Abstract
Elevated plasma von Willebrand factor (VWF) and low ADAMTS13 activity have been reported in several inflammatory states, including sepsis and acute respiratory distress syndrome. One hallmark of inflammation is neutrophil activation and production of reactive oxygen species, including superoxide radical, hydrogen peroxide, and hypochlorous acid (HOCl). HOCl is produced from hydrogen peroxide and chloride ions through the action of myeloperoxidase. HOCl can oxidize methionine to methionine sulfoxide and tyrosine to chlorotyrosine. This is of interest because the ADAMTS13 cleavage site in VWF, the Tyr1605-Met1606 peptide bond, contains both oxidation-prone residues. We hypothesized that HOCl would oxidize either or both of these residues and possibly inhibit ADAMTS13-mediated cleavage. We therefore treated ADAMTS13 substrates with HOCl and examined their oxidative modification by mass spectrometry. Met1606 was oxidized to the sulfoxide in a concentration-dependent manner, with complete oxidation at 75μM HOCl, whereas only a miniscule percentage of Tyr1605 was converted to chlorotyrosine. The oxidized substrates were cleaved much more slowly by ADAMTS13 than the nonoxidized substrates. A similar result was obtained with multimeric VWF. Taken together, these findings indicate that reactive oxygen species released by activated neutrophils have a prothrombotic effect, mediated in part by inhibition of VWF cleavage by ADAMTS13.
Introduction
During acute inflammation, endothelial cells are activated by inflammatory cytokines such as interleukin-1 and tumor necrosis factor-α. Activated endothelial cells release von Willebrand factor (VWF) and expose P-selectin on the cell surface, a result of exocytosis of Weibel-Palade bodies, the major endothelial storage granules.1,2 On a longer time scale, other adhesion molecules, such as E-selectin and intracellular adhesion molecule-1, are synthesized and exposed on the cell surface.3 These molecules provide an adhesive surface for neutrophils, which attach to and roll on the endothelium, then become activated and migrate through the vessel wall into the surrounding tissues.
Although a fraction of the VWF remains transiently attached to the activated endothelium, most is secreted into the plasma.4,5 Elevated VWF levels in plasma have been described in diseases associated with systemic inflammation, such as acute lung injury/acute respiratory distress syndrome6,7 and sepsis.8 In each of these disorders, increased plasma VWF levels correlated independently with increased risk of death. The elevated plasma VWF level is primarily a consequence of endothelial cell activation, but other mechanisms may be involved. Several recent studies have shown that systemic inflammation is also associated with reduced activity of the VWF-processing enzyme ADAMTS13 activity in syndromes such as acute systemic inflammation caused by endotoxin,9 acute pancreatitis,10 severe sepsis and septic shock,11 sepsis-induced disseminate intravascular coagulation,12 and sepsis-induced organ dysfunction.13-15 It is therefore likely that in addition to enhanced release of VWF, delayed or reduced VWF processing by ADAMTS13 contributes to the elevated level and adhesiveness of VWF in these inflammatory disorders.
The only known substrate for ADAMTS13 is VWF. The most important function of this metalloprotease is to cleave the newly released very large and hyperadhesive forms of VWF (known as ultralarge VWF or ULVWF) to the smaller, less adhesive multimeric forms that are normally found in the plasma. Failure of this process leads to ULVWF accumulation and formation of systemic microvascular thrombi, the unique characteristic of the microangiopathic disease thrombotic thrombocytopenic purpura.16 ULVWF spontaneously binds platelets and induces their clumping, whereas plasma VWF requires a modulator such as ristocetin or botrocetin to do so.17 Physiologically, the interaction of plasma VWF with platelets is also induced by pathologically elevated fluid shear stress18 or VWF immobilization on subendothelial collagen.19 The hyperadhesive nature of ULVWF is not merely a consequence of its large size and increased content of VWF dimers (the protomer for making large multimers), as single molecule experiments showed that ULVWF could form spontaneous, high-strength bonds with glycoprotein Ibα (GPIIbα), its platelet receptor, whereas plasma VWF only recognized GPIbα in the presence of ristocetin or botrocetin.17 Thus, the VWF A1 domain, which contains the GPIbα binding site, appears to exist in a different conformation in ULVWF than in plasma VWF. This gain-of-function conformation is recognized by the llama nanobody AU/VWFa-11, which displays enhanced binding to VWF from patients with type 2B von Willebrand disease, thrombotic thrombocytopenic purpura, HELLP syndrome (hemolysis, elevated liver enzymes, low platelets), malaria, and antiphospholipid syndrome.20 Therefore, an important function of ADAMTS13 is to convert hyperactive ULVWF to cryptic plasma VWF.
Because ADAMTS13 activity is often decreased in patients with acute systemic inflammation, the processing of ULVWF could also be impaired, especially if the secretion of ULVWF is increased over baseline because of endothelial cell activation. It therefore seems reasonable to speculate that systemic microvascular thrombosis caused by enhanced platelet aggregation could contribute to the pathology of systemic inflammation.
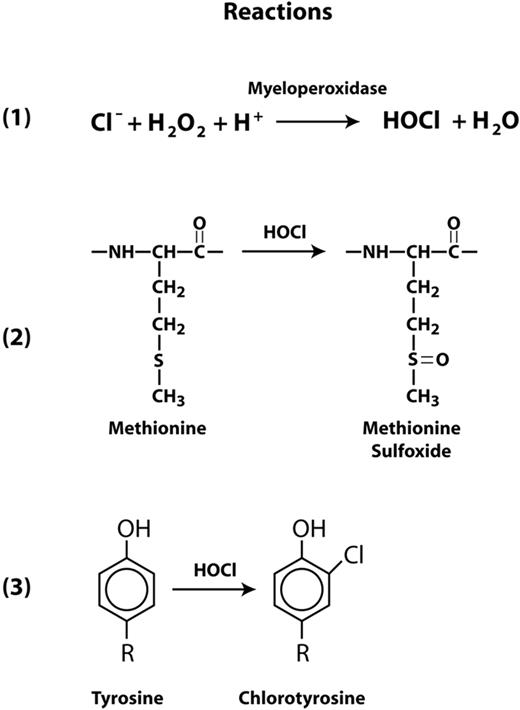
An important aspect of systemic inflammation is oxidative stress caused by the oxidative burst in activated neutrophils, which subsequently release several reactive oxygen species (ROS) that include superoxide radical, hydrogen peroxide, and hypochlorous acid (HOCl). Activated neutrophils also release myeloperoxidase (MPO), which generates HOCl from hydrogen peroxide and chloride ions (Figure 1 reaction 1). HOCl oxidizes cysteine and methionine residues 100 times faster than it oxidizes tyrosine, converting cysteine to cysteine sulfinic or sulfonic acids and methionine to methionine sulfoxide (Figure 1 reaction 2). HOCl can also oxidize tyrosine to chlorotyrosine (Figure 1 reaction 3). In other proteins, these oxidative modifications have been shown to profoundly influence function. For example, HOCl activates matrix metalloproteinase-7 from its latent proform by oxidizing a cysteine to cysteine sulfinic acid in the regulatory domain known as the cysteine switch.21 Methionine oxidation in α1-antitrypsin and thrombomodulin markedly reduces the functions of both proteins. The capacity of α1-antitrypsin to inhibit elastase was dramatically reduced after oxidation of methionine residue 351 or 358 to the sulfoxides.22 Similarly, oxidation of Met388 in thrombomodulin decreased the ability of the thrombomodulin-thrombin complex to activate protein C,23 a vital anticoagulant function. Conversion of Tyr192 to chlorotyrosine in apolipoprotein A1 of high density lipoprotein (HDL) interferes with HDL's ability to remove cholesterol from macrophages.24 Chlorotyrosine has been identified in HDL isolated from atherosclerotic lesions.25
Reactions for HOCl generation and oxidation of Met and Tyr residues. Myeloperoxidase catalyzes the conversion of hydrogen peroxide to HOCl and water in the presence of chloride ions (reaction 1). HOCl is capable of oxidizing methionine to methionine sulfoxide (reaction 2) and tyrosine to 3-chlorotyrosine (reaction 3).
Reactions for HOCl generation and oxidation of Met and Tyr residues. Myeloperoxidase catalyzes the conversion of hydrogen peroxide to HOCl and water in the presence of chloride ions (reaction 1). HOCl is capable of oxidizing methionine to methionine sulfoxide (reaction 2) and tyrosine to 3-chlorotyrosine (reaction 3).
Given these effects of HOCl on protein functions, we were intrigued by the fact that in the ADAMTS13 cleavage site in VWF, the Tyr1605-Met1606 peptide bond, both residues are potential targets for MPO-mediated oxidation. In the current study, we examined whether the ADAMTS13 cleavage site of VWF is susceptible to oxidation and, if so, whether the modification(s) interferes with proteolysis.
Methods
Materials
The following materials were used in this study: sodium hypochlorite (Red Bird Service); hydrogen peroxide (VWR International); methionine (MP Biomedicals); doxycycline, biotin, ammonium bicarbonate, and protease inhibitors (Sigma-Aldrich); sequencing-grade trypsin (Promega); acetonitrile (Mallinckrodt Baker Inc); iodoacetamide (Bio-Rad); trifluoroacetic acid and formic acid (EMD; Merck); FreeStyle 293 serum-free medium, puromycin, 16% tricine gels, and SYPRO orange (Invitrogen); rabbit anti–human VWF polyclonal antibody (DakoCytomation); myeloperoxidase (Lee Biosolutions); HEK 293 Tet-On cells (BD Clontech); and streptavidin–horseradish peroxidase (HRP; Pierce).
Preparation of VWF peptide substrate
An ADAMTS13 substrate peptide based on VWF A2 domain sequence was produced as a recombinant protein in Escherichia coli, as previously published.26,27 The peptide contains an N-terminal His-tag sequence, a 78-amino-acid sequence corresponding to the sequence Leu1591-Arg1668 of VWF, and a C-terminal biotin acceptor sequence. Two substitutions were introduced into the VWF-coding sequence: Ser1593Cys and Arg1668Lys. The Cys mutation was used for the unique attachment of HRP to the peptide in the previous study.26 However, the conjugated substrate was unsuitable for the present study because oxidation of HRP would also affect assays dependent on HRP activity. We therefore did not conjugate HRP to the peptide, but rather alkylated the Cys residue used for the conjugation to prevent its oxidation. The Lys substitution was originally made to conjugate biotin to the peptide. The peptide was isolated from E coli lysates by chromatography on a nickel column, reduced and reacted with iodoacetamide, and purified by reverse-phase high-performance liquid chromatography, as described previously.26,27
Recombinant human ADAMTS13
Recombinant ADAMTS13 (the Val900 polymorphic form) was produced in mammalian cells. The ADAMTS13 cDNA was ligated into expression plasmid pCBioSec, which contains a sequence encoding a biotin acceptor peptide (BioTag), producing a chimeric cDNA encoding full-length ADAMTS13 with the BioTag appended to its C terminus (ADAMTS13-Bio). The pCBioSec vector was produced by modification of the bicistronic expression unit of pCBio28 (GenBank No. DQ520291) to encode a secreted form of E coli biotin ligase. Recombinant ADAMTS13-Bio was enzymatically biotinylated by culturing cells in the presence of biotin. HEK 293 Tet-On cells transfected with the expression vector were selected in the presence of puromycin (2 μg/mL) for 2 weeks. After induction with doxycycline (2 μg/mL) in the presence of biotin (50μM), ADAMTS13-Bio expression in 23 stably transfected clones was evaluated by Western blotting of culture media with streptavidin-HRP. A stably transfected cell line, designated W1, expressing a high level of ADAMTS13-Bio was used for subsequent studies. W1 was cultured to confluence, and expression of ADAMTS13-Bio was induced for 72 hours by doxycycline (2 μg/mL) in FreeStyle 293 serum-free medium containing 50μM biotin. The medium containing the secreted biotinylated ADAMTS13-Bio was collected and treated with a mixture of protease inhibitors (1/100 dilution). The concentration of ADAMTS13-Bio in the medium was approximately 1 μg/mL, as determined by its proteolytic activity on HRP-H-A2-B.26 A Western blot probed with streptavidin-HRP showed that ADAMTS13-Bio migrated as a single species with an apparent mass of 190 kDa (data not shown). The medium containing ADAMTS13-Bio was stored at −70°C until use.
Purification of plasma VWF from cryoprecipitate
We purified plasma VWF from commercial cryoprecipitate. Briefly, cryoprecipitate was first resuspended in citrate buffer, and 2M glycine was added to precipitate the bulk of the fibrinogen. VWF was then precipitated by the addition of NaCl to a final concentration of 1.5M. The VWF precipitate was then suspended in 25mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid (HEPES) buffer, pH 6.8, before further purification on a size-exclusion column. Different fractions of the column flow-through were collected, and the VWF multimer composition was examined by 1% agarose gel electrophoreses and immunoblotting. The antigen concentration in each fraction was measured by ultraviolet absorption at 280 nm.
Oxidation reactions
Using HOCl.
HOCl was generated by the addition of 10 μL of stock sodium hypochlorite to 8 mL of doubly deionized water immediately before use. The absorption of HOCl was measured at wavelength 292 nm, and the concentration of HOCl was calculated using the absorption coefficient of 350 cm−1M−1.29 This HOCl solution was then further diluted to the concentrations used in individual experiments. In these experiments, the A2 substrate peptide concentration was 5μM, yielding a molar ratio of peptide to oxidant of 1:15 at the highest HOCl concentration (75μM). Purified plasma VWF was oxidized by HOCl in 10mM phosphate buffer (pH 7.4) for 30 minutes at 37°C either under constant rotation or in the presence of 1M urea. The reaction was terminated by the addition of excess methionine to quench the residual HOCl.
Using MPO plus hydrogen peroxide.
H2O2 was diluted from stock with doubly deionized water immediately before use. The absorption of diluted H2O2 was measured at 240 nm, and the concentration of H2O2 was calculated using an absorption coefficient of 39.4 cm−1M−1.30 The oxidation reaction was carried out in the presence of 50nM MPO and 100μM H2O2 in phosphate-buffered saline (pH 7.4) at 37°C for 1 hour. Methionine was added to quench HOCl at the end of the reaction.
Protein proteolysis
Before analysis of oxidation by mass spectrometry (MS), the A2 peptide substrate and plasma VWF were each first reduced for 20 minutes at 70 to 80°C with a buffer containing 4mM dithiothreitol, 50mM ammonium bicarbonate, and 5% acetonitrile, and then alkylated with 10mM iodoacetamide for 15 minutes at room temperature. The reduced and alkylated proteins were digested overnight at 37°C with trypsin (trypsin:protein, 1:20, w/w). The digestion was stopped with trifluoroacetic acid (pH 2-3). The tryptic peptides from the VWF peptide substrate were directly analyzed by MS. The tryptic peptides from plasma VWF were concentrated and desalted using a C18 column (Empore High Performance C18HD Extraction Disk Cartridges; 3M), dried under vacuum, and then resuspended in a solution of 0.1% formic acid and 5% acetonitrile before analysis by MS.
Oxidative modification examined by liquid chromatography–electrospray ionization–tandem MS
Liquid chromatography–electrospray ionization–tandem MS (LC-ESI-MS/MS).
LC/MS analyses of tryptic peptides were performed in the positive ion mode with a Thermo-Finnigan LCQ Deca XP Plus ion trap mass spectrometer coupled to an Agilent 1100 high-performance liquid chromatography system. Peptides were separated at a flow rate of 0.2 mL/min on a reverse-phase column (Vydac C18 MS column; 2.1 × 25 mm), using solvent A (0.1% formic acid in water) and solvent B (0.1% formic acid in CH3CN). Peptides were eluted using a linear gradient of 5% to 35% solvent B over 60 minutes (A2 peptide) or 140 minutes (plasma VWF). The spray voltage was 5 kV, and the temperature of the heated capillary was 220°C. The collision energy for MS/MS was 35%.
Nano-LC-MS/MS.
Nano-LC-MS analyses of tryptic peptides of plasma VWF were performed in the positive ion mode with a Thermo-Finnigan LTQ linear ion trap mass spectrometer coupled to a Waters nanoAcquity Ultra Performance LC (UPLC) system. Peptides were separated at a flow rate of 300 nL/min on a nanoUPLC BEH130 C18 column (100 × 0.075 mm, 1.7 μm; Waters), using solvent A and solvent B. Peptides were eluted using a linear gradient of 5% to 35% solvent B over 160 minutes. The spray voltage was 2.0 kV, and the temperature of the heated capillary was 200°C. The collision energy for MS/MS was 35%.
ADAMTS13 cleavage and cleavage product analysis
Oxidized or nonoxidized A2 substrate peptides (3.4μM) were incubated with ADAMTS13-Bio (0.1 μg/mL) at room temperature, and aliquots were removed at 15-minute intervals and proteolysis stopped by the addition of EDTA (ethylenediaminetetraacetic acid) to a final concentration of 10mM. ADAMTS13-mediated cleavage was monitored by 2 methods, with one fraction of the sample subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE), as previously described,27 and the remainder digested with trypsin and analyzed by LC-ESI-MS/MS, as described above. By MS, the rate was determined by assessment of production of the tryptic peptide EQAPNLVY, derived from the N-terminal ADAMTS13 cleavage product. This peptide is the same regardless of whether the parent substrate peptide being cleaved has the sequence YM or YM(O).
Oxidized or nonoxidized plasma VWF were incubated with ADAMTS13-Bio at 37°C in a buffer containing 10mM HEPES, 6.7mM BaCl2, and 1.8M urea, pH 7.4. The concentrations of VWF and ADAMTS13 were 10 μg/mL and 1 μg/mL, respectively. The cleavage reaction was stopped at either 2.5 or 5 hours, and the reaction mixtures were then digested with trypsin. The tryptic fragments of uncut substrates and cleavage products were analyzed and quantified by LC-ESI-MS/MS. The tryptic peptides derived from uncut substrates were EQAPNLVYMVTGNPASDEIK(R) and EQAPNLVYM(O)VTGNPASDEIK(R) for nonoxidized and oxidized VWF, respectively, and those from cleavage products were MVTGNPASDEIK(R) and M(O)VTGNPASDEIK(R) for nonoxidized and oxidized VWF, respectively. The percentage of products generated by ADAMTS13 was calculated by dividing the amount of products by the sum of uncut substrates and products at each time point.
Results
We tested whether HOCl could oxidize residues at the ADAMTS13 cleavage site of VWF using both an A2 peptide and multimeric VWF. In the experiments presented in Figures 2 through 4, VWF A2 peptides were first oxidized with various concentrations of HOCl, and the reactions were terminated at the specified time by the addition of methionine to quench the unreacted HOCl. An aliquot of the treated peptides was examined by MS to determine whether any residues were oxidized, and another aliquot was incubated with ADAMTS13 to determine the potential effect of oxidation on peptide cleavage by the metalloprotease. The cleavage products and cleavage rates were analyzed by both SDS-PAGE and MS.
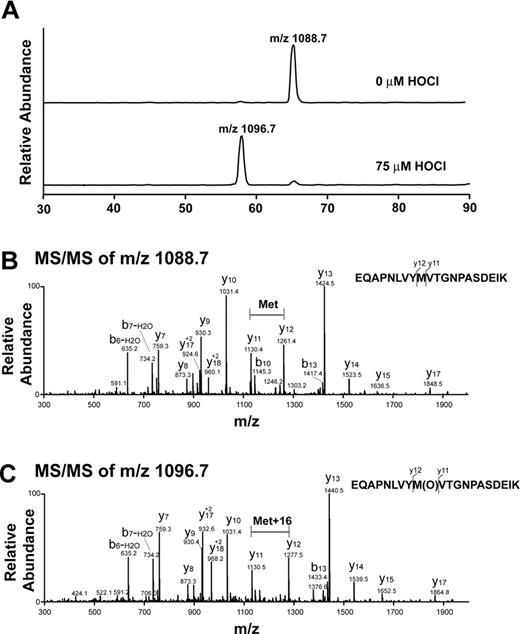
Analysis of oxidative modification of VWF A2 peptide by LC-ESI-MS/MS. (A) Reconstructed ion chromatograms extracted from the mass window corresponding to the expected mass/charge (m/z) ratios of the oxidized and unoxidized tryptic fragments. Note the almost complete disappearance of the m/z = 1088.7 peak with HOCl treatment and appearance of a peak with m/z = 1096.7. The increase in 8 atomic mass units in the doubly charged ion is consistent with the addition of 1 oxygen atom to the peptide. (B) MS/MS spectrum of nonoxidized peptide (m/z = 1088.7, doubly charged ion). (C) MS/MS spectrum of oxidized peptide at 75μM HOCl (m/z 1096.7, doubly charged ion). Note that the methionine residue in the oxidized peptide gained 16 atomic mass units.
Analysis of oxidative modification of VWF A2 peptide by LC-ESI-MS/MS. (A) Reconstructed ion chromatograms extracted from the mass window corresponding to the expected mass/charge (m/z) ratios of the oxidized and unoxidized tryptic fragments. Note the almost complete disappearance of the m/z = 1088.7 peak with HOCl treatment and appearance of a peak with m/z = 1096.7. The increase in 8 atomic mass units in the doubly charged ion is consistent with the addition of 1 oxygen atom to the peptide. (B) MS/MS spectrum of nonoxidized peptide (m/z = 1088.7, doubly charged ion). (C) MS/MS spectrum of oxidized peptide at 75μM HOCl (m/z 1096.7, doubly charged ion). Note that the methionine residue in the oxidized peptide gained 16 atomic mass units.
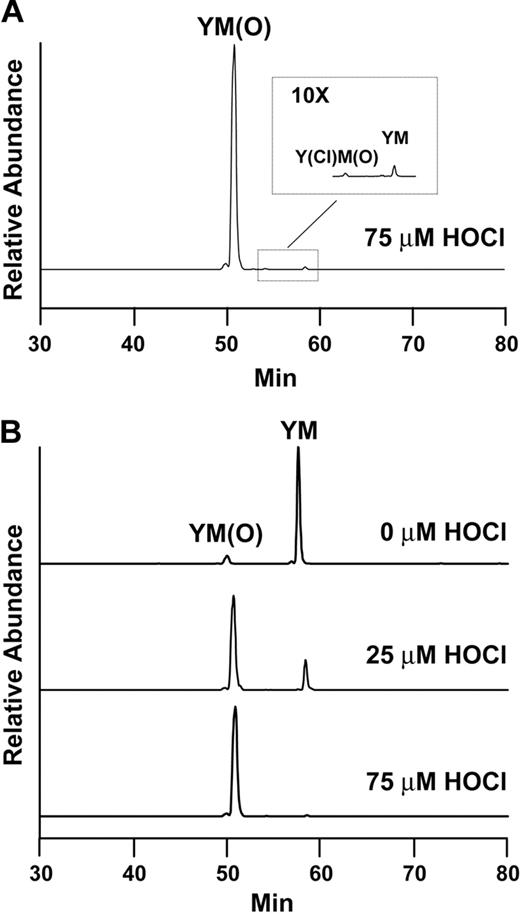
Reconstructed ion chromatograms of oxidized tryptic fragments. (A) In the presence of 75μM HOCl, approximately 99% of the A2 peptide contained methionine sulfoxide at position 1606, whereas only 0.3% contained both chlorotyrosine at position 1605 and methionine sulfoxide at 1606. (B) The content of methionine sulfoxide at position 1606 increased with increasing concentration of HOCl.
Reconstructed ion chromatograms of oxidized tryptic fragments. (A) In the presence of 75μM HOCl, approximately 99% of the A2 peptide contained methionine sulfoxide at position 1606, whereas only 0.3% contained both chlorotyrosine at position 1605 and methionine sulfoxide at 1606. (B) The content of methionine sulfoxide at position 1606 increased with increasing concentration of HOCl.
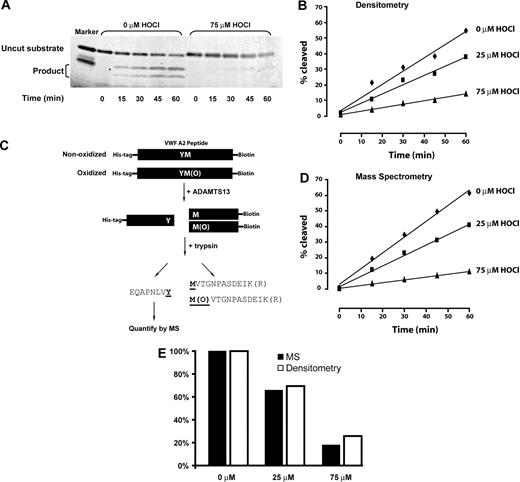
Time-dependent cleavage of oxidized or nonoxidized VWF A2 peptide by ADAMTS13. (A) Representative SDS-PAGE of ADAMTS13 cleavage of the A2 peptide substrate. In the unoxidized substrate, on the left, cleavage products of 9 kDa and 4 kDa accumulate over time, with concomitant disappearance of the 13-kDa substrate. Cleavage is markedly reduced after oxidation of the substrate peptide with 75μM HOCl (right side of gel). (B) Rate of cleavage quantified by densitometry. (C) Schematic depiction of the method used to quantify ADAMTS13 cleavage of the substrate peptide by MS. Note that the tryptic fragment that arises from the region immediately N-terminal to the ADAMTS13 cleavage site was analyzed. This fragment is identical whether or not the methionine has been oxidized. (D) ADAMTS13 cleavage of the unoxidized or oxidized substrate peptide as determined by MS. (E) Similar cleavage rates were obtained using SDS-PAGE/densitometry and MS.
Time-dependent cleavage of oxidized or nonoxidized VWF A2 peptide by ADAMTS13. (A) Representative SDS-PAGE of ADAMTS13 cleavage of the A2 peptide substrate. In the unoxidized substrate, on the left, cleavage products of 9 kDa and 4 kDa accumulate over time, with concomitant disappearance of the 13-kDa substrate. Cleavage is markedly reduced after oxidation of the substrate peptide with 75μM HOCl (right side of gel). (B) Rate of cleavage quantified by densitometry. (C) Schematic depiction of the method used to quantify ADAMTS13 cleavage of the substrate peptide by MS. Note that the tryptic fragment that arises from the region immediately N-terminal to the ADAMTS13 cleavage site was analyzed. This fragment is identical whether or not the methionine has been oxidized. (D) ADAMTS13 cleavage of the unoxidized or oxidized substrate peptide as determined by MS. (E) Similar cleavage rates were obtained using SDS-PAGE/densitometry and MS.
Methionine, but not tyrosine, at the ADAMTS13 cleavage site is oxidized by HOCl
We evaluated the VWF A2 peptide for oxidative modification by LC-ESI-MS/MS. Figure 2A is a reconstructed ion chromatogram comparing the A2 peptide tryptic fragments containing the ADAMTS13 cleavage site before and after HOCl treatment. In the treated peptide (75μM HOCl, peptide/oxidant molar ratio of 1:15), Met1606 was almost completely converted to the sulfoxide. The presence of the oxidative modification was confirmed by MS/MS (Figure 2B-C). The difference between the unoxidized and oxidized A2 peptides (Figure 2B-C, respectively) is revealed by comparing the differences in mass between the y11 and y12 fragment ions (derived from C-terminal fragmentation of the tryptic peptides depicted at the right side of the figures). In the oxidized peptide, this difference is 16 atomic mass units greater than in the unoxidized peptide, consistent with the addition of oxygen to the methionine residue. Under these conditions, only a miniscule fraction (0.3%) of the cleavage site tyrosine (corresponding to Tyr1605) was chlorinated (Figure 3A, Y[Cl]M[O]). We detected no peptides containing chlorotyrosine at position 1605 and unmodified methionine at 1606 (Figure 3A). HOCl oxidized Met1606 to methionine sulfoxide in a concentration-dependent manner; a small fraction of the methionine corresponding to Met1606 was oxidized in the untreated sample (Figure 3B).
Oxidation of VWF A2 peptide markedly impairs ADAMTS13 cleavage
The unoxidized or oxidized VWF A2 peptides were incubated with recombinant ADAMTS13-Bio. The reaction was sampled every 15 minutes for 1 hour. The cleavage products were analyzed by both electrophoresis and LC-ESI-MS. In the electrophoretic analysis, the cleavage products were first separated from uncut substrates by electrophoresis on SDS-PAGE (Figure 4A), and the amounts of both the uncut substrate and the cleavage products were quantified by densitometry (Figure 4B). ADAMTS13 cleaved the oxidized substrates much more slowly than the untreated substrate; the cleavage rates correlated inversely with the concentration of HOCl. We used MS as an independent, more quantitative means of determining proteolysis rates of the oxidized peptides. The method is depicted in Figure 4C, and the results are shown in Figure 4D. The results obtained with this method were almost identical to those obtained by electrophoresis and densitometry (Figure 4E). When we analyzed all of the cleavage products by LC-ESI-MS/MS, we detected product peptides with methionine sulfoxide at 1606, indicating that ADAMTS13 was able to cleave the oxidized peptide, albeit much less efficiently.
Oxidative modification by MPO/H2O2 was similar to that produced by HOCl
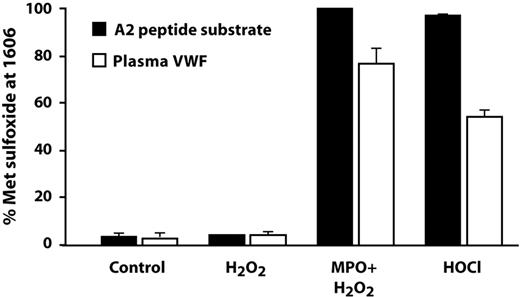
In vivo, HOCl is produced by the action of MPO on hydrogen peroxide, which is generated from superoxide by either enzyme-catalyzed or spontaneous dismutation. In this study, we used the combination of MPO and H2O2 to oxidize both the VWF A2 peptide and purified plasma VWF, and analyzed the extent of oxidation of Met1606 by LC-ESI-MS/MS. As with straight HOCl, incubation of the peptide with MPO plus H2O2 converted almost 100% of Met1606 to methionine sulfoxide (Figure 5). In multimeric plasma VWF, MPO and H2O2 converted 77% of Met1606 to methionine sulfoxide, whereas HOCl (100μM) converted 55% of Met1606 to the sulfoxide. Neither MPO (data not shown) nor H2O2, when used alone, generated methionine sulfoxide in either the peptide substrate or plasma VWF.
Oxidative modification by the combination of MPO and H2O2. An HOCl-generating system consisting of 50nM MPO plus 100μM H2O2 also completely oxidized Met1606 to methionine sulfoxide in the A2 substrate peptide (■). Hydrogen peroxide alone had no effect. Purified multimeric plasma VWF (100nM, molar concentration of VWF monomers) was also oxidized at Met1606 by HOCl (100μM) and by MPO/H2O2, although to a lesser extent than the substrate peptide (□).
Oxidative modification by the combination of MPO and H2O2. An HOCl-generating system consisting of 50nM MPO plus 100μM H2O2 also completely oxidized Met1606 to methionine sulfoxide in the A2 substrate peptide (■). Hydrogen peroxide alone had no effect. Purified multimeric plasma VWF (100nM, molar concentration of VWF monomers) was also oxidized at Met1606 by HOCl (100μM) and by MPO/H2O2, although to a lesser extent than the substrate peptide (□).
Oxidation of multimeric plasma VWF markedly impairs ADAMTS13 cleavage
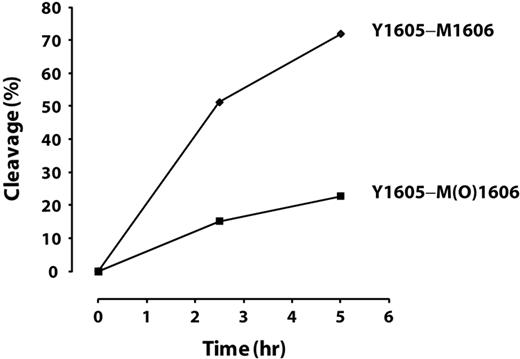
We further tested the effect of oxidation of Met1606 in intact VWF on ADAMTS13 cleavage. Purified plasma VWF was incubated with or without HOCl in the presence of 1M urea at 37°C for 30 minutes. The oxidation reaction was terminated by adding excess l-methionine. An aliquot of proteins was used to analyze oxidative modification at Met1606 by LC-MS/MS, and the remainder was subjected to ADAMTS13 cleavage. Aliquots of the cleavage reaction were withdrawn at 2.5 and 5 hours, the reactions were terminated, and the percentage of products at each time point was quantified by LC-MS/MS. Approximately 85% of Met1606 was oxidized to methionine sulfoxide. The cleavage of the Tyr1605-Met(O)1606 peptide bond by ADAMTS13 was markedly inhibited compared with the cleavage of Tyr1605-Met1606, with only 16% being cleaved versus 60% after 2.5 hours and 24% versus 80% after 5 hours (Figure 6). Comparing the initial rates of cleavage (slopes between 0 and 2.5 hours), the cleavage of the Tyr1605-Met(O)1606 peptide bond was less than 30% of that of the Tyr1605-Met1606 bond. This was consistent with our finding using the A2 peptide.
Time-dependent cleavage of oxidized or nonoxidized multimeric plasma VWF by ADAMTS13. The cleavage of the Tyr1605-Met(O)1606 peptide bond in multimeric VWF by ADAMTS13 was markedly inhibited compared with the cleavage of Tyr1605-Met1606.
Time-dependent cleavage of oxidized or nonoxidized multimeric plasma VWF by ADAMTS13. The cleavage of the Tyr1605-Met(O)1606 peptide bond in multimeric VWF by ADAMTS13 was markedly inhibited compared with the cleavage of Tyr1605-Met1606.
Discussion
We show in this study that neutrophil-derived oxidants are capable of readily oxidizing the methionine residue at the cleavage site for the VWF-processing enzyme ADAMTS13. This modification markedly reduces the ability of the enzyme to cleave the site, a process that would promote accumulation of the large and highly adhesive multimeric forms of VWF in vivo. Accumulation of highly adhesive VWF multimers during acute or chronic inflammation would also be facilitated by the reduced ADAMTS13 activity observed in inflammatory states. Inflammatory cytokines have been shown to interfere directly with ADAMTS13 cleavage of VWF,31 and to reduce synthesis of ADAMTS13 in hepatic stellate cells and endothelial cells.32
Of the 2 residues at the ADAMTS13 cleavage site in VWF, the tyrosyl-methionyl peptide bond at residues 1605 and 1606 (at the P1 and P′1 positions), only Met1606 was modified significantly by HOCl, although tyrosine is also susceptible to chlorination by this reagent. The modification occurred rapidly and in a concentration-dependent fashion, and to a much greater extent in a peptide substrate than in purified multimeric VWF from plasma. This fact suggests that exposure of the cleavage site enhances the ability of hypochlorite to react with the target site methionine. It is therefore likely that the VWF newly released from endothelial cells, ULVWF, would be more prone to cleavage-site oxidation, as this form of VWF is cleaved by ADAMTS13 much more rapidly than is plasma VWF,33 an indication that in ULVWF the cleavage site is exposed in some of the A2 domains. The binding site for platelet GPIbα also appears to be exposed in ULVWF, at least in a subset of A1 domains.17
In vivo, the impact of indiscriminate protein oxidation during inflammatory stress is most likely attenuated by the presence of many proteins in plasma, some of which, like albumin, are present at very high concentrations. Nevertheless, there are several factors that would render VWF (especially ULVWF) particularly sensitive to oxidation. The first and most important factor is location: the bulk of VWF is synthesized and released into the blood at the vessel wall, the site at which inflammatory cells, particularly neutrophils, initiate their egress from the bloodstream into the tissues. As neutrophils roll along the endothelium, engaging adhesion molecules such as P-selectin and VCAM-1 and immobilized chemokines,34 signals are transmitted into both the endothelial cells and the neutrophils. In neutrophils, these signals activate the respiratory burst and production of superoxide, and release of myeloperoxidase. The signals received by the endothelium stimulate additional Weibel-Palade body secretion and activation of the endothelium's own reduced nicotinamide adenine dinucleotide phosphate oxidase system,35 which generates more ROS. ROS can further stimulate VWF release,36 resulting in a vicious cycle of VWF release and oxidation.
Also favoring VWF oxidation is the fact that neutrophils not only use endothelial transmembrane proteins to adhere to the vessel wall, but they can also bind directly to surface-attached VWF via P-selectin glycoprotein ligand-1 and integrin αMβ2.37
Another factor that would favor VWF oxidation is the tendency of enzymes involved in ROS production to concentrate on the endothelial surface. Both MPO38 and xanthine oxidase39 are known to bind endothelial glycosaminoglycans, positioning them in close proximity to the site of VWF release. In fact, MPO has been shown to spread rapidly over the endothelial surface when neutrophils bind the endothelium.38 In the case of xanthine oxidase, the superoxide generated at the vessel surface acts as a nitric oxide (NO) scavenger, reacting with the gas to produce peroxynitrite, itself a potent oxidant.40 Removal of NO has another important consequence: increasing the reactivity of endothelial cells so they secrete VWF more readily, and making platelets more reactive. Also increasing oxidant stress and diminishing NO availability during inflammatory stress is the reaction of ROS at the vessel wall with tetrahydrobiopterin, a component of endothelial NO synthase.41,42 Tetrahydrobiopterin oxidation uncouples electron flow from l-arginine oxidation and NO production, and links it to superoxide production. Superoxide produced by any means yields hydrogen peroxide through the action of superoxide dismutase or by spontaneous dismutation.
Oxidation of the methionine at the ADAMTS13 cleavage site of VWF would be expected to produce a prothrombotic state, allowing the largest and most reactive multimers of VWF to persist in the circulation. It is of interest that another important regulator of thrombotic potential, thrombomodulin, can also be modified by methionine oxidation, abrogating its ability to act as a cofactor in the thrombin-catalyzed activation of protein C,23 also producing prothrombotic effects.
Because one of the major roles for ROS production by leukocytes is to aid in the killing of invading microorganisms, it is possible that induction of a prothrombotic state by these oxidants is one of the intended consequences of their production, serving as another weapon in the innate immunity arsenal. Indeed, the evolutionarily ancient clotting systems of the hemolymph of invertebrates, including insects and horseshoe crabs, appear to have been selected as means of battling invasive organisms.43-45 Recent studies in mice have also demonstrated that thrombosis can have beneficial effects in bacterial infection.46,47 What we have uncovered in the current study may be yet another example linking inflammation with thrombosis, 2 processes that appear intricately interwoven.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Drs Earl W. Davie, Jay W. Heinecke, and Adam D. Munday for intellectual input and helpful discussions. We also thank Jennie Le for assistance with the experiments.
This work was supported by National Institutes of Health grants P50HL65967, RO1HL091153, and R01HL075381, and a Bayer hemophilia award.
National Institutes of Health
Authorship
Contribution: J.C. designed and performed experiments, analyzed data, and cowrote the manuscript; X.F. designed and performed experiments, analyzed data, and edited the manuscript; Y.W. performed experiments and analyzed data; M.L., B.M., and J.K. provided vital reagents for experiments and contributed important intellectual insights into reagent purification and preparation and data interpretation; D.W.C. designed experiments, interpreted data, and edited the manuscript; and J.A.L. directed the project, designed experiments, interpreted experiments, and cowrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: José A. López, Puget Sound Blood Center, 921 Terry Ave, Seattle, WA 98104; e-mail: josel@psbcresearch.org.
References
Author notes
J.C. and X.F. contributed equally to this work.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal