Abstract
B-cell chronic lymphocytic leukemia (B-CLL) migration involves several molecules, including matrix metalloproteinase–9 (MMP-9) and vascular endothelial growth factor (VEGF). We have studied whether VEGF regulates MMP-9. VEGF significantly reduced MMP-9 protein expression in a dose-dependent manner, measured by gelatin zymography. Blocking the VEGFR2 receptor restored MMP-9 levels, implicating this receptor in the observed effect. Down-regulation of MMP-9 by VEGF resulted in significant inhibition of B-CLL cell migration through Matrigel or human umbilical vein endothelial cells, confirming the crucial role of MMP-9 in these processes. Reverse-transcription polymerase chain reaction analyses revealed that VEGF regulated MMP-9 at the transcriptional level. Indeed, VEGF induced STAT1 tyrosine phosphorylation, and this was blocked by inhibiting VEGFR2. STAT1 was responsible for MMP-9 down-regulation, as STAT1 gene silencing restored MMP-9 production and B-CLL cell migration in the presence of VEGF. Thus, the levels of VEGF and MMP-9 influence B-CLL cell expansion and both molecules could constitute therapeutic targets for this disease.
Introduction
B-cell chronic lymphocytic leukemia (B-CLL) extravasation and tissue infiltration involve several molecules, including vascular endothelial growth factor (VEGF) and matrix metalloproteinase–9 (MMP-9).1-6 B-CLL cells produce both molecules,7,8 and we have previously shown that, although constitutive MMP-9 expression is required for cell migration, increasing this expression by binding of exogenously added MMP-9 induces cell arrest.9 This suggests that MMP-9 levels play an important role in B-CLL cell expansion and localization in tissues. Understanding MMP-9 regulation is therefore crucial to define strategies that prevent B-CLL cell progression. Interestingly, anti-VEGF antibodies decreased MMP-9 expression,8 suggesting a link between both proteins. As several physiologic stimuli increase VEGF (both autocrine and paracrine) in the B-CLL cell microenvironment,1,2 we have studied the functional effects of elevated VEGF levels on MMP-9 expression and B-CLL cell migration.
Methods
Approval was obtained from the review boards of the Hospital Clínico Universitario, Valencia, Spain and the Hospital Universitario Puerta de Hierro, Madrid, Spain for these studies. Informed consent was obtained in accordance with the Declaration of Helsinki.
Patients and cells
CD5+ B lymphocytes were purified from peripheral blood samples from 15 B-CLL patients (supplemental Table 1, available on the Blood website; see the Supplemental Materials link at the top of the online article) using Ficoll-Hypaque (Nycomed) centrifugation and negative selection with anti–CD3-conjugated Dynabeads (Dynal Biotech ASA). The resulting B-cell population, determined by flow cytometry, was more than 95% CD19+ and more than 90% CD5+. Human umbilical vein endothelial cells (HUVECs) were provided by Dr M. L. Botella and cultured as described.5
Additional materials and methods are available as supplemental data.
Results and discussion
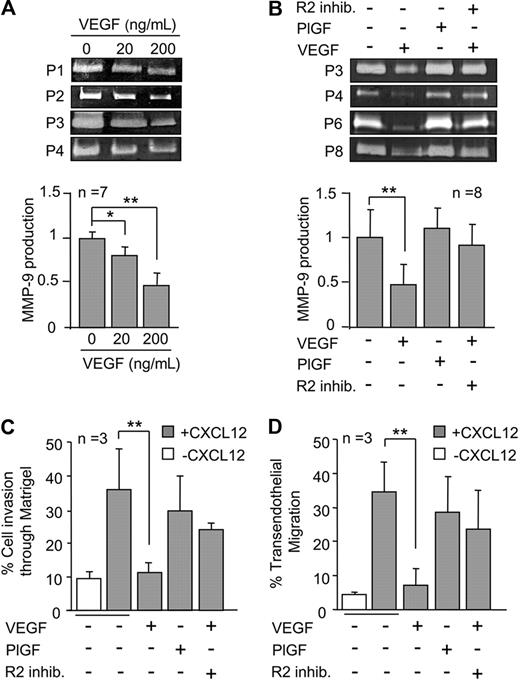
To determine whether MMP-9 was regulated by VEGF, B-CLL cells were incubated with or without 20 or 200 ng/mL VEGF for 24 hours and the conditioned media analyzed by gelatin zymography. As shown in Figure 1A for 4 representative samples and quantitated for 7, VEGF induced a significant, dose-dependent down-regulation of secreted MMP-9. At 200 ng/mL (or 150 ng/mL, not shown), MMP-9 average levels were reduced by 2.2-fold compared with untreated cells.
VEGF down-regulates MMP-9 and inhibits B-CLL cell migration. (A) A total of 3 × 106 B-CLL cells were incubated in RPMI/0.1% fetal bovine serum with the indicated concentrations of VEGF. After 24 hours, the conditioned media was concentrated and analyzed by gelatin zymography. MMP-9 levels on untreated cells were normalized to 1, and average values (n = 7) are shown. (B) B-CLL cells (n = 8) were untreated or treated with 200 ng/mL VEGF or PlGF, and in the presence or absence of 5μM VEGFR2 inhibitor I (R2 inhib.). After 24 hours, MMP-9 was analyzed and quantitated as explained. (C-D) A total of 5 × 105 B-CLL cells (n = 3) were untreated or treated with VEGF or PlGF for 30 minutes and added to Transwell filters (5 μm pore size) coated with Matrigel (A) or activated HUVECs (B) in the presence of the cytokines. Some cells were also pretreated with 5μM VEGFR2 inhibitor I as indicated. CXCL12 (150 ng/mL) was added to the medium in the bottom chamber, except for the control. After 24 hours, migrated cells were counted by flow cytometry. Expression of CD19 on transmigrated cells (> 90%) was also analyzed by flow cytometry. Values are the percentage of total cells added. Bars represent SD. *P ≤ .05, **P ≤ .01, calculated by the 2-tailed Student t test.
VEGF down-regulates MMP-9 and inhibits B-CLL cell migration. (A) A total of 3 × 106 B-CLL cells were incubated in RPMI/0.1% fetal bovine serum with the indicated concentrations of VEGF. After 24 hours, the conditioned media was concentrated and analyzed by gelatin zymography. MMP-9 levels on untreated cells were normalized to 1, and average values (n = 7) are shown. (B) B-CLL cells (n = 8) were untreated or treated with 200 ng/mL VEGF or PlGF, and in the presence or absence of 5μM VEGFR2 inhibitor I (R2 inhib.). After 24 hours, MMP-9 was analyzed and quantitated as explained. (C-D) A total of 5 × 105 B-CLL cells (n = 3) were untreated or treated with VEGF or PlGF for 30 minutes and added to Transwell filters (5 μm pore size) coated with Matrigel (A) or activated HUVECs (B) in the presence of the cytokines. Some cells were also pretreated with 5μM VEGFR2 inhibitor I as indicated. CXCL12 (150 ng/mL) was added to the medium in the bottom chamber, except for the control. After 24 hours, migrated cells were counted by flow cytometry. Expression of CD19 on transmigrated cells (> 90%) was also analyzed by flow cytometry. Values are the percentage of total cells added. Bars represent SD. *P ≤ .05, **P ≤ .01, calculated by the 2-tailed Student t test.
Because the VEGF isoform used in our study (VEGF165) binds VEGFR1 and VEGFR2,10,11 we aimed to identify the receptor involved in the observed effect. B-CLL cells were incubated with placenta growth factor-1 (PlGF), which selectively interacts with VEGFR1 but not VEGFR2.12,13 Figure 1B shows that these cells produced equal amounts of MMP-9 as untreated cells, thus excluding VEGFR1 from the down-modulatory effect. Accordingly, a specific VEGFR2 inhibitor14 restored the production of MMP-9 by VEGF-treated cells (Figure 1B).
We previously reported that MMP-9 is involved in B-CLL cell migration elicited by the CXCL12 or CCL21 chemokines.5,6 We therefore studied whether VEGF modulation of MMP-9 affected this migration. Figure 1C and D shows that B-CLL cells migrated through Matrigel or HUVECs (average 35.3% and 34.5%, respectively, > 90% CD19+) in response to CXCL12. VEGF significantly reduced this migration to the basal levels obtained in the absence of chemokine. In agreement with the results shown in Figure 1B, PlGF did not significantly affect cell migration, again implicating VGFR2 and not VEGFR1 in the effect. Indeed, adding the VEGFR2 inhibitor in combination with VEGF restored 70% of cell migration in both systems, compared with untreated cells (Figure 1C-D). Altogether, these results established that VEGFR2 is responsible for the decreased MMP-9 production in response to VEGF and the subsequent impairment of B-CLL cell migration.
Reverse-transcription polymerase chain reaction studies revealed that MMP-9 down-regulation was at the transcriptional level, as VEGF also significantly reduced MMP-9 mRNA after 1 to 3 hours of exposure (Figure 2A). VEGFR2 has been shown to bind and induce tyrosine phosphorylation of signal transducer and activator of transcription (STAT) factors 1 and 6, in bovine aortic endothelial cells.15 In B-CLL cells, the constitutive p-Ser727-STAT1 and p-Ser727-STAT3 interact with VEGFR2 (and VEGFR1), resulting in increased cell survival.16 STAT1 plays a critical role in MMP-9 gene transcriptional suppression in response to interferons in several cell systems.17-19 Interferons were also shown to down-regulate MMP-9 production by B-CLL cells, although the mechanism was not elucidated.8 We studied whether STAT1 was activated by VEGF in B-CLL cells and involved in the subsequent MMP-9 gene and protein suppression. VEGF induced STAT1 tyrosine phosphorylation, detected after 5 minutes of VEGF exposure (not shown) and with maximal levels after 30 minutes (Figure 2B), then decreasing after 60 minutes (not shown). In agreement with the results shown in Figure 1, this effect was completely inhibited by blocking VEGFR2 and was not induced by PlGF (Figure 2B). By performing cellular fractionation analyses, we further demonstrated that p-Y-STAT1 translocated to the nucleus in response to VEGF (Figure 2C). A previous study did not find p-Ser727-STAT1 in the nucleus after VEGF stimulation,16 suggesting that Ser727 phosphorylation (which amplifies STAT1 transcriptional activity) may not be necessary for VEGF-induced MMP-9 gene regulation.
STAT1 plays a critical role in VEGF-induced down-regulation of MMP-9. (A) A total of 107 B-CLL cells were untreated or treated with 200 ng/mL VEGF for the indicated times and mRNA expression analyzed by reverse-transcribed polymerase chain reaction. Quantitative values represent the average of 3 samples after normalizing untreated cells values to 1. (B) A total of 3 × 106 B-CLL cells were lysed before (constitutive [Const.]) or after 30 minutes of treatment with medium (untreated [Unt.]) or the indicated stimuli. STAT1 tyrosine 701 phosphorylation (p-Y-STAT1) and total STAT1 (t-STAT1) were analyzed by Western blotting, and normalized average values (n = 3) are shown. V+R2 inhib. indicates VEGF + VEGFR2 inhibitor I. (C) A total of 20 × 106 B-CLL cells treated with or without VEGF for 30 minutes were lysed, and the nuclear and cytosolic fractions separated and analyzed by Western blotting. The H4 histone was used as an internal nuclear marker. p-Y-STAT1 indicates tyrosine 701 phosphorylated STAT1. (D) B-CLL cells were untransfected (None) or transfected with STAT1 siRNA or control siRNA, lysed, and analyzed by Western blotting to determine STAT1 gene silencing efficiency. Actin was used as an internal loading control. Numbers represent the average t-STAT1/actin ratio after normalizing untransfected values to 1. *P ≤ .05 compared with untransfected, untreated control. (E) B-CLL cells untransfected or transfected with STAT1 or control siRNA were treated with medium or 200 ng/mL VEGF for 24 hours. The conditioned media was concentrated and MMP-9 levels analyzed by gelatin zymography. Normalized average values (n = 3) are shown. (F) B-CLL cells untransfected or transfected with STAT1 or control siRNA were treated with medium or VEGF and added to Transwell filters (5 μm pore size) coated with Matrigel. Cell migration was determined after 24 hours by flow cytometry. Values are the percentage of total cells added. *P ≤ .05. **P ≤ .01.
STAT1 plays a critical role in VEGF-induced down-regulation of MMP-9. (A) A total of 107 B-CLL cells were untreated or treated with 200 ng/mL VEGF for the indicated times and mRNA expression analyzed by reverse-transcribed polymerase chain reaction. Quantitative values represent the average of 3 samples after normalizing untreated cells values to 1. (B) A total of 3 × 106 B-CLL cells were lysed before (constitutive [Const.]) or after 30 minutes of treatment with medium (untreated [Unt.]) or the indicated stimuli. STAT1 tyrosine 701 phosphorylation (p-Y-STAT1) and total STAT1 (t-STAT1) were analyzed by Western blotting, and normalized average values (n = 3) are shown. V+R2 inhib. indicates VEGF + VEGFR2 inhibitor I. (C) A total of 20 × 106 B-CLL cells treated with or without VEGF for 30 minutes were lysed, and the nuclear and cytosolic fractions separated and analyzed by Western blotting. The H4 histone was used as an internal nuclear marker. p-Y-STAT1 indicates tyrosine 701 phosphorylated STAT1. (D) B-CLL cells were untransfected (None) or transfected with STAT1 siRNA or control siRNA, lysed, and analyzed by Western blotting to determine STAT1 gene silencing efficiency. Actin was used as an internal loading control. Numbers represent the average t-STAT1/actin ratio after normalizing untransfected values to 1. *P ≤ .05 compared with untransfected, untreated control. (E) B-CLL cells untransfected or transfected with STAT1 or control siRNA were treated with medium or 200 ng/mL VEGF for 24 hours. The conditioned media was concentrated and MMP-9 levels analyzed by gelatin zymography. Normalized average values (n = 3) are shown. (F) B-CLL cells untransfected or transfected with STAT1 or control siRNA were treated with medium or VEGF and added to Transwell filters (5 μm pore size) coated with Matrigel. Cell migration was determined after 24 hours by flow cytometry. Values are the percentage of total cells added. *P ≤ .05. **P ≤ .01.
To determine whether STAT1 activation was critical for MMP-9 regulation by VEGF, we transfected B-CLL cells with STAT1 siRNA and measured the levels of MMP-9 upon VEGF treatment. VEGF did not affect total STAT1 levels of untransfected cells (Figure 2D). STAT1 siRNA transfection reduced STAT1 expression by 40% (average of 3 patients; Figure 2D), and this was sufficient to restore production of MMP-9 (Figure 2E). This effect was not the result of the transfection procedure, as control siRNA-transfected cells had decreased MMP-9 expression on VEGF treatment (Figure 2E). STAT1 gene silencing also restored migration of VEGF-treated B-CLL cells, whereas migration remained inhibited on control siRNA-transfected cells (Figure 2F). These results therefore show, for the first time, that the VEGF/VEGFR2 axis induces STAT1 tyrosine phosphorylation and that this is critical for the VEGF effect on MMP-9 expression and cell migration.
Previous studies suggest that VEGF may have an important pathogenic role in B-CLL. Increased bone marrow angiogenesis20 and VEGF urine and serum levels have been found in B-CLL patients.7,20,21 Autocrine VEGF contributes to B-CLL cell motility3 as well as survival, as it mediates the antiapoptotic effect of CD15422 and up-regulates several antiapoptotic genes.16 We now report a novel role for VEGF in B-CLL, which affects malignant cell migration/arrest and thus disease expansion. The ability to block MMP-9 expression and cell migration was not observed in the absence of exogenously added VEGF, indicating that it requires higher VEGF levels than those constitutively produced by B-CLL cells. Several physiologic stimuli can increase endogenous (hypoxia,7 CD15422 ) or exogenous (stromal cells10,11 ) VEGF, and these may be particularly important in B-CLL cell niches. Elevated VEGF levels at these sites will therefore contribute to malignant cell accumulation and progression of the disease. The differential effect of low and high VEGF concentrations (perhaps resulting from different VEGFR2 responses) resembles the distinct behavior of high and low MMP-9 levels on B-CLL cell migration that we previously reported,9 and both molecules, VEGF and MMP-9, may constitute therapeutic targets for B-CLL.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the B-CLL patients who donated samples for this research and Dr Luisa Botella (Centro de Investigaciones Biológicas, Madrid) for HUVECs.
This work was supported by Ministerio de Ciencia e Innovación, Spain (grants PI060400, SAF2009-07035, and RTICC RD06/0020/0011, A.G.-P.; and PI061637 and RTICC RD06/0020/0080, M.J.T.) and by the Fundación de Investigación Médica Mutua Madrileña (A.G.-P.). J.R.-M. was supported by the Fundación Ramón Areces. P.E. was supported by the Ministerio de Ciencia e Innovación.
Authorship
Contribution: E.U.-B. and J.R.-M. performed research and designed some experiments; P.E. contributed some reagents and helpful suggestions; M.H.d.C. purified and maintained cells; J.A.G.-M. and M.J.T. contributed patient samples and data; and A.G.-P. designed and supervised research and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Angeles García-Pardo, Cellular and Molecular Medicine Programme, Centro de Investigaciones Biológicas, Consejo Superior de Investigaciones Cientificas, Ramiro de Maeztu 9, 28040 Madrid, Spain; e-mail: agarciapardo@cib.csic.es.

![Figure 2. STAT1 plays a critical role in VEGF-induced down-regulation of MMP-9. (A) A total of 107 B-CLL cells were untreated or treated with 200 ng/mL VEGF for the indicated times and mRNA expression analyzed by reverse-transcribed polymerase chain reaction. Quantitative values represent the average of 3 samples after normalizing untreated cells values to 1. (B) A total of 3 × 106 B-CLL cells were lysed before (constitutive [Const.]) or after 30 minutes of treatment with medium (untreated [Unt.]) or the indicated stimuli. STAT1 tyrosine 701 phosphorylation (p-Y-STAT1) and total STAT1 (t-STAT1) were analyzed by Western blotting, and normalized average values (n = 3) are shown. V+R2 inhib. indicates VEGF + VEGFR2 inhibitor I. (C) A total of 20 × 106 B-CLL cells treated with or without VEGF for 30 minutes were lysed, and the nuclear and cytosolic fractions separated and analyzed by Western blotting. The H4 histone was used as an internal nuclear marker. p-Y-STAT1 indicates tyrosine 701 phosphorylated STAT1. (D) B-CLL cells were untransfected (None) or transfected with STAT1 siRNA or control siRNA, lysed, and analyzed by Western blotting to determine STAT1 gene silencing efficiency. Actin was used as an internal loading control. Numbers represent the average t-STAT1/actin ratio after normalizing untransfected values to 1. *P ≤ .05 compared with untransfected, untreated control. (E) B-CLL cells untransfected or transfected with STAT1 or control siRNA were treated with medium or 200 ng/mL VEGF for 24 hours. The conditioned media was concentrated and MMP-9 levels analyzed by gelatin zymography. Normalized average values (n = 3) are shown. (F) B-CLL cells untransfected or transfected with STAT1 or control siRNA were treated with medium or VEGF and added to Transwell filters (5 μm pore size) coated with Matrigel. Cell migration was determined after 24 hours by flow cytometry. Values are the percentage of total cells added. *P ≤ .05. **P ≤ .01.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/115/4/10.1182_blood-2009-08-239426/4/m_zh89990947500002.jpeg?Expires=1769095114&Signature=nUBVZG3BIhP2wm~Ox4L7OP2-iUzVR0cD3CcPRWixbdzYnLR0IKW2CV2TIIW66YUdJdFM-97i0fuikSuHiCerIci25-YKGJru1W~nvI~Yy9oKMSj2VcpbivCgTqyCnWNbYKzgXd1OSmJHaSPkYNLaj38wyJpWUBkyh817ax-4FCQ2WX2PRb1e2KrNg1wc27YWl8kDLjRAktxchYheaCoIkSqFKtVYqe3NtBCx1vjKGodggCI0TJFZpv21jzYq1x0biyjcsISRpPFSUMTCgeGx0YYAii-742PjmScK0IO1tF7b~uICJXzcW7wslxnAa1Q7gbq0BaB4yuW7vjXf9Ciusw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal