Abstract
The small GTPase Rap1 and its effector RAPL regulate lymphocyte adhesion and motility. However, their precise regulatory roles in the adhesion cascade preceding entry into lymph nodes and during interstitial migration are unclear. Here, we show that Rap1 is indispensably required for the chemokine-triggered initial arrest step of rolling lymphocytes through LFA-1, whereas RAPL is not involved in rapid arrest. RAPL and talin play a critical role in stabilizing lymphocyte arrest to the endothelium of blood vessels under flow or to the high endothelial venules of peripheral lymph nodes in vivo. Further, mutagenesis and peptide studies suggest that release of a trans-acting restraint from the β2 cytoplasmic region of LFA-1 is critical for Rap1-dependent initial arrest. Rap1 or RAPL deficiency severely impaired lymphocyte motility over lymph node stromal cells in vitro, and RAPL deficiency impaired high-velocity directional movement within lymph nodes. These findings reveal the several critical steps of Rap1, which are RAPL-dependent and -independent, in lymphocyte trafficking.
Introduction
Lymphocyte trafficking plays important roles in immune surveillance and in adaptive immune responses. To better understand the regulatory mechanisms of these processes, it is important to determine how each trafficking step is controlled. Integrins are major adhesion molecules involved in dynamic lymphocyte trafficking. Central to these functions is the unique ability of integrins to regulate their adhesive activity by a process termed inside-out signaling. In addition, ligand-bound integrins transmit signals to the cytoplasm in an outside-in direction (outside-in signals), leading to stabilized adhesion, cell spreading, and modulated cellular functions.1 It has been well established that temporal and spatial regulation of integrins through bidirectional signaling is important in adhesion-related cellular processes.2,3
Several well-characterized cellular processes critically involve integrins, including leukocyte trafficking to inflammatory sites and lymphocyte homing to secondary lymphoid organs. In the case of naive lymphocytes immigrating across high endothelial venules (HEVs) into peripheral lymph nodes (LNs), lymphocytes are first captured by weak binding between L-selectin and a sulfated sialyl Lex-related carbohydrate, resulting in rolling on the HEV. When rolling lymphocytes are exposed to chemokines present on the luminal side of the HEV, chemokine signaling coupled with Gi proteins activates LFA-1 in less than 1 second, resulting in a complete stop (arrest).4 Within seconds to minutes, lymphocyte adhesion is stabilized and these cells transmigrate into the tissues. The transition of integrin external domains from a bent to an extended conformation, which is triggered by separation of the α and β cytoplasmic domains,5 has been proposed to transform rolling into arrest/adhesion.6 However, our understanding of the molecular mechanisms of integrin regulation under physiologic conditions, where they occur over a broad range with a timescale of less than 1 second to minutes or hours, is still limited.
After transmigration, T cells enter the T cell–rich paracortex of the LN, which contains an elaborate network of fibroblastic reticular cells (FRCs), surrounding the HEVs and extending from the capsule to the medulla.7 B cells migrate into the follicles where a dense network of follicular dendritic cells (FDCs) is organized. FRCs and FDCs produce homeostatic chemokines (CCL21, CXCL13, and CXCL12) and abundantly express integrin ligands, such as ICAM-1 and VCAM-1 as well as MAdCAM-1 in some areas.7 Live imaging of the LN by 2-photon laser scanning microscopy has demonstrated a robust migration of lymphocytes in the paracortex and follicles.8 FRCs and FDCs appear to support rapid movement of lymphocytes.9 Although many studies reported that chemokines control integrin-dependent cell migration in vitro, it has not been determined whether integrins and chemokines coordinately regulate lymphocyte interstitial migration in lymphoid tissues. A recent study showed that immobilized chemokines sufficiently support T-cell migration without a major contribution by β2 and α4 integrins under shear-free conditions,10 suggesting integrin-independent migration within the LN. DOCK2 is a Rac-GEF that is critical for actin cytoskeletal rearrangements in lymphocytes,11 integrin activation and trafficking in B cells, and directional high-velocity lymphocyte movement in the LN.12 However, the molecular mechanisms controlling interstitial migration of lymphocytes are largely unknown.
The small GTPase Rap1 is a potent activation signal for β1, β2, and β3 integrins and enhances cellular adhesion in both immune and nonimmune cells.13 Lymphocyte Rap1, which is rapidly activated by chemokines and cognate antigens, increases the adhesiveness of integrins to their ligands by modulating affinity and avidity, induces polarized cell shape, and facilitates cell migration.14 Targeted deletion of Rap1a and Rap1b resulted in impaired activation of lymphocyte and platelet integrins.15-18 Although the phenotype of Rap1a and Rap1b double-knockout mice has not yet been reported, both Rap1a and Rap1b probably contribute to integrin activation. A deficiency in the Rap1-specific GEF, calcium, and diacylglycerol (CalDAG)–GEFI caused defective β1, β2, and β3 integrin activation in platelets and leukocytes in mice.19-21 A splice junction mutation in this gene was reported in some LAD-III patients.20 The signaling pathway of Rap1 activation by chemokines depends on PLC activity through CalDAG-GEFI to mediate human T-cell adhesion by LFA-1, but not VLA-4,21 suggesting that multiple pathways regulate integrins. RAPL is highly expressed in immune cells where it functions as a Rap1-GTP–binding protein that mediates Rap1 functions on integrins.22 Targeted deletion of the rapl gene impaired chemokine-induced lymphocyte adhesion and trafficking to peripheral LNs.23 However, the exact roles of Rap1 and RAPL signaling in lymphocyte adhesion cascades on endothelial cells and interstitial migration after entering the LNs are unknown. Here we demonstrate the essential steps of Rap1 and RAPL signaling in lymphocyte trafficking.
Methods
Methods for mice, plasmids, cell transfection, shRNA knockdown, flow and motility assays, intravital microscopy, and flow cytometric analysis are provided in detail in supplemental Methods (available on the Blood website; see the Supplemental Materials link at the top of the online article). Mice were housed in specific pathogen-free conditions, and all experiments were conducted in accordance with protocols approved by the Animal Care and Use Committee of Kansai Medical University (Osaka, Japan).
Results
Establishment of an in vitro system to reproduce the lymphocyte adhesion cascade
Most of the previous studies examining LFA-1 activation processes were performed by first reproducing the transition of selectin-dependent rolling into chemokine-triggered arrest by integrins. Therefore, our approach was to reconstitute the lymphocyte adhesion cascade mediated by L-selectin and LFA-1 using a BAF pro-B-cell line expressing human L-selectin and LFA-1 (BAF/LFA-1/L-selectin) and LS12 endothelial cells expressing tumor necrosis factor-α (TNF-α)–induced ICAM-1, as well as the L-selectin ligand, PNAd, which was produced by introducing HEV-specific carbohydrate modification enzymes.24 When infused over the monolayer of LS12 cells immobilized with CXCL12 in the parallel plate flow chamber at various shear stresses, a fraction of BAF/LFA-1/L-selectin cells exhibited rolling at various velocities and stopped at the shear stress between 1 to 5 dyne/cm2. We found that shear stress less than 0.6 dyne/cm2 did not support lymphocyte rolling, consistent with a previous report demonstrated for primary T cells.25
Because the transition from rolling to arrest was efficiently observed when cells were infused at 2 dyne/cm2 into this system, the experiments hereafter were performed at this shear stress. The images of cellular interactions were acquired by a CCD camera and video-recorded, and then digitized at 30 frames/second and subjected to an auto-cell tracking analysis. The representative adhesive profiles are shown (Figure 1). The attachment sustained for more than 10 seconds (typically longer than 1 minute) is hereafter termed “stable arrest” (Figure 1B-C; supplemental Video 1). Treatment with pertussis toxin severely inhibited “transient arrest,” attachment of less than 10 seconds, suggesting that it is a Gi-dependent process (data not shown). In the absence of chemokines, the majority of cells just rolled (Figure 3B; supplemental Video 10). Anti–L-selectin antibody (MEL-14) treatment abolished all cellular adhesion events (Figure 1B,D; supplemental Video 2). Treatment with anti–LFA-1 monoclonal antibody (TS1/22) abrogated arrest without affecting rolling (Figure 1B-D; supplemental Video 3). A similar result was also obtained with anti–ICAM-1 mAb (data not shown). Collectively, these results confirm that the in vitro flow system using BAF/LFA-1/L-selectin and LS12 cells recapitulates the lymphocyte adhesion cascade on HEV in peripheral LN in which endothelial chemokines convert L-selectin–mediated rolling into arrest by activating LFA-1.
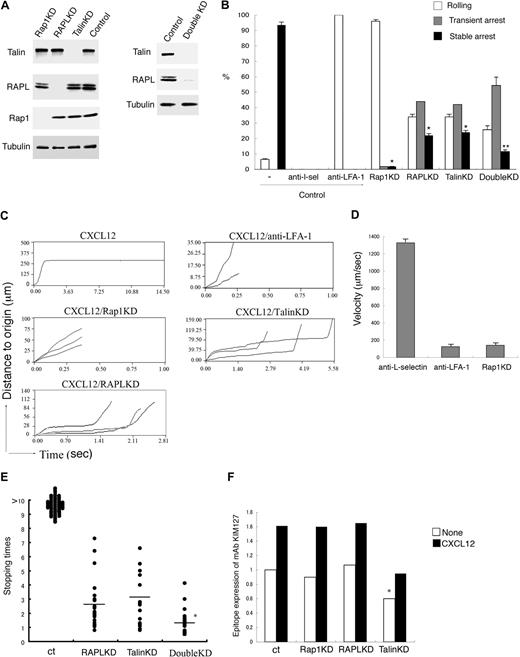
Requirement for Rap1, RAPL, and talin in LFA-1–mediated arrest under shear flow. (A) Knockdown of talin, RAPL, and Rap1 by shRNA. Western blots of total cell lysates from BAF/LFA-1/L-selectin cells with lentiviruses encoding control shRNA or shRNA targeting Rap1a/b-specific (Rap1KD), RAPL (RAPLKD), talin (TalinKD) are shown. Tubulin was used as a loading control. Western blots of total lysates from the double knockdown cells with lentiviruses encoding RAPL (RAPLKD) and talin (TalinKD) are also shown in the right panel. (B) Effects of anti–L-selectin, anti–LFA-1, Rap1KD, RAPLKD, TalinKD, and double KD on the interactions of BAF/LFA-1/L-selectin cells with LS12 endothelial cells. Control cells were pretreated with or without anti–L-selectin or anti–LFA-1 antibody. Then the cells perfused at 2 dyne/cm2 on LS12 monolayers, which were immobilized with CXCL12. The digital images of interactions of BAF/LFA-1/L-selectin cells with LS12 endothelial cells were taken at 30 frames/second. The adhesive events of more than 100 cells were measured and categorized as described in the supplemental Methods. Data represent the mean ± SD of 3 independent experiments. *P < .001, compared with control cells. **P < .01, compared with RAPL or Talin KD cells. (C) Time-displacement profiles of individual cell movement over LS12 endothelial monolayers under shear flow. BAF/LFA-1/L-selectin cells were perfused at 2 dyne/cm2 on LS12 monolayers immobilized with CXCL21. Representative profiles of the displacements over time are shown for “stable arrest” of the cells on the CXCL12 immobilized endothelium, “rolling” in the presence of anti–LFA-1 antibody (CXCL12/anti–LFA-1), “rolling” of those depleting Rap1 (CXCL12/Rap1KD), and “transient arrest” of those depleting talin (CXCL12/TalinKD) and depleting RAPL (CXCL12/RAPL). Each line represents individual cell tracking. (D) The noninteracting and rolling velocities of control BAF/LFA-1/L-selectin cell movements on LS12 in the presence of anti–L-selectin (anti–L-selectin) and anti–LFA-1 (anti–LFA-1) antibodies as well as the Rap1 knockdown cells (Rap1KD). Data represent the mean ± SD of 3 independent experiments. (E) Stopping time of control (ct), RAPLKD or talinKD, or RAPL/talin double KD BAF/LFA-1/L-selectin cells arrested on LS12 endothelial cells are shown. More than 100 cells were measured in 3 independent experiments, and representative distribution of stopping time is shown. *P < .02, compared with RAPL or talin KD cells. (F) Epitope expression of mAb KIM127 on control (ct) BAF/LFA-1 cells and the Rap1 (Rap1KD), RAPL (RAPLKD), or talin (Talin KD) knockdown cells in the absence (None) or presence of CXCL12. *P < .05, compared with control cells. Data are normalized for LFA-1 expression detected by TS1/18.
Requirement for Rap1, RAPL, and talin in LFA-1–mediated arrest under shear flow. (A) Knockdown of talin, RAPL, and Rap1 by shRNA. Western blots of total cell lysates from BAF/LFA-1/L-selectin cells with lentiviruses encoding control shRNA or shRNA targeting Rap1a/b-specific (Rap1KD), RAPL (RAPLKD), talin (TalinKD) are shown. Tubulin was used as a loading control. Western blots of total lysates from the double knockdown cells with lentiviruses encoding RAPL (RAPLKD) and talin (TalinKD) are also shown in the right panel. (B) Effects of anti–L-selectin, anti–LFA-1, Rap1KD, RAPLKD, TalinKD, and double KD on the interactions of BAF/LFA-1/L-selectin cells with LS12 endothelial cells. Control cells were pretreated with or without anti–L-selectin or anti–LFA-1 antibody. Then the cells perfused at 2 dyne/cm2 on LS12 monolayers, which were immobilized with CXCL12. The digital images of interactions of BAF/LFA-1/L-selectin cells with LS12 endothelial cells were taken at 30 frames/second. The adhesive events of more than 100 cells were measured and categorized as described in the supplemental Methods. Data represent the mean ± SD of 3 independent experiments. *P < .001, compared with control cells. **P < .01, compared with RAPL or Talin KD cells. (C) Time-displacement profiles of individual cell movement over LS12 endothelial monolayers under shear flow. BAF/LFA-1/L-selectin cells were perfused at 2 dyne/cm2 on LS12 monolayers immobilized with CXCL21. Representative profiles of the displacements over time are shown for “stable arrest” of the cells on the CXCL12 immobilized endothelium, “rolling” in the presence of anti–LFA-1 antibody (CXCL12/anti–LFA-1), “rolling” of those depleting Rap1 (CXCL12/Rap1KD), and “transient arrest” of those depleting talin (CXCL12/TalinKD) and depleting RAPL (CXCL12/RAPL). Each line represents individual cell tracking. (D) The noninteracting and rolling velocities of control BAF/LFA-1/L-selectin cell movements on LS12 in the presence of anti–L-selectin (anti–L-selectin) and anti–LFA-1 (anti–LFA-1) antibodies as well as the Rap1 knockdown cells (Rap1KD). Data represent the mean ± SD of 3 independent experiments. (E) Stopping time of control (ct), RAPLKD or talinKD, or RAPL/talin double KD BAF/LFA-1/L-selectin cells arrested on LS12 endothelial cells are shown. More than 100 cells were measured in 3 independent experiments, and representative distribution of stopping time is shown. *P < .02, compared with RAPL or talin KD cells. (F) Epitope expression of mAb KIM127 on control (ct) BAF/LFA-1 cells and the Rap1 (Rap1KD), RAPL (RAPLKD), or talin (Talin KD) knockdown cells in the absence (None) or presence of CXCL12. *P < .05, compared with control cells. Data are normalized for LFA-1 expression detected by TS1/18.
Requirement for Rap1, RAPL, and talin in arrest by LFA-1 and ICAM-1
To clarify the roles of Rap1, RAPL, and talin in the arrest step, we knocked them down by more than 95% using lentiviral transduction of shRNAs specific for Rap1a, Rap1b, RAPL, and talin (Figure 1A). We also knocked down both RAPL and talin (Figure 1A right). The depletion of Rap1 in BAF/LFA-1/L-selectin completely inhibited chemokine-induced arrest without affecting rolling (Figure 1B-C; supplemental Video 4). The range of rolling velocities of Rap1 knockdown cells was almost identical to that of control cells treated with the anti–LFA-1 mAb (Figure 1D; supplemental Videos 3-4), indicating that Rap1 is critically involved in the transition from rolling to arrest. On the other hand, the knockdown of RAPL or talin did not significantly affect the transition to arrest, as the majority of cells stopped on the monolayer. However, the attached cells were easily dislodged, resulting in an increased transient arrest population with a reciprocal decreased stable arrest population (Figure 1B-C; supplemental Figure 1). The stopping time of cells depleted of RAPL or talin was less than 10 seconds with an average of 2.6 and 3.1 seconds, respectively (Figure 1B,C,E; supplemental Video 5). Double knockdown of RAPL and talin synergistically decreased the conversion to stable arrest and stopping times, suggesting that they have distinct effects on the arrest (Figure 1B,E). These results suggest that LFA-1–dependent arrest can be divided into 2 sequential steps. The initial step is a transition from rolling to arrest, occurring within 1 second, and requires Rap1. The subsequent stabilization occurs within 10 seconds and requires both RAPL and talin.
Previous studies suggest that conformational changes alter the affinity of LFA-1 and are required for LFA-1–dependent arrest. The conversion from the low affinity bent conformation to the intermediate affinity extended conformation induces the transition from rolling to arrest.6,26 We examined whether the extension status of LFA-1 could be regulated by Rap1, RAPL, and talin using the antibody KIM127, which recognizes an extension reporter epitope on the β2 subunit. The expression levels of the extension reporter epitope were significantly up-regulated by CXCL12, as previously reported,27 indicating that this chemokine induces or shifts equilibrium to the extended conformation (Figure 1F; supplemental Figure 2). Neither Rap1 nor RAPL depletion affected KIM127 epitope expression. Depletion of talin significantly decreased both the basal and chemokine-stimulated KIM127 epitope levels but still exhibited CXCL12-induced up-regulation of the KIM127 epitope to an extent similar to that of control cells (1.6-fold, Figure 1F; supplemental Figure 2). The effects of deletion of RAPL and talin on the KIM127 expression were similar to that of talin deletion (data not shown). On treatment with Mg2+ and ethyleneglycoltetraacetic acid, BAF/LFA-1/L-selectin cells depleted of Rap1, RAPL, or talin expressed the high affinity conformation of LFA-1, as evidenced by mAb24 binding,28 and adhered to ICAM-1 (supplemental Figure 3). These results suggest that Rap1 exerts a different regulatory effect distinct from induction of the extension of LFA-1 by chemokine.
Requirement for RAPL in stable arrest of mouse lymphocytes on HEV
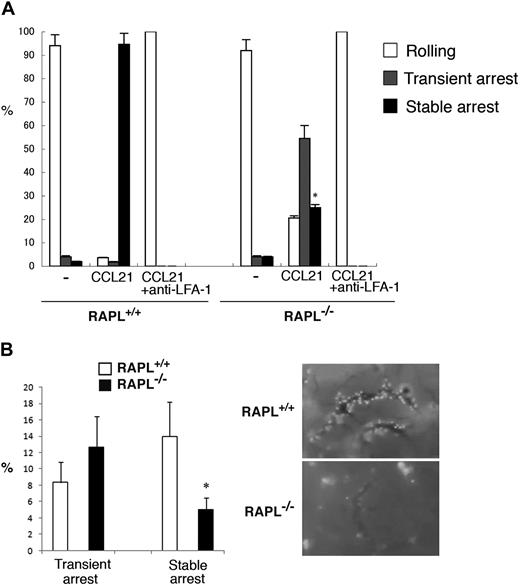
To further confirm whether RAPL plays an important role in stable attachment, but not during the initial arrest, we examined RAPL−/− T lymphocytes using the flow adhesion assay with LS12 cells expressing mouse ICAM-1. Control T cells efficiently roll on the LS12 monolayer and arrest in the presence of immobilized CCL21 (Figure 2A). This arrest was inhibited by anti–LFA-1 mAb (FD441). Although CCL21-induced transient arrest was increased, RAPL−/− T cells did not stably attach. The majority of RAPL−/− T cells were dislodged within 10 seconds at the shear stress of 2 dyne/cm2 (Figure 2A; supplemental Videos 6-7). To confirm the in vivo relevance of this result, we labeled RAPL−/− lymphocytes with CMTMR and injected them intravenously into normal mice and then examined lymphocyte interactions with the HEV in the mesenteric LN using intravital microscopy. Injected wild-type lymphocytes interacted with HEVs, resulting in firm attachment and subsequent accumulation of attached lymphocytes along the venules within 10 minutes (Figure 2B; supplemental Video 8). RAPL−/− lymphocytes appeared to tether/roll and stop normally on HEV. However, attached RAPL−/− lymphocytes were easily detached and accumulated poorly along the HEV (Figure 2B; supplemental Video 9). The number of stably attached cells was severely decreased, whereas the number of transiently adherent cells were not reduced (Figure 2B). These in vivo and in vitro results indicate that RAPL is not critical in the initial step of arrest but is required for subsequent adhesion stabilization.
RAPL is required for stable adhesion. (A) The adhesion of wild-type and RAPL-deficient T lymphocytes with LS12 cells expressing mouse ICAM-1 under shear flow. Adhesive interactions were measured as in Figure 1. Data represent the mean ± SD of 3 independent experiments. *P < .001, compared with wild-type lymphocytes. (B) Intravital microscopic analysis of wild-type and RAPL-deficient lymphocytes in HEVs. The percentages of transient arrest and stable arrest of adoptively transferred lymphocytes from control and RAPL−/− mice passing through HEVs in the MLN are shown (left). The y-axis indicates the ratios of the cells, which stopped more than 0.5 seconds, but detached within 10 seconds (transient arrest) or adhered more than 10 seconds (stable arrest) against total cells interacting with the vessel wall. Data represent the mean ± SD of 4 independent experiments. Representative cell interactions with MLN HEVs are shown (right). Image acquisition information is available in the supplemental Methods.
RAPL is required for stable adhesion. (A) The adhesion of wild-type and RAPL-deficient T lymphocytes with LS12 cells expressing mouse ICAM-1 under shear flow. Adhesive interactions were measured as in Figure 1. Data represent the mean ± SD of 3 independent experiments. *P < .001, compared with wild-type lymphocytes. (B) Intravital microscopic analysis of wild-type and RAPL-deficient lymphocytes in HEVs. The percentages of transient arrest and stable arrest of adoptively transferred lymphocytes from control and RAPL−/− mice passing through HEVs in the MLN are shown (left). The y-axis indicates the ratios of the cells, which stopped more than 0.5 seconds, but detached within 10 seconds (transient arrest) or adhered more than 10 seconds (stable arrest) against total cells interacting with the vessel wall. Data represent the mean ± SD of 4 independent experiments. Representative cell interactions with MLN HEVs are shown (right). Image acquisition information is available in the supplemental Methods.
Roles of the αL and β2 cytoplasmic domains in arrest
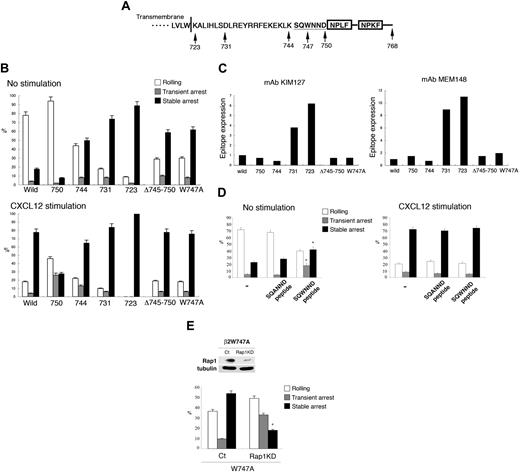
To identify the cytoplasmic region of LFA-1 critical for Rap1-dependent arrest, a series of deletion mutants of the β2 tail were introduced into the BAF/L-selectin cells (Figure 3A). Stable transfectants at comparable expression levels of LFA-1 (data not shown) were chosen and examined by the flow adhesion assay with LS12 cells. Carboxyl-terminal deletion of the β2 tail after amino acid 750 (Δ750) predominantly showed rolling without chemokines and decreased the chemokine-triggered stable arrest with a reciprocal increase of the transient arrest. This indicated that the region downstream of 750, containing the talin binding NPXF/Y motif, was important for stable arrest. However, the Δ750 was still capable to respond to CXCL12, indicating that the region downstream of 750 is not critical for chemokine-induced arrest. In contrast, deletion after amino acid 744 (Δ744), 731 (Δ731), or 723 (Δ723) induced stable arrest independently of chemokines (Figure 3B). These findings suggested that the region between 745 and 750 could suppress cell arrest in the absence of activation. To examine this possibility, an internal deletion mutant lacking amino acids 745 to 750 was made. The β2Δ745-750 also induced arrest without chemokines (Figure 3B). Because tryptophan 747 is the only conserved aromatic amino acid among β integrins in this region, this W747 residue was mutated to alanine. The W747A mutation efficiently induced the stable arrest with no further augmentation by chemokines (Figure 3B; supplemental Videos 10–11). There was a slight increase of the transient arrest of Δ744 in the presence of chemokine compared with that of wild-type, implying the reduced adhesion stability of Δ744. In contrast to the β2 integrin, deletion of the αL cytoplasmic region after the GFFKR motif did not induce spontaneous arrest (supplemental Figure 4). Rather, the αLΔ1095 mutation converted the stable arrest into transient arrest with similar frequencies of the total arrest (transient plus stable), indicating that a region downstream of 1095 in αL is also critical for the stabilization of this arrest. This result is in agreement with the previous study showing that RAPL interacted with the αL cytoplasmic region.22
LFA-1 cytoplasmic regions regulate transient and stable arrest. (A) Schematic representation of the cytoplasmic region of human β2 subunit. Deletions and point mutation were made at the indicated amino acid residues. Deletion mutants include the indicated amino acids. The boxes indicate the NPXF/Y motif, which is the talin-binding region. (B) Under flow adhesion of BAF cells expressing L-selectin and LFA-1 mutants with or without CXCL12. Data represent the mean ± SD of 3 independent experiments. (C) Expression of activation epitopes detected by mAb KIM127 (left) and MEM148 (right) in unstimulated LFA-1 mutants. (D) Induction of the arrest by a penetratin-1 fusion peptide corresponding to β2/745-750. Treatment with peptide β2/745-750 (SQWNND), but not with the control peptide (SQANND; 100 μg/mL), induced the stable arrest of BAF/L-selectin/LFA-1 cells. The cells were treated with the peptides in the absence or presence of CXCL12 as described in the supplemental Methods. Data represent the mean ± SD of 3 independent experiments. *P < .01, compared with the cells with control peptide. (E) Knockdown of Rap1 in BAF cells/L-selectin expressing αL/β2 W747A. (Top panel) Western blotting of vector alone (ct) or vector encoding Rap1-specific shRNA (Rap1 KD). Tubulin served as a loading control. (Bottom panel) Under flow adhesion of BAF cells/L-selectin expressing αL/β2 W747A mutation infected with lentiviruses encoding GFP alone (ct) or Rap1a/b-specific shRNA (KD). Data represent the mean ± SD of 3 independent experiments. *P < .005, compared with control lymphocytes.
LFA-1 cytoplasmic regions regulate transient and stable arrest. (A) Schematic representation of the cytoplasmic region of human β2 subunit. Deletions and point mutation were made at the indicated amino acid residues. Deletion mutants include the indicated amino acids. The boxes indicate the NPXF/Y motif, which is the talin-binding region. (B) Under flow adhesion of BAF cells expressing L-selectin and LFA-1 mutants with or without CXCL12. Data represent the mean ± SD of 3 independent experiments. (C) Expression of activation epitopes detected by mAb KIM127 (left) and MEM148 (right) in unstimulated LFA-1 mutants. (D) Induction of the arrest by a penetratin-1 fusion peptide corresponding to β2/745-750. Treatment with peptide β2/745-750 (SQWNND), but not with the control peptide (SQANND; 100 μg/mL), induced the stable arrest of BAF/L-selectin/LFA-1 cells. The cells were treated with the peptides in the absence or presence of CXCL12 as described in the supplemental Methods. Data represent the mean ± SD of 3 independent experiments. *P < .01, compared with the cells with control peptide. (E) Knockdown of Rap1 in BAF cells/L-selectin expressing αL/β2 W747A. (Top panel) Western blotting of vector alone (ct) or vector encoding Rap1-specific shRNA (Rap1 KD). Tubulin served as a loading control. (Bottom panel) Under flow adhesion of BAF cells/L-selectin expressing αL/β2 W747A mutation infected with lentiviruses encoding GFP alone (ct) or Rap1a/b-specific shRNA (KD). Data represent the mean ± SD of 3 independent experiments. *P < .005, compared with control lymphocytes.
We also examined conformations of the β2 mutants showing spontaneous arrest with KIM127 β2 extension reporter mAb29 and MEM148 open, high-affinity reporter mAb.30 Deletion of LFA-1 after amino acids 723 and 731 (Δ723 and Δ731) led to dramatic increases in the expression of both epitopes (Figure 3C; supplemental Figure 5), indicating extended open conformations. A similar result was also obtained with the high-affinity conformation reporter mAb24 (data not shown). This result in agreement with previous studies showing that mutations in the membrane proximal region of the β subunit and the GFFKR motif of the α subunit are important to keep integrins inactive.31,32 However, Δ744 as well as W747A mutation did not affect the expression levels of activation epitopes (Figure 3C; supplemental Figure 5), suggesting that these mutants are not the extended and open conformation of LFA-1. These results indicate that the region between 731 and 743 is sufficient for the inhibition of spontaneous extended conformations of LFA-1, and suggest that there is another critical step of the suppression of LFA-1 activation requiring the region between 745 and 755, specifically tryptophan 747.
Because the mutagenesis study suggested that the β2 cytoplasmic domain negatively regulates arrest through the region containing W747, we reasoned that similar regulation could be achieved by a trans-acting “restraint” that suppresses LFA-1 in low-affinity states by interacting with the β2/745 to 750 region. The restraint model predicts that the peptide sequence of this region can competitively inhibit interactions with a restraint resulting in stable arrest. To examine this possibility, we used the penetratin1 (P1) system. P1 fusion peptides are used as “Trojans” to efficiently deliver target peptides across the plasma membrane; and on entry into cells, the linking disulfide bond is cleaved in the cytoplasm to release the target peptide.33 A P1 peptide linked to β2/745-750 (SQWNND) and a control peptide in which W747 was replaced with alanine (SQANND) were synthesized. When BAF/L-selectin/LFA-1 cells were treated with these peptides and infused over an LS12 monolayer, the β2/745-750 peptide but not the control peptide substantially induced transient and stable arrest without CXCL12 (Figure 3D; supplemental Videos 12-13), supporting the notion that releasing the restraint induces arrest. The peptide did not augment the arrest by CXCL12 (Figure 3D). This suggests that the intracellular peptide concentration might not be sufficient for complete release of the trans-acting restraint, or the release of restraint may not be enough for the induction of arrest. It should be noted that the treatment with the β2/745-750 peptide as well as CXCL12 did not increase the spontaneous arrest of the cells with W747A mutation (Figure 3B; supplemental Figure 6), suggesting that they might act on the same pathway by a release of the restraint in the induction of the transient arrest.
Because β2/W747A induced stable arrest as efficiently as that triggered by chemokines, we next examined the Rap1 dependency for this spontaneous arrest. Rap1 depletion by shRNA inhibited stable arrest in cells expressing β2/W747A but did not reduce transient arrest (Figure 3E); however, Rap1 depletion in cells expressing wild-type LFA-1 demonstrated rolling without arrest (Figure 1B). Taken together, these results support the notion that, although the stabilization of the nascent ligand-bound LFA-1 still requires Rap1, the W747A mutant can substitute for Rap1 function to activate LFA-1–mediated transient arrest, possibly through the release a restraint from the β2 cytoplasmic domain.
Roles of Rap1 and RAPL in cell migration over FRCs
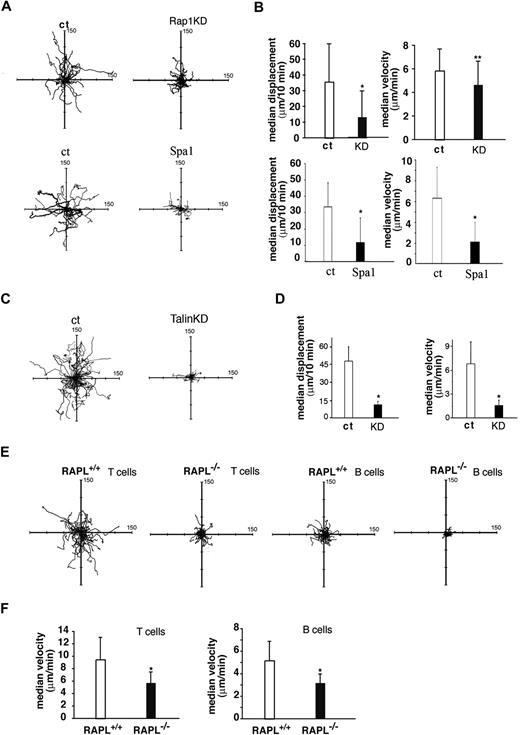
We previously demonstrated that Rap1 and RAPL signaling induced integrin-dependent migration as well as adhesion.34 We examined whether Rap1 and RAPL are also involved in interstitial migration after entering the LN. As a first approach to clarify the involvement of Rap1-RAPL signaling in this type of lymphocyte motility, we used the FRC cell line, BLS4, which was established from peripheral LNs and can develop ER-TR7+ reticular networks in vitro.7 BLS4 constitutively expresses VCAM-1 on the cell surface, whereas ICAM-1 is induced by TNF-α. Likewise, BLS4 cells produce CXCL12 constitutively, whereas the production of several other chemokines, including CCL4, CCL5, CCL20, and CXCL10, are augmented via TNF-α stimulation.7 When incubated over a TNF-α–stimulated BLS4 monolayer, BAF cells exhibited polarized morphologies with a leading edge and uropod and actively migrated (Figure 4A-D). Adhesion was largely dependent on the VLA-4–VCAM-1 system because VLA-4 or VCAM-1 mAb treatment reduced adhesion levels by approximately 70% (data not shown). We then examined the contribution of Rap1 and talin. Rap1-depleted BAF cells largely retained unpolarized shapes and showed marked reduction in migration velocities and displacements, compared with those of control BAF cells (Figure 4A-B). Spa1, a Rap1GAP, suppressed cell motility more severely. The knockdown of talin did not affect cell polarization but decreased adhesion and migration of BAF/LFA-1 cells on a BLS4 monolayer (Figure 4C-D).
Cell motility over the monolayer of BLS4 cells, an ER-TR7+ FRC cell line. (A) Representative cell tracks of BAF/L-selectin/LFA-1 cells transfected with vector (ct), Rap1 -specific shRNA (Rap1KD), or Spa1 over BLS4 cells. Each line represents a single-cell track. (B) Displacement and velocity of BAF/L-selectin/LFA-1 cells transfected with vector (ct,  ) or Rap1-specific shRNA (KD, ■) or Spa1 (■) over BLS4 cells. Each line represents a single-cell track. *P < .001. **P < .05. (C) Representative tracks of BAF/L-selectin/LFA-1 cells transfected with vector (ct) or talin-specific shRNA (KD) over BLS4 cells. Each line represents a single-cell track. (D) Displacement and velocity of BAF/L-selectin/LFA-1 cells transfected with vector (ct,
) or Rap1-specific shRNA (KD, ■) or Spa1 (■) over BLS4 cells. Each line represents a single-cell track. *P < .001. **P < .05. (C) Representative tracks of BAF/L-selectin/LFA-1 cells transfected with vector (ct) or talin-specific shRNA (KD) over BLS4 cells. Each line represents a single-cell track. (D) Displacement and velocity of BAF/L-selectin/LFA-1 cells transfected with vector (ct,  ) or Talin-specific shRNA (KD, ■) over BLS4 cells. *P < .001. (E) Cell motility of wild-type and RAPL−/− lymphocytes over the BLS4 monolayer. Representative tracks of wild-type and RAPL−/− T-cell blasts and B-cell blasts over BLS4 cells as indicated. Each line represents a single-cell track on the monolayer recorded every 30 seconds for 10 minutes. The median displacement was 35.1 μm/10 minutes for wild-type T cells and 10.5 μm/10 minutes for RAPL−/− T cells. The median displacement was 19.4 μm/10 minutes for wild-type B cells and 6.9 μm/10 minutes for RAPL−/− B cells. (F) Median velocity of wild-type and RAPL−/− T and B cells shown in panel E. The velocity data were obtained from movements measured every 30 seconds. *P < .001
) or Talin-specific shRNA (KD, ■) over BLS4 cells. *P < .001. (E) Cell motility of wild-type and RAPL−/− lymphocytes over the BLS4 monolayer. Representative tracks of wild-type and RAPL−/− T-cell blasts and B-cell blasts over BLS4 cells as indicated. Each line represents a single-cell track on the monolayer recorded every 30 seconds for 10 minutes. The median displacement was 35.1 μm/10 minutes for wild-type T cells and 10.5 μm/10 minutes for RAPL−/− T cells. The median displacement was 19.4 μm/10 minutes for wild-type B cells and 6.9 μm/10 minutes for RAPL−/− B cells. (F) Median velocity of wild-type and RAPL−/− T and B cells shown in panel E. The velocity data were obtained from movements measured every 30 seconds. *P < .001
Cell motility over the monolayer of BLS4 cells, an ER-TR7+ FRC cell line. (A) Representative cell tracks of BAF/L-selectin/LFA-1 cells transfected with vector (ct), Rap1 -specific shRNA (Rap1KD), or Spa1 over BLS4 cells. Each line represents a single-cell track. (B) Displacement and velocity of BAF/L-selectin/LFA-1 cells transfected with vector (ct,  ) or Rap1-specific shRNA (KD, ■) or Spa1 (■) over BLS4 cells. Each line represents a single-cell track. *P < .001. **P < .05. (C) Representative tracks of BAF/L-selectin/LFA-1 cells transfected with vector (ct) or talin-specific shRNA (KD) over BLS4 cells. Each line represents a single-cell track. (D) Displacement and velocity of BAF/L-selectin/LFA-1 cells transfected with vector (ct,
) or Rap1-specific shRNA (KD, ■) or Spa1 (■) over BLS4 cells. Each line represents a single-cell track. *P < .001. **P < .05. (C) Representative tracks of BAF/L-selectin/LFA-1 cells transfected with vector (ct) or talin-specific shRNA (KD) over BLS4 cells. Each line represents a single-cell track. (D) Displacement and velocity of BAF/L-selectin/LFA-1 cells transfected with vector (ct,  ) or Talin-specific shRNA (KD, ■) over BLS4 cells. *P < .001. (E) Cell motility of wild-type and RAPL−/− lymphocytes over the BLS4 monolayer. Representative tracks of wild-type and RAPL−/− T-cell blasts and B-cell blasts over BLS4 cells as indicated. Each line represents a single-cell track on the monolayer recorded every 30 seconds for 10 minutes. The median displacement was 35.1 μm/10 minutes for wild-type T cells and 10.5 μm/10 minutes for RAPL−/− T cells. The median displacement was 19.4 μm/10 minutes for wild-type B cells and 6.9 μm/10 minutes for RAPL−/− B cells. (F) Median velocity of wild-type and RAPL−/− T and B cells shown in panel E. The velocity data were obtained from movements measured every 30 seconds. *P < .001
) or Talin-specific shRNA (KD, ■) over BLS4 cells. *P < .001. (E) Cell motility of wild-type and RAPL−/− lymphocytes over the BLS4 monolayer. Representative tracks of wild-type and RAPL−/− T-cell blasts and B-cell blasts over BLS4 cells as indicated. Each line represents a single-cell track on the monolayer recorded every 30 seconds for 10 minutes. The median displacement was 35.1 μm/10 minutes for wild-type T cells and 10.5 μm/10 minutes for RAPL−/− T cells. The median displacement was 19.4 μm/10 minutes for wild-type B cells and 6.9 μm/10 minutes for RAPL−/− B cells. (F) Median velocity of wild-type and RAPL−/− T and B cells shown in panel E. The velocity data were obtained from movements measured every 30 seconds. *P < .001
To address whether RAPL is also involved in lymphocyte motility on FRCs, we used T and B lymphocytes from RAPL−/− mice. Because primary lymphocytes show only a weak motility on BLS4 cells, T or B cells were activated with an anti-CD3 mAb or LPS for several days. In this system, wild-type T cells showed active migration with an average velocity and displacement rate of 9.5 μm/minute and 44.0 μm/10 minutes, respectively (Figure 4E-F; supplemental Video 14). Treating with mAbs for LFA-1 and VLA-4 or ICAM-1 and VCAM-1 reduced the adhesion levels of CD3-stimulated lymphoblasts by 70%, the velocity by 20% to 30%, and the displacement by 30% to 40% (data not shown). When RAPL−/− T cells were applied to a BLS4 monolayer, they could adhere to the monolayer, but a large fraction of them retained unpolarized shapes and migrated within a limited area with an average velocity of 5.7 μm/minute. Trajectory analysis showed that a RAPL deficiency affected movements with high velocities and displacement rates (Figure 4E-F; supplemental Video 15). RAPL−/− B cells migrated poorly on the BLS4 monolayer with reduced velocity and minimum displacement (Figure 4E-F). Compared with T cells, B cells exhibited slower movement on the BLS4 monolayer with a 5.2 μm/minute velocity (Figure 4E-F). These results indicate that Rap1-RAPL signaling is required for efficient directional movement of cultured lymphoblasts on the FRC monolayer through regulation of both cell polarization and integrin adhesion.
Defective interstitial migration of RAPL−/− lymphocytes
To further investigate whether RAPL plays an important role in interstitial migration in vivo, we recorded the movement of adoptively transferred splenic lymphocytes within the inguinal LN by intravital epifluorescence microscopy and a high-sensitivity digital CCD camera. The cortex side of the inguinal LN was exposed from the skin and set under the microscope. Preliminary experiments confirmed that this experimental setting could detect active B-cell movement in follicles but not T-cell motility in the paracortex area. Wild-type lymphocytes actively migrated with an average velocity and displacement rate of 8.5 μm/minute and 38.1 μm/10 minutes, respectively. Intraperitoneally administered anti–VCAM-1 and anti–ICAM-1 mAbs bound to the FRC and FDC networks (supplemental Figure 7) and significantly retarded movement characterized by a decreased velocity and displacement rate, whereas control rat IgG had no significant effects (Figure 5), indicating that integrin-dependent adhesion facilitates lymphocyte motility within the LN. Further, lymphocyte motility was inhibited severely by pertussis toxin treatment (Figure 5), consistent with previous reports.35,36 Although lymphocytes were detected regardless of RAPL expression, RAPL−/− lymphocytes were inefficient in trafficking to the LN and required adoptive transfer of a greater numbers of lymphocytes to be tracked for 18 hours after transfer. In contrast to wild-type lymphocytes, most RAPL−/− lymphocytes remained near the original tracking sites with reduced average velocities (1.8 μm/minute) and displacement rates (10.9 μm/10 minutes; Figure 5). Most RAPL−/− lymphocytes, which either constantly changed cell shape or remained round, failed to initiate steady movement.
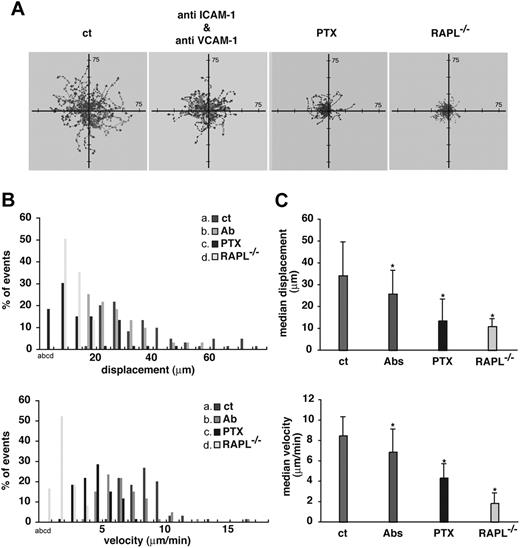
Contribution of Gi, integrin ligands, and RAPL to cell migration within the LN. (A) Lymphocyte movement in B-cell follicles observed by intravital microscopy of inguinal LN. Representative tracks of wild-type lymphocytes in recipient mice injected with control rat IgG (ct) (green), anti–ICAM-1 and anti–VCAM-1 mAbs (red), or pertussis toxin (blue). Representative tracks of RAPL-deficient lymphocytes (yellow) in untreated, normal recipient mice are shown. (B) Displacement and velocity profiles of wild-type and RAPL−/− lymphocytes in recipient mice shown in panel A. Fifty-nine cells were tracked for 10 minutes for each dataset. The velocity data were obtained from movements measured every 30 seconds. (C) Median displacement and velocity of populations shown in panel B. Statistical significance was determined by a t test. *P < .001 compared with lymphocytes in rat IgG-administered recipient mice.
Contribution of Gi, integrin ligands, and RAPL to cell migration within the LN. (A) Lymphocyte movement in B-cell follicles observed by intravital microscopy of inguinal LN. Representative tracks of wild-type lymphocytes in recipient mice injected with control rat IgG (ct) (green), anti–ICAM-1 and anti–VCAM-1 mAbs (red), or pertussis toxin (blue). Representative tracks of RAPL-deficient lymphocytes (yellow) in untreated, normal recipient mice are shown. (B) Displacement and velocity profiles of wild-type and RAPL−/− lymphocytes in recipient mice shown in panel A. Fifty-nine cells were tracked for 10 minutes for each dataset. The velocity data were obtained from movements measured every 30 seconds. (C) Median displacement and velocity of populations shown in panel B. Statistical significance was determined by a t test. *P < .001 compared with lymphocytes in rat IgG-administered recipient mice.
To examine the movement of T cells that localize primarily in the paracortical areas (100-300 μm below the capsule), intravital imaging of popliteal LN was performed using a multiphoton laser microscope 24 hours after adoptively transfer of differentially labeled wild-type and RAPL−/− T cells into the same recipient mice. RAPL−/− T cells exhibited a significant reduction in mean velocity compared with wild-type T cells. Trajectory analysis indicated that the 3-dimensional displacement of RAPL−/− T cells during the observation period was severely compromised (Figure 6; supplemental Video 16). Consistent with this observation, RAPL-deficient lymphocytes were distributed in limited areas within the LN compared with wild-type lymphocytes after adoptive transfer (data not shown). Collectively, these results reveal that RAPL contributes to efficient interstitial migration.
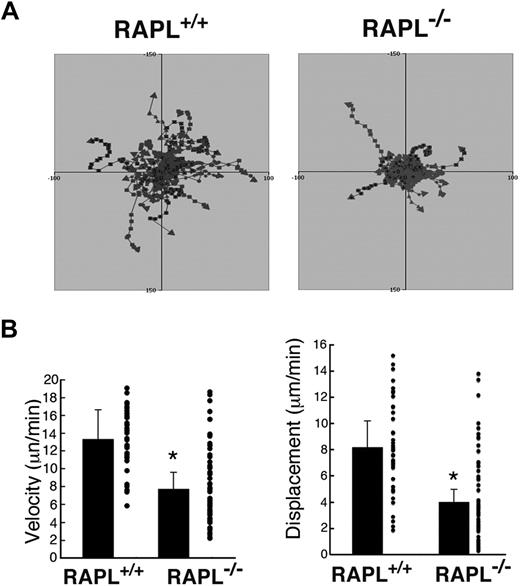
Interstitial migration of wild-type and RAPL-deficient T cells analyzed by intravital 2-photon microscopy. (A) Representative tracks of wild-type T cells (red) and RAPL−/− T cells (green) in popliteal LN. Each line represents a single T-cell track. (B) Displacement and velocity profiles of wild-type T cells and RAPL-deficient T cells. Forty cells of each type were tracked for 30 minutes for each dataset. *P < .001, compared with wild-type cells.
Interstitial migration of wild-type and RAPL-deficient T cells analyzed by intravital 2-photon microscopy. (A) Representative tracks of wild-type T cells (red) and RAPL−/− T cells (green) in popliteal LN. Each line represents a single T-cell track. (B) Displacement and velocity profiles of wild-type T cells and RAPL-deficient T cells. Forty cells of each type were tracked for 30 minutes for each dataset. *P < .001, compared with wild-type cells.
Discussion
This study provides novel information on the roles of Rap1 and RAPL signaling during entry into and trafficking within the LN. Rap1 is necessary for the rapid, chemokine-induced activation of LFA-1 that mediates arrest, but RAPL is not involved in the Rap1-dependent initial step of this arrest. Subsequently, Rap1, together with RAPL, stabilizes adhesion. They are also required for the motility of cultured lymphoblasts on FRC cells in vitro and contribute to the high-velocity directional movements of primary T and B cells mediated, at least in part, by ICAM-1 and VCAM-1 within the LN.
Because LFA-1 can act as the rolling receptor in some cellular contexts and under low shear flow, allowing pre-engagement of LFA-1 before the arrest,6,26 it is difficult to distinguish intracellular signaling required for the initial arrest from the subsequent stabilization of engaged LFA-1. We confirmed that, in our experimental system reproducing L-selectin–dependent rolling to arrest by LFA-1 under shear flow at 2 dyne/cm2, the blockade of LFA-1 or ICAM-1 did not affect rolling frequencies and velocities, ruling out the possibility of the pre-engagement of LFA-1 before the arrest. Under this condition, it is predicted that, when inside-out signaling by chemokines is blocked, the cells keep rolling through L-selectin engagement, whereas inhibition of the outside-in signaling should result in unstable arrest by LFA-1. The experiments with shRNA-mediated knockdown demonstrate that Rap1 acts through inside-out signaling and RAPL through outside-in signaling during arrest. This raises an issue as to how Rap1 possibly regulates subsecond activation independently of RAPL. There are other Rap1-binding proteins potentially involved in lymphocyte adhesion, including RIAM.37 However, CCL25-triggered adhesion of a human T-cell line to VCAM-1 through VLA-4 was inhibited by knockdown of Rap1a/b and RAPL, but not RIAM.38 Therefore, it is an important issue to be solved in the future, as to the mechanism by which Rap1 regulates subsecond LFA-1 activation.
The W747A mutation of the cytoplasmic region of the β2 tail induced spontaneous arrest independent of chemokines. It is widely thought that the association (clasping) and separation (unclasping) of the α and β tail is translated into inactive, bent low-affinity conformations and active, extended high-affinity conformations, respectively, and that the inside-out and outside-in signaling induces the separation.2 The mutational and structural studies have demonstrated that the membrane proximal “hinge” region, GFFKR“ in the α and the corresponding β cytoplasmic region, plays a critical role for clasping and restraining the integrin in a resting state.31,32 Therefore, it is possible that the W747A mutation causes unclasping, leading to constitutive activation of LFA-1. This is doubtful for several reasons. First, W747 is far from the hinge region (corresponding to β2 723-731), and the tryptophan residue at the homologous position in αIIbβ3 is not involved in the α and β cytoplasmic tail contacts.39 Second, deletion after the GFFKR motif did not result in constitutive activation. Finally, the W747 mutation did not induce extended, open conformations of LFA-1, which are induced by hinge-disrupting mutations, such as Δ723 and Δ731 mutations in this study. Thus, the chemokine-independent arrest by the W747A mutation is not simply explained by unclasping of the αL and β2 subunit. Because the β2/745-750 region overlaps the talin interacting region,40 talin might act as a restraint as well as a positive regulator.41 However, knockdown of talin1, the major isotype expressed in splenic lymphocytes and BAF cells, with talin2 below detectable levels (data not shown), failed to increase arrest adhesion without chemokine but severely inhibited stable arrest, thus indicating that talin does not act as restraint but plays the important role in stable adhesion.27,42 There has been so far no reports of β integrin-associated molecules interacting with the W747 residue. Immunoprecipitation experiments are so far unsuccessful to detect the putative restraint. Further studies with improved methods are needed to clarify the molecular characterization of restraint.
The result showing that the short peptide (S745-D750) covering the critical W747 induced the spontaneous arrest suggests that a trans-acting “restraint” binds to this region, keeping LFA-1 inactive, and release of the restraint by competitive inhibition with the peptide could lead to activation of LFA-1 for the initial arrest. It is conceivable that Rap1 could release the restraint on chemokine stimulation and induce the initial arrest because the knockdown of Rap1 in W747A mutants did not abrogate transient arrest (Figure 3). We could not clarify what effect a release of the restraint has on LFA-1. Previous studies suggest that LFA-1 extension with intermediate to high affinities induces arrest under shear flow,6,26,27 and in human T cells LFA-1 unbending by chemokine was suppressed by inhibition of Rap1 activation. However, chemokine-triggered LFA-1 extension did not require Rap1 in our system, suggesting other compensatory mechanisms through Rap1-related GTPases, such as Rap2, or through Rho-PI5kinase,43 operating for LFA-1 extension in a cellular context-dependent manner. Our results suggest that the function of Rap1 in the initial arrest occurs independently of extension conformational changes; instead, chemokine signaling coordinates the Rap1 and Rho signaling to initiate arrest.
Our results showed that RAPL and talin acted at the stabilization step but did not play a critical role in initial arrest. We previously showed that RAPL could associate with LFA-1 depending on activated Rap1 and the αL cytoplasmic tail, which was necessary for binding to ICAM-1.22 We could not detect the association of RAPL and talin, suggesting that they act independently through binding the αL and β2 tail, respectively. The arrest of cells expressing β2/W747A or Δ745-750 also required RAPL and talin for stabilization (data not shown). Further, knockdown of Rap1 converts stable arrest into transient arrest in W747A mutants, indicating that stabilization of ligand-engaged LFA-1 still requires Rap1. Because Rap1 is also activated by ligand-bound LFA-1 in lymphocytes,44 sequential Rap1 activation might occur by chemokine and ligand-bound LFA-1 to stabilize the arrest through RAPL, together with talin. Based on these findings, we propose sequential Rap1-mediated regulatory steps that control arrest and firm adhesion (supplemental Figure 8). LFA-1 is kept in a low-affinity bent-conformation state by the binding of a restraint to the β2 tail. Rap1 activation by chemokine initiates the adhesion cascade by release of the restraint, which could induce subsecond arrest adhesion (step 1). Transient adhesion is stabilized through the continuing activity of Rap1 by the binding of talin or other β2 tail interactors and RAPL (step 2), perhaps through separation of cytoplasmic tails.5 In addition, shear force facilitates affinity conformation of the I domain and enforced stabilized ligand-bound extended conformation.27 Thus, our results support a model that arrest adhesion is regulated by bidirectional, inside-out and outside-in signaling,45 and Rap1 activation is required throughout these processes.
Rap1 and RAPL function uniquely to induce migratory cell shapes and facilitate cell migration through endothelial cells under shear flow or on ICAM-1–coated surfaces.23,34,46 However, whether this signaling also plays a role in dynamic lymphocyte interstitial migration in lymphoid tissues is not yet known. Lymphoblasts exhibited active migration in vitro, as seen on the BLS4 FRC monolayer in this study. This motility was mediated mostly by LFA-1 and VLA-4 and severely impaired by Rap1 inhibition. The inhibitory effect of Rap1 depletion was also confirmed by Rap1 inhibition by Spa1. The inhibitory levels by Spa1 were more evident than Rap1 depletion. This suggests that the weak adhesive interactions resulting from incomplete Rap1 depletion can still support cell migration under nonflow conditions, or other signaling pathways could be involved in cell motility. RAPL−/− T and B lymphoblasts exhibited reduced motility and displacement with poor development of cell polarity. In vivo, primary RAPL−/− lymphocytes remained motile, to some extent, in the B-cell follicle and T-cell areas. However, they changed orientation frequently with reduced average velocities, resulting in defective long-distance migration. We showed that administering ICAM-1 and VCAM-1 antibodies in vivo decreased only high-velocity directional movements of lymphocytes. A small reduction in average velocity was also observed by CD18−/− lymphocytes.10 Nevertheless, the contribution of integrins to overall cell motility is relatively small compared with that of Gi signaling and RAPL, suggesting that other adhesion molecules and/or immobilized chemokines by themselves10,47 support cell motility in the LN environment. This implies that Rap1 and RAPL play more fundamental roles in cell motility in addition to integrin regulation. For example, chemokines stimulate lymphocyte polarization with a leading edge and uropod, a process that is independent of cell attachment but requires Rap1 and RAPL.22,34 Thus, Gi signaling followed by Rap1 and RAPL triggered by homeostatic chemokines presented on the LN stromal network controls the basic cellular machinery of movement in which integrins contribute to fast-speed movement, probably along the LN stromal network.9,10
Our study provides new evidence that Rap1 and RAPL signaling plays critical roles from LN entry to interstitial migration. Future studies are needed to elucidate how Rap1 and RAPL operate at the molecular level. In addition to trafficking and antigen recognition, LFA-1 has been suggested to play a role in regulatory T-cell development and function.48,49 Therefore, impairment of integrin regulation might lead to impairment of tolerance, leading to autoimmunity. The effects of Rap1 and RAPL on homeostasis of the immune system need detailed analysis.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Ms R. Hamaguchi for technical assistance and Dr N. O'Reilly for peptide synthesis.
This work was supported in part by the Ministry of Education, Culture, Sports, Science and Technology of Japan (grant-in-aid), the Toray Science Foundation, and the Naito Foundation.
Authorship
Contribution: Y.E. and K.K. performed the experiments, analyzed the data, and wrote some parts of the paper; T. Katakai and Y.U. performed some parts of the experiments; T.N., H.I., J.N., and T.O. supported the experiments using 2-photon microscopy; R.K. provided essential reagents; T.T. and M.M. supported the observation of lymphocyte interaction with HEV by intravital microscopy; N.H. provided essential reagents, discussed the results, and edited the paper; and T. Kinashi designed and supported the project and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for K.K. is Laboratory of Immunology, Department of Life Science, School of Science and Technology, Kwanseigakuin University, Osaka, Japan.
Correspondence: Tatsuo Kinashi, Department of Molecular Genetics, Institute of Biomedical Science, Kansai Medical University, Fumizono-cho 10-15, Moriguch, Osaka, 570-8506, Japan; e-mail: kinashi@takii.kmu.ac.jp.
References
Author notes
*Y.E. and K.K. contributed equally to this study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal