MM.1S cells were pretreated with lenalidomide for 12 hours, and then NPI-0052 was added for an additional 6 hours; the cells were more than 90% viable at this time point. The cells were washed and cultured in serum-free medium. After 2 hours of incubation, cells (viability > 90%) were plated on a fibronectin-coated polycarbonate membrane in the upper chamber of Transwell inserts and exposed for 4 hours to serum-containing medium in the lower chamber. Cells migrating to the bottom face of the membrane were fixed with 90% ethanol and stained with crystal violet (original magnification, 10×/0.25 numeric aperture [NA] oil). A total of 3 randomly selected fields were examined for cells that had migrated from top to bottom chambers. (Left panel) Image is representative of 2 experiments with similar results. (Right panel) The bar graph represents quantification of migrated cells. Data are mean ± SD (n = 2; P < .05 for control vs NPI-0052 plus lenalidomide–treated cells). (B) HUVECs were cultured in the presence or absence of combined low doses of NPI-0052 plus lenalidomide for 48 hours, and then assessed for in vitro angiogenesis using Matrigel capillary-like tube structure formation assays (original magnification, 4×/0.10 NA oil, media: EBM-2). (Left panel) Image is representative from 3 experiments with similar results. The in vitro angiogenesis is reflected by capillary tube branch formation (dark brown). (Right panel) The bar graph represents quantification of capillary-like tube structure formation in response to indicated agents: Branch points in several random view fields/well were counted, values were averaged, and statistically significant differences were measured using Student t test. (C-D) MM.1S and RPMI-8226 cells were cultured for 48 hours in BMSC-coated or noncoated wells with control media, NPI-0052, lenalidomide, or NPI-0052 plus lenalidomide. Cell proliferation was assessed by the nonradioactive WST-1 colorimetric assay. Data are mean ± SD of 2 independent experiments. Error bars represent SD.
We next used in vitro capillary-like tube structure formation assays to determine whether NPI-0052 plus lenalidomide triggers antiangiogenic effects. Angiogenesis was measured in vitro using Matrigel capillary-like tube structure formation assays: HUVECs plated onto Matrigel differentiate and form capillary-like tube structures similar to in vivo neovascularization, a process dependent on cell-matrix interaction, cellular communication, and cellular motility. This assay therefore provides evidence for antiangiogenic effects of drugs/agents. HUVECs were seeded in 96-well culture plates precoated with Matrigel; treated with vehicle (dimethyl sulfoxide), NPI-0052 (2nM), lenalidomide (3μM), or NPI-0052 (2nM) plus lenalidomide (3μM) for 8 hours; and then examined for tube formation using an inverted microscope. As seen in Figure 3B, tubule formation was markedly decreased in the NPI-0052 plus lenalidomide–treated cells, but not after treatment with either agent alone. HUVEC viability was assessed using Trypan blue exclusion assay, and less than 10% cell death was observed with single or combined treatment. Together, these findings suggest that the combination of low doses of NPI-0052 and lenalidomide blocks migration and angiogenesis.
Because our previous studies have shown that the MM-host bone marrow (BM) microenvironment confers growth, survival, and drug resistance in MM cells,28,29 we next examined whether the combination of low-dose NPI-0052 plus lenalidomide retains its ability to trigger MM-cell death in the BM milieu. MM.1S, RPMI-8226, and KMS12PE cells were cultured with or without BM stromal cells (BMSCs) in the presence or absence of NPI-0052, lenalidomide, or NPI-0052 plus lenalidomide. Low doses of NPI-0052 plus lenalidomide significantly inhibited BMSC-induced proliferation of all cell lines, as assessed by WST-1 colorimetric assay (Figure 3C-D; supplemental Figure 2). These findings suggest that the low-dose NPI-0052 plus lenalidomide combination not only directly targets MM cells but also overcomes the cytoprotective effects of the MM-host BM microenvironment.
Mechanisms mediating anti-MM activity of NPI-0052 plus lenalidomide
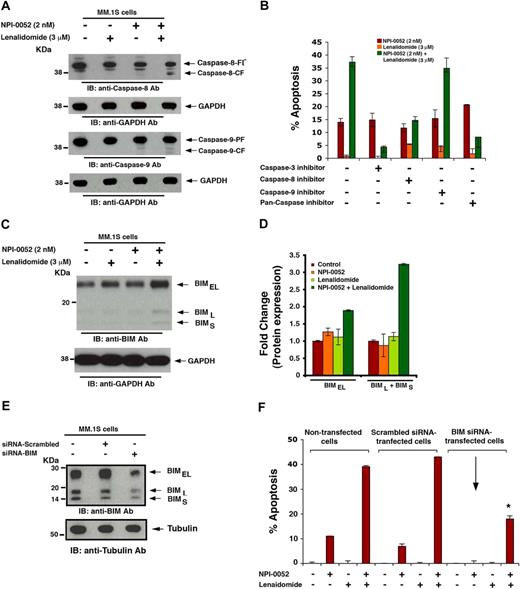
Our earlier studies showed that higher doses of NPI-0052 (7-24nM) efficiently triggered both intrinsic and extrinsic cell death pathways in various MM-cell lines.9 Here we asked whether the combination of low concentrations of each agent induced extrinsic and/or intrinsic apoptotic signaling pathways. Our results showed that NPI-0052 plus lenalidomide, but not NPI-0052 or lenalidomide alone, induced activation of caspase-8 (extrinsic), casapse-9 (intrinsic), and caspase-3 (Figure 4A). Because our data also showed a more robust cleavage of caspase-8 than caspase-9, we examined the requirement for caspase-8 versus caspase-9. Indeed, inhibition of caspase-8 (IETD-FMK) led to a significant decrease in NPI-0052 plus lenalidomide–triggered cell death (P < .05), whereas inhibition of caspase-9 (LEHD-FMK) only moderately blocked NPI-0052 plus lenalidomide–triggered MM.1S cell death (Figure 4B). Moreover, incubation of MM.1S cells with pan-caspase inhibitor (Z-VAD-FMK) markedly abrogated NPI-0052 plus lenalidomide–induced apoptosis (Figure 4B). These biochemical data suggest that NPI-0052 plus lenalidomide–induced MM-cell apoptosis occurs predominantly via caspase-8–mediated signaling pathway.
Mechanisms mediating anti-MM activity of NPI-0052 plus lenalidomide. (A) MM.1S cells were pretreated with or without lenalidomide for 24 hours, and then NPI-0052 was added for an additional 24 hours. Cells were harvested, and whole cell lysates were subjected to immunoblot analysis with anticaspase-8 or anticaspase-9 Abs. FL indicates full length; and CF, cleaved fragment. Blots shown are representative of 3 independent experiments. (B) MM.1S cells were treated with indicated agents (as in panel A) in the presence or absence of biochemical inhibitors of caspase-3, caspase-8, or caspase-9, and then analyzed for apoptosis using annexin V/propidium iodide staining assay. Data are mean ± SD (n = 3; P < .005). (C) MM.1S cells were pretreated with or without lenalidomide for 24 hours, and then NPI-0052 was added for an additional 24 hours. Cells were harvested, and whole cell lysates were subjected to immunoblot analysis with anti-BIM or anti-GAPDH Abs. (D) Bar graph showing quantification by densitometry of BIM protein bands in panel C: A 2- to 3-fold increase in BIMEL and BIM(L+S), respectively, was noted in NPI-0052 plus lenalidomide–treated versus untreated cells. Samples were normalized to GAPDH. (E) MM.1S cells were transfected with siRNA BIM or scrambled siRNA for 24 hours and harvested; whole cell lysates were subjected to immunoblot analysis with anti-BIM or antitubulin Abs. Blots shown are representative of 2 independent experiments. (F) MM.1S cells were transfected with siRNA BIM or scrambled siRNA for 24 hours and treated with indicated agents (as in panel A), followed by analysis for apoptosis by annexin V/propidium iodide staining. As a control, nontransfected cells were also treated with indicated drugs and similarly analyzed. Data are mean ± SD (n = 2; *P > .004). Error bars represent SD.
Mechanisms mediating anti-MM activity of NPI-0052 plus lenalidomide. (A) MM.1S cells were pretreated with or without lenalidomide for 24 hours, and then NPI-0052 was added for an additional 24 hours. Cells were harvested, and whole cell lysates were subjected to immunoblot analysis with anticaspase-8 or anticaspase-9 Abs. FL indicates full length; and CF, cleaved fragment. Blots shown are representative of 3 independent experiments. (B) MM.1S cells were treated with indicated agents (as in panel A) in the presence or absence of biochemical inhibitors of caspase-3, caspase-8, or caspase-9, and then analyzed for apoptosis using annexin V/propidium iodide staining assay. Data are mean ± SD (n = 3; P < .005). (C) MM.1S cells were pretreated with or without lenalidomide for 24 hours, and then NPI-0052 was added for an additional 24 hours. Cells were harvested, and whole cell lysates were subjected to immunoblot analysis with anti-BIM or anti-GAPDH Abs. (D) Bar graph showing quantification by densitometry of BIM protein bands in panel C: A 2- to 3-fold increase in BIMEL and BIM(L+S), respectively, was noted in NPI-0052 plus lenalidomide–treated versus untreated cells. Samples were normalized to GAPDH. (E) MM.1S cells were transfected with siRNA BIM or scrambled siRNA for 24 hours and harvested; whole cell lysates were subjected to immunoblot analysis with anti-BIM or antitubulin Abs. Blots shown are representative of 2 independent experiments. (F) MM.1S cells were transfected with siRNA BIM or scrambled siRNA for 24 hours and treated with indicated agents (as in panel A), followed by analysis for apoptosis by annexin V/propidium iodide staining. As a control, nontransfected cells were also treated with indicated drugs and similarly analyzed. Data are mean ± SD (n = 2; *P > .004). Error bars represent SD.
Previous studies have established a role of BH3-only Bcl-2 family protein BIM during apoptosis.30 Our data showed that combined NPI-0052 plus lenalidomide, but not either agent alone, significantly up-regulated all 3 isoforms of BIM protein (Figure 4C-D). In contrast, examination of another proapoptotic BH3-only family protein NOXA showed little, if any, alteration in response to NPI-0052 plus lenalidomide treatment (data not shown). To determine whether BIM mediates NPI-0052 plus lenalidomide–induced apoptosis, we knocked down BIM expression using the siRNA strategy.21 The functional specificity of BIM siRNA was confirmed by a marked decrease in protein levels of all 3 isoforms of BIM, without altering Bcl-2 levels (Figure 4E; and data not shown). Specifically, quantification of protein bands by densitometry reveals 43% total BIM knockdown, with more than half of BIML and BIMS isoforms reduced compared with negative control siRNA. Samples were normalized to tubulin. Importantly, transfection of siRNA BIM, but not negative-control (scrambled) siRNA, significantly inhibited NPI-0052 plus lenalidomide–induced apoptosis in MM.1S cells (Figure 4F; P < .05). These findings suggest that NPI-0052 plus lenalidomide–triggered apoptosis is mediated, at least in part, via BIM.
NPI-0052 plus lenalidomide up-regulates BIM localization to ER and triggers ER stress
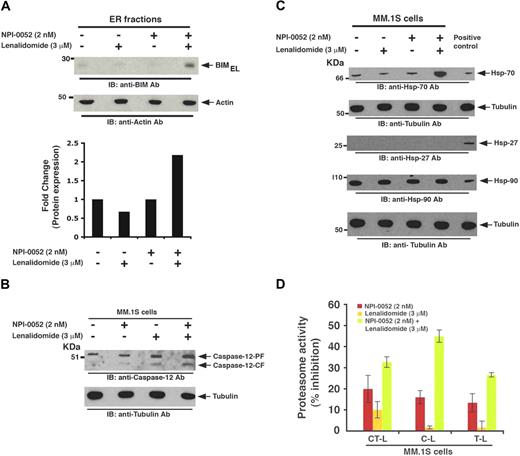
BIM plays a role in initiating mitochondrial apoptotic signaling cascade via cytochrome-c release and caspase-9 activation.30 Our data show that, although NPI-0052 plus lenalidomide trigger BIM induction in MM cells (Figure 4C), only a modest caspase-9 activation is observed (Figure 4A). Moreover, NPI-0052 plus lenalidomide–induced apoptosis is less dependent on caspase-9–mediated signaling (Figure 4B). These findings suggested that BIM probably activates other downstream apoptotic signaling cascades during NPI-0052 plus lenalidomide–induced apoptosis. In this context, previous studies showed that BIM also triggers apoptosis by translocating to the ER and inducing ER stress signaling via activation of caspase-12.20,31,32 We therefore next examined whether NPI-0052 plus lenalidomide triggered BIM translocation to ER, thereby initiating ER-mediated apoptotic signaling. To examine this issue, we prepared ER fractions from MM.1S cells treated with NPI-0052 (2nM), lenalidomide (3μM), or NPI-0052 (2nM) plus lenalidomide (3μM), and levels of all the BIM were analyzed by immunoblot analysis. A significant accumulation of BIMEL isoform (2.2-fold increase) was noted in the ER fraction from NPI-0052 plus lenalidomide–treated cells, but no increase was detected in the ER fractions from cells treated with either agent alone, demonstrating specific translocation of BIMEL to the ER (Figure 5A and bottom bar graph). No increase in BIMS or BIML isoforms was observed (data not shown). Additional time-course experiments to examine the alterations in BIMEL expression in response to combined treatment with lenalidomide and NPI-0052 showed an increase in BIM levels as early as 24 hours (pretreatment with lenalidomide for 12 hours, followed by addition of NPI-0052 for another 12 hours); however, maximal induction of BIMEL was noted only at 48 hours (24-hour pretreatment with lenalidomide, followed by addition of NPI-0052 for another 24 hours).
Effects of NPI-0052 plus lenalidomide on ER stress signaling, heat shock proteins, and proteasome activity. (A) MM.1S cells were pretreated with or without lenalidomide for 24 hours, and then NPI-0052 was added for an additional 24 hours. Cells were harvested, and ER-protein fractions were subjected to immunoblot analysis with anti-BIM or antiactin Abs. Bar graph (bottom) showing quantification of BIMEL protein bands in immunoblot by densitometry: A 2.2-fold increase in BIMEL isoform was noted in NPI-0052 plus lenalidomide–treated versus untreated cells. Samples were normalized to actin. (B) MM.1S cells were treated with indicated agents (as in panel A); whole cell lysates were subjected to immunoblot analysis with anticaspase-12 or tubulin Abs. (C) MM.1S cells were treated with indicated agents (as in panel A); whole cell lysates were subjected to immunoblot analysis with anti-Hsp-70, anti-Hsp-27, anti-Hsp-90, or antitubulin Abs. Lysates from HeLA cells served as a positive control for Hsp Abs. Blots shown are representative of 2 independent experiments. (D) MM.1S cells were pretreated with or without lenalidomide for 6 hours, and then NPI-0052 was added for an additional 6 hours and harvested; cytosolic extracts were then analyzed for CT-L, C-L, and T-L proteasome activities. Results are represented as percentage inhibition in proteasome activities in drug-treated versus vehicle control. Data are mean ± SD (n = 3, P < .05).
Effects of NPI-0052 plus lenalidomide on ER stress signaling, heat shock proteins, and proteasome activity. (A) MM.1S cells were pretreated with or without lenalidomide for 24 hours, and then NPI-0052 was added for an additional 24 hours. Cells were harvested, and ER-protein fractions were subjected to immunoblot analysis with anti-BIM or antiactin Abs. Bar graph (bottom) showing quantification of BIMEL protein bands in immunoblot by densitometry: A 2.2-fold increase in BIMEL isoform was noted in NPI-0052 plus lenalidomide–treated versus untreated cells. Samples were normalized to actin. (B) MM.1S cells were treated with indicated agents (as in panel A); whole cell lysates were subjected to immunoblot analysis with anticaspase-12 or tubulin Abs. (C) MM.1S cells were treated with indicated agents (as in panel A); whole cell lysates were subjected to immunoblot analysis with anti-Hsp-70, anti-Hsp-27, anti-Hsp-90, or antitubulin Abs. Lysates from HeLA cells served as a positive control for Hsp Abs. Blots shown are representative of 2 independent experiments. (D) MM.1S cells were pretreated with or without lenalidomide for 6 hours, and then NPI-0052 was added for an additional 6 hours and harvested; cytosolic extracts were then analyzed for CT-L, C-L, and T-L proteasome activities. Results are represented as percentage inhibition in proteasome activities in drug-treated versus vehicle control. Data are mean ± SD (n = 3, P < .05).
We next determined whether BIMEL translocation to ER is associated with caspase-12 cleavage, a hallmark event during ER stress.20,31,32 Low-dose combination of NPI-0052 and lenalidomide indeed triggered activation of caspase-12 (Figure 5B): densitometry of immunoblot showed a 1.9-fold increase in cleaved caspase-12 in lenalidomide plus NPI-0052–treated versus control protein lysates (Figure 5B). Although lenalidomide alone increases caspase-12 cleavage, a significant increase (30%, assessed by densitometry) was noted only in protein extracts from lenalidomide plus NPI-0052–treated cells.
Multiple previous studies link bortezomib-induced apoptosis with the up-regulation of heat shock proteins (Hsp),9,33,34 which confer drug resistance.9,35 Based on these findings, bortezomib has been combined with Hsp-90 inhibitor in clinical trials to overcome Hsp-mediated drug resistance. Interestingly, NPI-0052 plus lenalidomide triggered an increase in Hsp-70, but not Hsp-27 or Hsp-90, protein levels (Figure 5C). Our findings have clinical implications because combined low-dose NPI-0052 with lenalidomide does not induce 2 of the 3 Hsp, suggesting that drug resistance may be less frequent in patients given combined low-dose regimens.
Effects of NPI-0052 plus lenalidomide on CT-L, C-L, and T-L proteolytic activities
Although proteasome inhibitors induce apoptotic signaling cascades, the primary target of these agents is the proteasome. The proteolytic activity of proteasomes is mediated by 3 active sites: CT-L, T-L, and C-L.36,37 In the present study, treatment of MM.1S cells with low-dose NPI-0052 (2nM) inhibits 20% CT-L, 13% C-L, and 11% T-L activity, whereas lenalidomide (3μM) had little effect on these activities. Although our earlier study9 showed minimal effect of low-dose NPI-0052 on C-L activity, the difference may be the result of analysis using MM cell–derived versus isolated human red blood cell–derived proteasomes. Moreover, the differences in C-L activity profile may be the result of differential proteasome composition or levels of constitutive activities, including immunoproteasomes, in MM cells. Importantly, combined low doses of these agents resulted in a significant blockade of all 3 proteasomal activities (Figure 5D). Our data therefore suggest that lenalidomide enhances the ability of NPI-0052, even at low doses, to effectively target all 3 proteasome activities and trigger potent anti-MM activity.
NPI-0052 and lenalidomide synergize to suppress human MM-cell growth in vivo
Having shown that combined NPI-0052 plus lenalidomide induced synergistic apoptosis in MM cells in vitro, we next examined the in vivo efficacy of low-dose combination NPI-0052 and lenalidomide treatment using the human plasmacytoma MM.1S xenograft mouse model.19,22 For these studies, we first used low doses of either NPI-0052 (0.15 mg/kg) or lenalidomide (2.5 mg/kg) administered orally. As seen in Figure 6A, low doses of either agent had minimal effect on the growth of tumors, which increased as in control mice. Importantly, when NPI-0052 was combined with lenalidomide, there was a significant reduction (P = .002) in tumor growth relative to untreated mice (Figure 6A). As an additional control, we also treated mice with higher doses of NPI-0052 (0.25 mg/kg, oral on a similar dosing schedule). As in our previous study,9 a significant reduction in tumor growth was noted in NPI-0052–treated cohorts (data not shown). Lenalidomide alone at 5.0 mg/kg (oral) showed a modest reduction in tumor growth (Figure 6A). Importantly, the extent of tumor growth inhibition was similar in mice treated with low-dose combination NPI-0052 plus lenalidomide versus mice treated with the maximum tolerated dose of NPI-0052.
Combination of low doses of NPI-0052 and lenalidomide inhibits human plasmacytoma growth in CB-17 SCID mice. (A) Average and SD of tumor volume (mm3) from group of mice (n = 5/group) versus time (days) when tumor was measured. Mice were treated with vehicle, NPI-0052 (orally), lenalidomide (orally), or NPI-0052 plus lenalidomide (orally) at the indicated doses for 24 days on a twice-weekly schedule for NPI-0052 and 4 consecutive days weekly for lenalidomide. A significant delay in tumor growth in NPI-0052 plus lenalidomide–treated mice was noted compared with vehicle-treated control mice (P = .002). Bars represent mean ± SD. (B) Micrographs show apoptotic cells in tumors sectioned on day 24 (endpoint) from untreated or NPI-0052 (0.15 mg/kg) plus lenalidomide (2.5 mg/kg)- treated mice as identified by caspase-3 cleavage (dark brown cells). Photographs are representative of similar observations in 2 different mice receiving treatment. Images were obtained with a Zeiss Axioimager M1 microscope (63×/1.4 Plan-Apochromat objective), axioCam HRc camera, a Axiovision Version 4.6 software, and permount imaging solution. (C) Kaplan-Meier plots showing survival for mice treated with NPI-0052, lenalidomide, or NPI-0052 plus lenalidomide at the indicated concentrations. NPI-0052 plus lenalidomide–treated mice show significantly increased survival (P < .002) compared with the untreated group. The mean overall survival (OS) was 48 days (95% confidence interval, 35-60) in the untreated or single agent–treated cohorts versus 135 days (95% confidence interval, 120-150) in groups treated with combination of NPI-0052 and lenalidomide (0.25 or 0.5 mg/kg). Overall, a 67% increase in survival was observed in mice receiving combined low dose of NPI-0052 (0.15 mg/kg plus lenalidomide (0.5 mg/mg) versus mice receiving either agent alone at these doses. A statistically significant prolongation in mean OS compared with control mice was observed in animals treated with 0.25 mg/kg (P < .002) and 0.5 mg/kg (P > .007). (D) Mice in vehicle-treated controls, NPI-0052–, lenalidomide-, or NPI-0052 plus lenalidomide–treated group were weighed every week. The average changes in body weight are shown. (E) Mice were treated with vehicle, NPI-0052, lenalidomide, or NPI-0052 plus lenalidomide (as in panel A) for 24 days; blood samples were obtained and subjected to analysis for serum bilirubin, hemoglobin, and creatine levels using Quantichrom Creatinine, Bilirubin, and Hemoglobin Assay kit (BioAssay Systems).
Combination of low doses of NPI-0052 and lenalidomide inhibits human plasmacytoma growth in CB-17 SCID mice. (A) Average and SD of tumor volume (mm3) from group of mice (n = 5/group) versus time (days) when tumor was measured. Mice were treated with vehicle, NPI-0052 (orally), lenalidomide (orally), or NPI-0052 plus lenalidomide (orally) at the indicated doses for 24 days on a twice-weekly schedule for NPI-0052 and 4 consecutive days weekly for lenalidomide. A significant delay in tumor growth in NPI-0052 plus lenalidomide–treated mice was noted compared with vehicle-treated control mice (P = .002). Bars represent mean ± SD. (B) Micrographs show apoptotic cells in tumors sectioned on day 24 (endpoint) from untreated or NPI-0052 (0.15 mg/kg) plus lenalidomide (2.5 mg/kg)- treated mice as identified by caspase-3 cleavage (dark brown cells). Photographs are representative of similar observations in 2 different mice receiving treatment. Images were obtained with a Zeiss Axioimager M1 microscope (63×/1.4 Plan-Apochromat objective), axioCam HRc camera, a Axiovision Version 4.6 software, and permount imaging solution. (C) Kaplan-Meier plots showing survival for mice treated with NPI-0052, lenalidomide, or NPI-0052 plus lenalidomide at the indicated concentrations. NPI-0052 plus lenalidomide–treated mice show significantly increased survival (P < .002) compared with the untreated group. The mean overall survival (OS) was 48 days (95% confidence interval, 35-60) in the untreated or single agent–treated cohorts versus 135 days (95% confidence interval, 120-150) in groups treated with combination of NPI-0052 and lenalidomide (0.25 or 0.5 mg/kg). Overall, a 67% increase in survival was observed in mice receiving combined low dose of NPI-0052 (0.15 mg/kg plus lenalidomide (0.5 mg/mg) versus mice receiving either agent alone at these doses. A statistically significant prolongation in mean OS compared with control mice was observed in animals treated with 0.25 mg/kg (P < .002) and 0.5 mg/kg (P > .007). (D) Mice in vehicle-treated controls, NPI-0052–, lenalidomide-, or NPI-0052 plus lenalidomide–treated group were weighed every week. The average changes in body weight are shown. (E) Mice were treated with vehicle, NPI-0052, lenalidomide, or NPI-0052 plus lenalidomide (as in panel A) for 24 days; blood samples were obtained and subjected to analysis for serum bilirubin, hemoglobin, and creatine levels using Quantichrom Creatinine, Bilirubin, and Hemoglobin Assay kit (BioAssay Systems).
We next investigated the effect of the drug combination on in vivo apoptosis using staining of paraffin-embedded sections of xenografted tumors for caspase-3 activation. The combination of NPI-0052 plus lenalidomide dramatically increased the number of caspase-3 cleavage-positive cells compared with either treatment alone (Figure 6B). Importantly, treatment of tumor-bearing mice with NPI-0052 plus lenalidomide, but not vehicle alone, significantly prolonged survival (P < .002; Figure 6C). NPI-0052 alone at 0.15 mg/kg oral dose showed a modest prolongation of survival (10-15 days) compared with vehicle alone (Figure 6C). Analysis at day 50 showed no recurrence of tumor in 67% of the NPI-0052 (0.15 mg/kg) plus lenalidomide (0.5 mg/kg)–treated mice (Figure 6C). These data demonstrate the potent antitumor activity of NPI-0052 plus lenalidomide in vivo. Importantly, these findings also show that both NPI-0052 and lenalidomide are orally bioactive in combination and provide the preclinical framework for their evaluation as an oral agent combination regimen in phase 1 trials in MM.
Low-dose combination NPI-0052 plus lenalidomide treatment was well tolerated, as evidenced by the lack of weight loss and neurologic changes even after 4 weeks of treatment (Figure 6D; and data not shown). As seen in Figure 6E, the blood chemistry profiles of NPI-0052 plus lenalidomide–treated mice showed normal levels of creatinine, hemoglobin, and bilirubin (Figure 6E). No leucopenia/neutropenia and thrombocytopenia were noted at these low doses of either agent (data not shown). These findings suggest that combining NPI-0052 with lenalidomide markedly reduces tumor growth and is well tolerated in vivo.
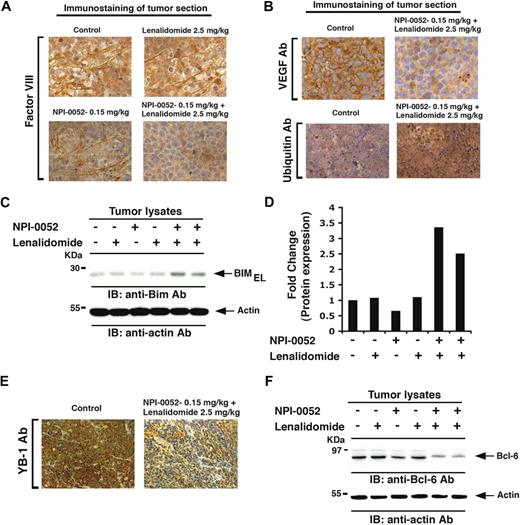
Our in vitro data indicated antiangiogenic activity of NPI-0052 plus lenalidomide, and we therefore next evaluated tumors harvested from mice by immunostaining for factor VIII, a marker of angiogenesis. As seen in Figure 7A, low doses of NPI-0052 or lenalidomide alone triggered a very modest decrease in the number of factor VIII–positive cells compared with sections from control-treated tumors, whereas combination treatment dramatically decreased the number of factor VIII–positive cells (Figure 7A). Similarly, NPI-0052 plus lenalidomide markedly decreased the number of VEGFR1-positive cells (Figure 7B top panel). These findings suggest a probable mechanism mediating NPI-0052 plus lenalidomide–triggered inhibition of migration and angiogenesis.
Effect of NPI-0052 plus lenalidomide on neovascularization, ubiquitination, BIM, Bcl-6, and YB-1 in vivo in xenografted MM tumors. Tumor sections from mice receiving indicated treatment were immunostained with factor VIII (A), VEGFR1, or ubiquitin Abs (B). (C) Tumor lysates from control and drug-treated mice were subjected to immunoblot analysis using anti-BIM or antiactin Abs. Lanes 1 through 6 represent tumor lysates from mice receiving the following treatments: lane 1, vehicle alone (control); lane 2, NPI-0052 (0.15 mg/kg); lane 3, lenalidomide (2.5 mg/kg); lane 4, lenalidomide (5.0 mg/kg); lane 5, NPI-0052 (0.15 mg/kg) plus lenalidomide (2.5 mg/kg); and lane 6, NPI-0052 (0.15 mg/kg) plus lenalidomide (5.0 mg/kg). (D) Bar graph represents quantification of BIMEL protein bands in immunoblot shown in Figure 4C by densitometry: a 3.3-fold increase in BIMEL isoform was noted in NPI-0052 plus lenalidomide–treated versus untreated cells. Samples were normalized to actin. (E) Tumor sections from control and NPI-0052 plus lenalidomide–treated mice were immunostained with YB-1 Ab. (F) Tumor lysates from control and drug-treated mice were subjected to immunoblot analysis using anti–Bcl-6 or antiactin Abs. Lanes 1 through 6 are the same as in panel C. (A-B,E) Representative of similar observations in 2 different mice receiving the same treatment. For panels A, B, and E, images were obtained with a Zeiss Axioimager M1 microscope (63×/1.4 Plan-Apochromat objective), a AxioCam HRc camera, Axiovision Version 4.6 software, and permount imaging solution.
Effect of NPI-0052 plus lenalidomide on neovascularization, ubiquitination, BIM, Bcl-6, and YB-1 in vivo in xenografted MM tumors. Tumor sections from mice receiving indicated treatment were immunostained with factor VIII (A), VEGFR1, or ubiquitin Abs (B). (C) Tumor lysates from control and drug-treated mice were subjected to immunoblot analysis using anti-BIM or antiactin Abs. Lanes 1 through 6 represent tumor lysates from mice receiving the following treatments: lane 1, vehicle alone (control); lane 2, NPI-0052 (0.15 mg/kg); lane 3, lenalidomide (2.5 mg/kg); lane 4, lenalidomide (5.0 mg/kg); lane 5, NPI-0052 (0.15 mg/kg) plus lenalidomide (2.5 mg/kg); and lane 6, NPI-0052 (0.15 mg/kg) plus lenalidomide (5.0 mg/kg). (D) Bar graph represents quantification of BIMEL protein bands in immunoblot shown in Figure 4C by densitometry: a 3.3-fold increase in BIMEL isoform was noted in NPI-0052 plus lenalidomide–treated versus untreated cells. Samples were normalized to actin. (E) Tumor sections from control and NPI-0052 plus lenalidomide–treated mice were immunostained with YB-1 Ab. (F) Tumor lysates from control and drug-treated mice were subjected to immunoblot analysis using anti–Bcl-6 or antiactin Abs. Lanes 1 through 6 are the same as in panel C. (A-B,E) Representative of similar observations in 2 different mice receiving the same treatment. For panels A, B, and E, images were obtained with a Zeiss Axioimager M1 microscope (63×/1.4 Plan-Apochromat objective), a AxioCam HRc camera, Axiovision Version 4.6 software, and permount imaging solution.
Given that NPI-0052 plus lenalidomide inhibits proteasome activity (Figure 5D), coupled with the fact that proteasome inhibition results in accumulation of ubiquitinated proteins, we examined tumor sections from mice for alterations in the ubiquitination pattern. Tumors were excised 3 hours after the last dose was administered, and IHC was performed using ubiquitin Abs. Combined NPI-0052 and lenalidomide treatment markedly increased ubiquitin staining versus control (Figure 7B bottom panel). These in vivo data, together with our in vitro results, confirm that anti-MM activity of NPI-0052 plus lenalidomide is associated with enhanced inhibition of proteasome activity.
We next examined whether in vivo antitumor activity of NPI-0052 plus lenalidomide is associated with induction of BIM. In accord with our in vitro data (Figure 4C), tumors from mice treated with NPI-0052 and lenalidomide, but not with either agent alone, showed significantly increased BIMEL levels (3.3-fold increase; Figure 7C-D). We also observed a concurrent decrease in prosurvival signaling: for example, NPI-0052 and lenalidomide decreased levels of MM survival proteins YB-123 and Bcl-638 (Figure 7E-F). Together, these findings demonstrate potent in vivo anti-MM activity of NPI-0052 combined with lenalidomide at doses that are well tolerated in a human plasmacytoma xenograft mouse model, supporting the potential clinical evaluation of combined NPI-0052 plus lenalidomide treatment in MM.
Discussion
We first showed that the combination of low doses of NPI-0052 and lenalidomide decreases viability of MM cells without affecting normal lymphocyte viability. For example, combined low doses of NPI-0052 (2nM) and lenalidomide (3μM) triggered a degree of apoptosis in MM.1S cells that is achievable only at much higher doses of either agent alone. Because each of these MM-cell lines shows different sensitivity in response to single agent, the additive doses of agents required to achieve a significant degree of cell death also vary for each cell line. Genetic heterogeneity and drug resistance are a hallmark of MM,39-42 which may explain, at least in part, these differences in cytotoxic activity against different agents. Importantly, our data demonstrate anti-MM activity of low-dose combination of NPI-0052 and lenalidomide in a panel of MM-cell lines, including those sensitive and resistant to therapies, as well as representing cytogenetically distinct MM cells. For example, we studied isogenic cell lines Dex-sensitive MM.1S and Dex-resistant MM.1R with t(14;16) translocation and c-maf overexpression; RPMI-8266 with TP53, K-Ras, and EGFR mutations; INA-6, an IL-6–dependent cell line with N-Ras activating mutation; and KMS12PE t(11:14) with cyclin D1 dysregulation.39-45 We also observed similar responses even in patient-derived MM cells resistant to anti-MM therapies, such as Dex, Dox, melphalan, lenalidomide, or bortezomib. Moreover, combination, but not NPI-0052 or lenalidomide alone, overcame the MM-cell growth advantage conferred by adherence to BMSCs.
Mechanistic studies showed that anti-MM activity of NPI-0052 plus lenalidomide is associated with activation of the caspase cascade, BIM, Hsp-70, and ER stress response. Of note, the combination of NPI-0052 and lenalidomide triggers robust caspase-8 cleavage; however, a very modest caspase-9 cleavage was observed. These data are consistent with our findings using biochemical inhibitors that blockade of caspase-8, but not caspase-9, significantly abrogates anti-MM activity of NPI-0052 plus lenalidomide. Similarly, lenalidomide plus NPI-0052–induced MM-cell apoptosis is associated with significant accumulation of BIMEL isoform in the ER-enriched fraction without detectable changes in the other 2 isoforms of BIM, BIMS or BIML. In this context, our earlier findings using a combination of 2 proteasome inhibitors NPI-0052 and bortezomib19 also showed a role of BIM; however, in contrast to NPI-0052 and lenalidomide, the combination of NPI-0052 with bortezomib did not induce BIM translocation to ER (data not shown). Thus, although BIM plays a role in mediating apoptosis triggered by these combination regimens, the apoptotic signaling route of BIM is distinct. It is probable that other differential apoptotic signaling pathways are involved in mediating apoptosis by these combination regimens. Our ongoing studies are focused on delineating the mechanism of action of these combination regimens. Nonetheless, our present findings suggest a predominant link of BIMEL isoform in ER-mediated stress signaling during lenalidomide plus NPI-0052–induced apoptosis in MM cells. Overall, our mechanistic studies suggest that the synergistic anti-MM activity of NPI-0052 plus lenalidomide predominantly relies on caspase-8 and BIM > ER > caspase-12 signaling axis.
Although proteasome inhibitors induce apoptotic signaling cascades, the primary target of these agents is the proteasome. Interestingly, although NPI-0052 is known to inhibit CT-L, C-L, and T-L activity, its combination with lenalidomide blocks all of these activities to a greater extent. The mechanism whereby lenalidomide enhances the ability of NPI-0052 to potently inhibit proteasome activity remains to be defined. One possibility is that lenalidomide may also affect immunoproteasome activities (CT-Li, C-Li, and T-Li), which in turn may alter function of constitutive proteasome activities. Indeed, a previous study showed that proteasome active sites allosterically regulate each other: for example, a biochemical study showed that occupancy of C-L sites induces the T-L activity of proteasomes.46 In addition, our previous studies have shown that NPI-0052 also inhibits the immunoproteasome in MM cells.9 Although definitive evidence for the role of individual proteasomal activities requires further genetic studies involving specific inhibition of β subunits or their corresponding immunoproteasome components, it is now clear from our data that (1) lenalidomide enhances NPI-0052–induced inhibition of proteasome function and (2) even 25% to 35% proteasome inhibition, albeit of all 3 activities, is sufficient to trigger significant anti-MM activity.
Besides our in vitro studies, we also examined anti-MM activity of NPI-0052 and lenalidomide in vivo using human MM xenograft mouse model. To demonstrate synergistic activity, NPI-0052 and lenalidomide were used at doses that were at least 2 times lower than their individual maximum tolerated dose. No significant tumor growth inhibition was noted at low-dose single-agent treatment, but a marked growth inhibitory effect was observed with the combination regimen. NPI-0052 plus lenalidomide treatment was not associated with any toxicity because no differences in body weight and overall appearance were noted. The remarkable anti-MM activity of NPI-0052 plus lenalidomide in vivo was confirmed by IHC analysis of tumors harvested from control and NPI-0052 plus lenalidomide–treated mice using molecular markers of apoptosis (caspase-3 cleavage), survival (Bcl-6 and YB-1), and associated angiogenesis (factor VIII and VEGFR1). Therefore, these findings demonstrated a dual effect of combining NPI-0052 and lenalidomide: increased apoptosis and decreased MM-cell proliferation.
Direct analysis of tumor cells from mice showed synergistic accumulation of ubiquitinated proteins in NPI-0052 plus lenalidomide–treated mice, but not in mice treated with low doses of either agent alone, consistent with blockade of proteasome function in tumor cells. These in vivo findings, coupled with our in vitro data showing minimal toxicity of combined NPI-0052 plus lenalidomide against normal cells, confirmed that MM cells are more sensitive to proteasome inhibition than normal cells.
Collectively, our preclinical studies demonstrate potent in vitro and in vivo anti-MM activity of NPI-0052 combined with lenalidomide at doses that are well tolerated in a human plasmacytoma xenograft mouse model. These findings provide the framework for clinical trials of low-dose combination of NPI-0052 and lenalidomide to increase response, overcome drug resistance, reduce side effects, and improve patient outcome in MM.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgment
This work was supported by the National Institutes of Health (grants SPORE-P50100707, PO1-CA078378, and RO1CA050947).
National Institutes of Health
Authorship
Contribution: D.C. designed research, analyzed data, and wrote the manuscript; A.V.S. performed most of the experiments and interpreted data; B.C. and P.G.R. provided clinical samples; M.A.P. provided NPI-0052; and K.C.A. analyzed data and wrote the manuscript.
Conflict-of-interest disclosure: D.C. and K.C.A. are consultants to Nereus Pharmaceuticals. The remaining authors declare no competing financial interests.
Correspondence: Kenneth C. Anderson or Dharminder Chauhan, Dana-Farber Cancer Institute, Mayer Bldg Rm 561, 44 Binney St, Boston, MA 02115; e-mail: Kenneth_Anderson@dfci.harvard.edu or Dharminder_Chauhan@dfci.harvard.edu.
References
Author notes
*D.C. and A.V.S. contributed equally to this study.

![Figure 3. Combined low doses of NPI-0052 and lenalidomide block migration and tubule formation. (A) For migration assay, MM.1S cells were pretreated with lenalidomide for 12 hours, and then NPI-0052 was added for an additional 6 hours; the cells were more than 90% viable at this time point. The cells were washed and cultured in serum-free medium. After 2 hours of incubation, cells (viability > 90%) were plated on a fibronectin-coated polycarbonate membrane in the upper chamber of Transwell inserts and exposed for 4 hours to serum-containing medium in the lower chamber. Cells migrating to the bottom face of the membrane were fixed with 90% ethanol and stained with crystal violet (original magnification, 10×/0.25 numeric aperture [NA] oil). A total of 3 randomly selected fields were examined for cells that had migrated from top to bottom chambers. (Left panel) Image is representative of 2 experiments with similar results. (Right panel) The bar graph represents quantification of migrated cells. Data are mean ± SD (n = 2; P < .05 for control vs NPI-0052 plus lenalidomide–treated cells). (B) HUVECs were cultured in the presence or absence of combined low doses of NPI-0052 plus lenalidomide for 48 hours, and then assessed for in vitro angiogenesis using Matrigel capillary-like tube structure formation assays (original magnification, 4×/0.10 NA oil, media: EBM-2). (Left panel) Image is representative from 3 experiments with similar results. The in vitro angiogenesis is reflected by capillary tube branch formation (dark brown). (Right panel) The bar graph represents quantification of capillary-like tube structure formation in response to indicated agents: Branch points in several random view fields/well were counted, values were averaged, and statistically significant differences were measured using Student t test. (C-D) MM.1S and RPMI-8226 cells were cultured for 48 hours in BMSC-coated or noncoated wells with control media, NPI-0052, lenalidomide, or NPI-0052 plus lenalidomide. Cell proliferation was assessed by the nonradioactive WST-1 colorimetric assay. Data are mean ± SD of 2 independent experiments. Error bars represent SD.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/115/4/10.1182_blood-2009-03-213009/4/m_zh89990947210003.jpeg?Expires=1767750494&Signature=4VBwJkS3drJiT3QxD-zdjyuzyPBHodZdFAfP0N6-THITL48OkH8mXrGZxBmdwo2WkZJ9TBif2gR2cF~G58SGQZOcKoHasudQQLe4h4BmhtJr8dP7mphQ0wJ4bK6yM06Czs-~QaqzYDykRC1yPYywm16Rh7X0e4O2kp5eJjtmYcQGCsXYWirRW0kWTKAbeDBqZw-z92bpRRz9cj-Gzc-pxG-imtVZ7qlCfEsWkusHlG~6gRUzm5qV7tYNh-~GhhcOy8QhYRDuyQWjNrQK~-7cfcC9W6B1F3xDgnO3Y0iUQdkaomOQWVHQWMQQLzbVA7uUByzwQTYlBxpI2xSvCdV0Eg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal