Abstract
Interleukin-21 (IL-21), a member of the IL-2 cytokine family, has diverse regulatory effects on natural killer (NK), T, and B cells. In contrast to other cytokines that are usually immunostimulatory, IL-21 can induce apoptosis of murine B cells at specific activation-differentiation stages. This effect may be used for treatment of B-cell malignancies. Herein we report that diffuse large B-cell lymphoma (DLBCL) cell lines exhibit widespread expression of the IL-21 receptor (IL-21R) and that IL-21 stimulation leads to cell-cycle arrest and caspase-dependent apoptosis. IL-21 also induces apoptosis in de novo DLBCL primary tumors but does not affect viability of human healthy B cells. Furthermore, IL-21 promotes tumor regression and prolongs survival of mice harboring xenograft DLBCL tumors. The antilymphoma effects of this cytokine are dependent on a mechanism involving IL-21–activated signal transducer and activator of transcription 3 (STAT3) up-regulating expression of c-Myc. This up-regulation promotes a decrease in expression of antiapoptotic Bcl-2 and Bcl-XL proteins triggering cell death. Our results represent one of the first examples in which the STAT3–c-Myc signaling pathway, which can promote survival and oncogenesis, can induce apoptosis in neoplastic cells. Moreover, based on IL-21's potency in vitro and in animal models, our findings indicate that this cytokine should be examined in clinical studies of DLBCL.
Introduction
Interleukin-21 (IL-21) is a member of type I cytokine family that uses the shared γ-common receptor chain for signaling.1,2 It is predominantly secreted by activated CD4+ T and natural killer (NK) T cells and induces pleiotropic effects on the immune system by regulating functions of T, B, NK, and myeloid cells.1,3,4 The IL-21 receptor (IL-21R) has been reported to be present on almost all mature lymphocytes, with the highest expression on activated B cells.5,6
The nature of IL-21's effects on B cells depends on the organism, specific cellular context (eg, activation and developmental stages), and presence of costimulatory factors.7,8 IL-21 increases growth and differentiation of murine B lymphocytes that received both B-cell receptor (BCR) and T-cell help mediating signals while inducing apoptosis in cells lacking the concomitant BCR activation.5 Although there have been several reports of IL-21 inducing apoptosis in murine B cells, the effects of IL-21 on human nonneoplastic B cells have been confined to regulation of B-cell activation and differentiation. Specifically, IL-21 has been shown to costimulate human B-cell proliferation induced by anti-CD40 antibody, yet inhibit proliferation induced by IL-4 and BCR stimulation.1,6 IL-21 was also reported to have a central role in the differentiation of human primary B cells into plasma cells9 and in promoting class-switch recombination and secretion of immunoglobulin G (IgG) and IgA in postswitch IgM+ memory B cells.10
Since IL-21 was shown to stimulate the immune system, its effects on some tumors have been explored. IL-21 was reported to have potent antitumor activity in a variety of solid tumor models in mice.11 Because solid tumors do not express IL-21R, these effects are likely to be indirect and mediated by IL-21–induced terminal differentiation of NK cells, regulation of T-cell differentiation and proliferation, and induction of cytotoxic T-cell responses.12
In contrast to indirect immune-mediating effects of IL-21 on solid tumors, IL-21 may have direct effects on IL-21R–expressing malignancies originating from B lymphocytes. It was reported that IL-21 enhanced growth of multiple myeloma (MM) cells13 yet induced apoptosis in chronic lymphocytic leukemia (CLL) B cells.14,15
Diffuse large B-cell lymphoma (DLBCL), the most common subtype of non-Hodgkin lymphoma, is characterized by heterogeneity in clinical course and response to therapy. With the recent introduction of the anti-CD20 antibody rituximab into clinical practice, the “gold standard” therapy of DLBCL has evolved to include rituximab with cyclophosphamide, doxorubicin, vincristine, and prednisone. This has resulted in significant improvement in patient outcome, with 5-year survival reaching 50% to 60%. However, a significant proportion of patients still succumb to DLBCL and an urgent need for new therapies exists.
Because IL-21 may be proapoptotic for certain B cells, we have examined the direct effects of IL-21 on DLBCL cell lines and primary tumors. We show that IL-21R is expressed on DLBCL cells and that IL-21 stimulation activates signal transducer and activator of transcription (STAT) proteins STAT1, STAT3, and STAT5. STAT activation is followed by proliferation arrest and caspase-dependent apoptosis in a majority of DLBCL cell lines and primary tumors. Furthermore, we show that IL-21 induces tumor regression and prolongs survival of mice bearing xenograft DLBCL tumors. Finally, we propose a novel IL-21 proapoptotic signaling pathway that is dependent on STAT3-induced up-regulation of c-Myc expression. To our knowledge, this is the first evidence that the STAT-3–c-Myc pathway, which has been implicated in B-cell tumorigenesis, can be used to mediate tumor cell death by a therapeutic agent.
Methods
Reagents
Recombinant IL-21 and biotinylated anti–IL-21R antibody were kindly provided by Zymogenetics. Biotinylated isotype control and streptavidin–fluorescein isothiocyanate (FITC) used for receptor staining were purchased from BD Biosciences. Anti-Bax, Bcl-2 (50E3), c-Myc, pSTAT1 (Tyr701), STAT1, pSTAT3 (Tyr705), STAT3, pSTAT5 (Tyr694), and STAT5 antibodies were from Cell Signaling Technology; anti–glyceraldehyde-3-phosphate dehydrogenase (GAPDH) antibody was from Ambion; anti–Bcl-XL (YTH-2H12) antibody was from Trevigen; and anti-Bim and anti–rat horseradish peroxidase antibodies were from Calbiochem. Anti–Bcl-6 (N-3), Mcl-1 (22), and Bid (FL-195) antibodies were purchased from Santa Cruz Biotechnology. The pan-caspase inhibitor Z-VAD-FMK, caspase-8 inhibitor Z-IETD-FMK, and caspase-9 inhibitor Z-LEHD-FMK were purchased from R&D Systems and doxorubicin was from Sigma-Aldrich.
Cell lines and primary tumor cells
The following cell lines were selected for this study: OCI-LY-3, OCI-LY-10, OCI-LY-19, RC-K8, SU-DHL-4, SU-DHL-6, VAL, MC116, HBL-1 (DLBCL); RPMI-8226, U-266 (multiple myeloma); UPN-1 (mantle cell lymphoma [MCL]); HeLa, and 293T. MC-116, RC-K8, RPMI-8226, SU-DHL-4, SU-DHL-6, U-266, UPN-1, HBL-1, and VAL cell lines were grown in RPMI 1640 medium (Mediatech) supplemented with 10% fetal bovine serum (FBS; Mediatech), 2nM glutamine (Gibco BRL), and penicillin/streptomycin (Gibco BRL). OCILY-3, OCILY-10, and OCILY-19 cell lines were grown in Iscove Modified Dulbecco Medium (Mediatech) supplemented with 20% human plasma, 2nM glutamine, penicillin/streptomycin, and 50μM 2-β mercaptoethanol (Gibco BRL). HeLa and 293T cells were grown in Dulbecco modified Eagle medium (Mediatech) supplemented with 10% FBS, 2nM glutamine, and penicillin/streptomycin. The RC-K8–resistant (RC-K8R) cell line was generated by treating IL-21–sensitive RC-K8 cells with IL-21 at escalating concentrations from 10 ng/mL to 100 ng/mL weekly for a period of 10 weeks.
Fresh primary tumors, obtained from routine biopsies after patients signed an informed consent approved by the Institutional Review Board in accordance with the Declaration of Helsinki, were used for preparation of viable single-cell suspensions. The lymph nodes were cut sterilely and forced through a metal sieve. Mononuclear cells were obtained after centrifugation of the cell suspension over Ficoll/Hypaque gradient. B-cell purification was performed by negative selection using a cocktail of biotinylated CD2, CD14, CD16, CD36, CD43, and CD235a (glycophorin A) antibodies (Miltenyi Biotec). Magnetically labeled cells were separated using an autoMACS magnetic sorter (Miltenyi Biotec). Cell viability was assessed with YO-PRO (Invitrogen) and propidium iodide (PI; Invitrogen) staining. Purity was assessed by anti-CD19 (BD Biosciences) staining and analysis on a Becton Dickinson LSR analyzer (BD Biosciences). Samples with at least 80% viability and 95% B-cell purity were cultured in RPMI 1640 medium (Fisher Scientific) supplemented with 10% FBS, 2nM glutamine, and penicillin/streptomycin and used for subsequent experiments.
Cell-surface receptor staining
For cell-surface receptor staining, 5 × 106 cells were washed with 1× phosphate-buffered saline (PBS) and resuspended in blocking buffer (Hanks balanced salt solution [Mediatech], 2% FBS, 2% normal goat serum [Rockland Immunochemicals], 3% human AB sera) for 10 minutes. Cells were pelleted and resuspended in cold staining buffer (Hanks balanced salt solution, 2% FBS). Biotinylated anti–IL-21R antibody or isotype control was added for 30 minutes followed by 3 washes and resuspension in cold staining buffer with streptavidin-FITC (BD Biosciences) for 30 minutes. After 3 additional washes, the cells were resuspended in 2% paraformaldehyde (Sigma-Aldrich) for 10 minutes, washed, and resuspended in cold staining buffer. Cells were analyzed on a BD LSR Analyzer (BD Biosciences).
Proliferation and apoptosis studies
For proliferation studies, 105 cells/mL were incubated with or without IL-21 (100 ng/mL) for specified time periods. Concentration-corrected aliquots were transferred into a 96-well plate and incubated with 3H-thymidine at a final concentration of 2 μCi/mL (0.074 MBq; PerkinElmer) for 4 hours. Cells were transferred onto fiberglass filters and radioactivity was measured by a TopCount-NXT scintillation counter (PerkinElmer).
For apoptosis studies, 105 cells/mL were incubated with or without IL-21 (10 or 100 ng/mL) for specified time periods, collected, washed with 1× PBS, and stained with PI and YO-PRO as per the manufacturer's instructions. Analysis was performed on a Becton Dickinson LSR analyzer (BD Biosciences).
To assay caspase activation, cells were treated with IL-21 (100 ng/mL) for 48 hours. Caspase-Glo 3 reagent (Promega) was added for 30 minutes and luminescence was measured on a Luminoskan Ascent (Thermo Labsystems) luminometer. Readings were normalized to total amount of protein.
Whole-cell extract preparation and Western blot analysis
Whole-cell extracts for Western blot analysis were prepared by lysing 5 × 106 cells as reported previously.16 Protein concentrations in cell lysates were determined using Coomassie Protein Assay Reagent (Pierce) and a Genesys 10UV Spectrophotometer (Thermo Labsystems). Whole-cell lysates (20 μg per experimental condition) were separated by electrophoresis on 10% sodium dodecyl sulfate (SDS)–polyacrylamide gel, transferred to nitrocellulose membrane (Bio-Rad), and immunoblotted with specified antibodies. Supersignal West Pico chemiluminescent substrate (Thermo Scientific) was added to visualize protein levels with light-sensitive film (Phenix Research). Film was then scanned and data were subjected to densitometric analysis using Scion Image Software (National Institutes of Health [NIH]). Protein levels were normalized to the corresponding loading controls and reported as ratios.
Transfection of cell lines
RC-K8 and MC-116 cells were transfected by Amaxa electroporation using solution L, program H-024 and solution C, program D-024, respectively, as specified by the manufacturer (Amaxa). Nontargeting control small interefering RNA (siRNA) as well as pools of siRNAs targeting c-Myc, STAT1, STAT3, Bax, and Bim were purchased from Dharmacon. Control shRNA and small hairpin RNA (shRNA) targeting c-Myc were purchased from Origene. PCDNA3.1 empty vector, PCDNA3.1–BCL-XL, and PCDNA3.1–MCL-1 were kindly provided by Dr Lawrence Boise (University of Miami). PCDNA3.1–BCL-2 (Addgene plasmid 8768) was purchased from Addgene.17
DLBCL xenograft tumor studies
Six- to 8-week-old female nonobese diabetic/severe combined immunodeficient mice (The Jackson Laboratory) were inoculated subcutaneously in the flank with 5 × 106 RC-K8 or MC116 cells. Mice were monitored daily for tumor growth. When the tumor area reached 25 mm2, the mice were treated intratumorally once daily with IL-21 (10 μg) or PBS for 7 consecutive days. Tumors were assessed using the 2 largest perpendicular axes measured with standard calipers. Tumor-bearing mice were assessed for weight loss and tumor size at least twice weekly. Animals were killed when tumor area exceeded 100 mm2 or after loss of more than 10% body weight in accordance with institutional guidelines. All procedures with animals were conducted in conformity with an approved institutional animal protocol.
Microarray hybridization and analysis
OCI-LY-3, RC-K8, MC-116, OCI-LY-10, and IL-21–resistant RC-K8 cell lines were treated with IL-21 for 6 hours and harvested. RNA was isolated by TRIzol reagent (Invitrogen) according to the manufacturer's instructions. Total RNA yield was determined spectrophotometrically.
RNA quality was assessed on the Agilent 2100 Bioanalyzer. Total sample RNA or Human Reference Total RNA (10-20 μg; Stratagene) was used for performance of gene expression studies on Agilent Oligo microarrays, as reported previously.18
The microarrays were scanned at 10-μm resolution using a GenePix 4000A scanner (Molecular Devices) and the resulting images were analyzed with the software package GenePix Pro 5.1 (Molecular Devices). Data extracted from the images were transferred to the software package Acuity 4.0 (Molecular Devices) for normalization and statistical analysis. Each array was normalized for signal intensities across the whole array and locally, using Lowess normalization. Features for further analysis were selected according to the following quality criteria: (1) at least 90% of the pixels in the spot had intensity higher than background plus 2 SDs; (2) there were less than 2% saturated pixels in the spot; (3) signal-to-noise ratio (defined as ratio of the background subtracted mean pixel intensity to SD of background) was 3 or above for each channel; (4) the spot diameter was between 110 and 150 μm; and (5) the regression coefficient of ratios of pixel intensity was 0.6 or above. cDNA array results were filtered for absolute fold change of greater than 2 and genes that were modulated similarly by IL-21 across similarly responding cell lines were identified. All microarray data can be found at the GEO public database under accession number GSE18967.19
Statistical analysis
To test the differences in IL-21 responses we used the 2-tailed Student t test. Mice survival curves were estimated using the product-limit method of Kaplan-Meier and were compared using the log-rank test. P values less than .05 were considered statistically significant.
Results
IL-21 blocks proliferation and induces apoptosis of DLBCL cell lines
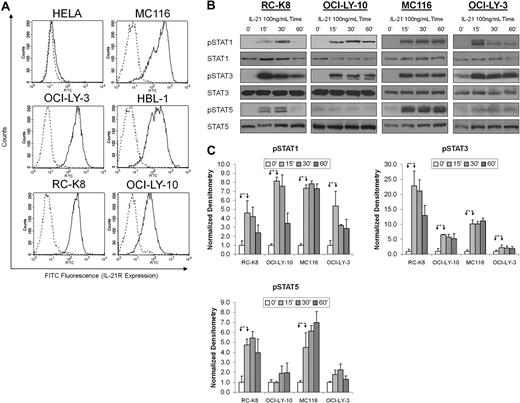
Analysis of cell-surface IL-21R expression in a panel of cell lines demonstrated that all tested DLBCL cell lines displayed the receptor, with the most prominent expression on RC-K8, OCI-LY-3, HBL-1, OCI-LY-10, and MC116 (Figure 1A). Neither HeLa nor 293T cells expressed IL-21R (Figure 1A and supplemental Figure 1, available on the Blood website; see the Supplemental Materials link at the top of the online article), whereas MM cell lines expressed low levels (not shown).
IL-21R is expressed on DLBCL cell lines and mediates IL-21–induced activation of STAT1, STAT3, and STAT5. (A) DLBCL cell lines were stained for IL-21R cell-surface expression as described in “Cell-surface receptor staining.” Solid histograms represent staining with biotinylated anti–IL-21R antibody and dashed histograms represent isotype control. (B) RC-K8, OCI-LY-10, MC116, and OCI-LY-3 DLBCL cells were treated with IL-21 (100 ng/mL) for the indicated time periods and STAT activation was measured by immunoblotting with phosphorylation-specific antibodies. Immunoblotting for nonphosphorylated STATs served as loading controls. Results in panels A and B are representative of 3 independent experiments. (C) Densitometric analysis of STAT activation from 3 independent experiments. The values in specimens at time point 0 were arbitrarily defined as 1. Error bars represent SE. *A statistically significant difference (P < .05) between experimental conditions, marked by arrowheads.
IL-21R is expressed on DLBCL cell lines and mediates IL-21–induced activation of STAT1, STAT3, and STAT5. (A) DLBCL cell lines were stained for IL-21R cell-surface expression as described in “Cell-surface receptor staining.” Solid histograms represent staining with biotinylated anti–IL-21R antibody and dashed histograms represent isotype control. (B) RC-K8, OCI-LY-10, MC116, and OCI-LY-3 DLBCL cells were treated with IL-21 (100 ng/mL) for the indicated time periods and STAT activation was measured by immunoblotting with phosphorylation-specific antibodies. Immunoblotting for nonphosphorylated STATs served as loading controls. Results in panels A and B are representative of 3 independent experiments. (C) Densitometric analysis of STAT activation from 3 independent experiments. The values in specimens at time point 0 were arbitrarily defined as 1. Error bars represent SE. *A statistically significant difference (P < .05) between experimental conditions, marked by arrowheads.
Next, we investigated the functionality of the IL-21R in the DLBCL cell lines by stimulating cells with IL-21. It has been reported that IL-21 can activate STAT1, STAT3, and to a lesser degree STAT5 in B cells.14,20 STAT3 phosphorylation was rapidly induced in all investigated DLBCL cell lines (Figure 1B-C). OCI-LY-3 cells, which exhibit constitutive activation of STAT3, also showed increased STAT3 phosphorylation after IL-21 stimulation, yet the level of induction was lower than in other analyzed cell lines. IL-21 also induced phosphorylation of STAT1 and STAT5.
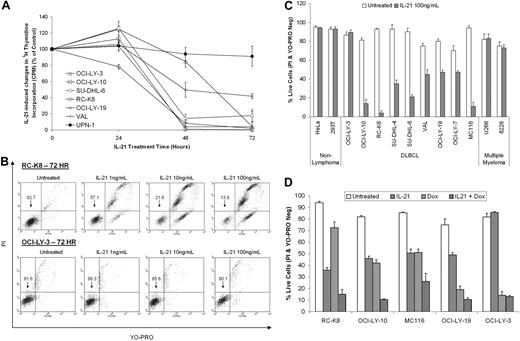
Having shown functionality of the IL-21R in DLBCL cell lines, we investigated the effects of IL-21 on DLBCL cell proliferation and survival. IL-21 markedly inhibited cellular proliferation at 48 hours and induced almost complete proliferation arrest at 72 hours in DLBCL cell lines (Figure 2A), whereas the MCL cell line UPN-1, which does not express IL-21R (supplemental Figure 1), was not affected. YO-PRO and PI staining for cell viability revealed that IL-21 treatment induced apoptosis and decreased cell viability in all but one (OCI-LY-3) of the examined DLBCL cell lines in a time-dependent (not shown) and dose-dependent (Figure 2B-C) manner. The largest decreases in cell survival upon treatment with IL-21 were observed in the RC-K8, MC116, and OCI-LY-10 cell lines. IL-21 did not induce apoptosis in MM, HeLa, or 293T cell lines.
IL-21 has potent antiproliferative and proapoptotic effects on DLBCL cell lines. (A) Proliferative responses of DLBCL cell lines and an MCL cell line (UPN-1) after treatment with IL-21. After treatment for specified time period with IL-21, cells were pulsed for another 4 hours with 3H-thymidine and were harvested for scintillation counting. Results are shown as the means of 3H-thymidine incorporation (± SD) and are representative of 3 independent experiments. Counts of treated cells are given relative to counts of untreated cells, which were set arbitrarily at 100% for each cell line. (B-C) Cells were stimulated with IL-21 at indicated doses for 72 hours and cell viability was assayed by YO-PRO/PI staining. Cells were considered “live” if negative for both YO-PRO and PI staining. Data in panel B demonstrate dose response in the RC-K8 and OCI-LY-3 cells that is representative of 3 independent experiments. Data in panel C represent means ± SE from 3 independent experiments. (D) Cells were treated with IL-21 (100 ng/mL) and/or doxorubicin (100 ng/mL) for 48 hours and cell viability was assayed by YO-PRO/PI staining. Data in panel D represent means ± SE from 3 independent experiments.
IL-21 has potent antiproliferative and proapoptotic effects on DLBCL cell lines. (A) Proliferative responses of DLBCL cell lines and an MCL cell line (UPN-1) after treatment with IL-21. After treatment for specified time period with IL-21, cells were pulsed for another 4 hours with 3H-thymidine and were harvested for scintillation counting. Results are shown as the means of 3H-thymidine incorporation (± SD) and are representative of 3 independent experiments. Counts of treated cells are given relative to counts of untreated cells, which were set arbitrarily at 100% for each cell line. (B-C) Cells were stimulated with IL-21 at indicated doses for 72 hours and cell viability was assayed by YO-PRO/PI staining. Cells were considered “live” if negative for both YO-PRO and PI staining. Data in panel B demonstrate dose response in the RC-K8 and OCI-LY-3 cells that is representative of 3 independent experiments. Data in panel C represent means ± SE from 3 independent experiments. (D) Cells were treated with IL-21 (100 ng/mL) and/or doxorubicin (100 ng/mL) for 48 hours and cell viability was assayed by YO-PRO/PI staining. Data in panel D represent means ± SE from 3 independent experiments.
We next examined the effects of IL-21 on cell viability when used concurrently with doxorubicin, a major component of the standard clinical therapy for DLBCL. Interestingly, the levels of apoptosis induced by IL-21 were comparable with or higher than those induced by doxorubicin in the RC-K8, OCI-LY-10, and MC116 cell lines (Figure 2D). In addition, IL-21 treatment augmented the cytotoxic effects of doxorubicin in all cell lines except OCI-LY-3, which is resistant to IL-21.
IL-21 induces apoptosis of primary B-cell lymphomas
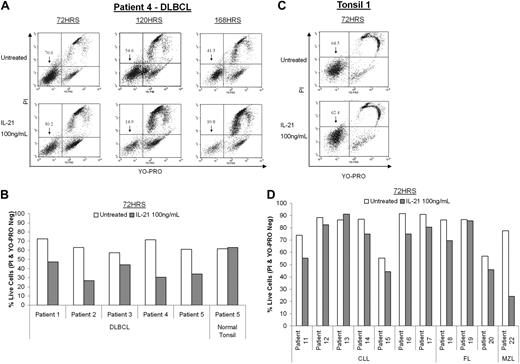
To demonstrate that the IL-21–induced cytotoxicity was not restricted to established DLBCL cell lines, we evaluated IL-21 effects on 5 fresh de novo untreated primary DLBCL tumors. All analyzed primary tumors expressed IL-21R (supplemental Figure 1). Notably, IL-21R was expressed at higher levels on the tumor cells compared with normal B cells obtained from nonmalignant tonsil or lymph node in the same patients (supplemental Figure 1). IL-21 treatment induced marked apoptosis and cell death in all examined primary DLBCL tumors (Figure 3A-B). The magnitude of the IL-21–induced DLBCL apoptosis increased over time, starting at 48 hours after treatment and reaching maximum cell death at 120 to 168 hours (Figure 3A). Of note, 1 tumor was obtained from a bilateral tonsillectomy and was localized only to 1 of the 2 tonsils. Although IL-21 treatment induced apoptosis of 44% of these tumor cells, no apoptosis was observed in normal B cells derived from the uninvolved tonsil from the same patient (Figure 3B, patient 5). In addition, IL-21 did not induce cell death of normal B cells derived from peripheral blood or nonmalignant tonsils and lymph nodes from other patients (Figure 3C).
IL-21 induces apoptosis in DLBCL primary tumors. Neoplastic or healthy B cells isolated from primary de novo tumors or normal tonsils were treated with IL-21 (100 ng/mL) for the specified time period and viability was assayed using YO-PRO/PI staining. (A) Flow cytometric profiles of B cells isolated from one representative DLBCL primary tumor originating from patient 4 and stained with YO-PRO/PI. (B) Compilation of viability data for B cells isolated from 5 DLBCL primary tumors and 1 healthy tonsil tested by flow cytometry after 72 hours of IL-21 treatment. (C) Flow cytometric profiles of healthy B cells isolated from a representative normal tonsil (tonsil 1) and stained with YO-PRO/PI. (D) Compilation of viability data for B cells isolated from 7 chronic lymphocytic leukemia (CLL), 3 follicular lymphoma (FL), and 1 marginal zone lymphoma (MZL) patient samples tested by flow cytometry after 72 hours of IL-21 treatment.
IL-21 induces apoptosis in DLBCL primary tumors. Neoplastic or healthy B cells isolated from primary de novo tumors or normal tonsils were treated with IL-21 (100 ng/mL) for the specified time period and viability was assayed using YO-PRO/PI staining. (A) Flow cytometric profiles of B cells isolated from one representative DLBCL primary tumor originating from patient 4 and stained with YO-PRO/PI. (B) Compilation of viability data for B cells isolated from 5 DLBCL primary tumors and 1 healthy tonsil tested by flow cytometry after 72 hours of IL-21 treatment. (C) Flow cytometric profiles of healthy B cells isolated from a representative normal tonsil (tonsil 1) and stained with YO-PRO/PI. (D) Compilation of viability data for B cells isolated from 7 chronic lymphocytic leukemia (CLL), 3 follicular lymphoma (FL), and 1 marginal zone lymphoma (MZL) patient samples tested by flow cytometry after 72 hours of IL-21 treatment.
We also examined whether IL-21 can induce apoptosis of primary neoplastic B cells derived from other lymphoproliferative disorders. Consistent with previous reports, IL-21 induced mostly mild decreases in cell viability in 5 of 7 CLL cases (Figure 3D). In addition, IL-21 induced apoptosis and cell death in 2 of the 3 primary follicular lymphomas and in one marginal zone lymphoma.
IL-21 induces regression of in vivo DLBCL xenograft tumors
The proapoptotic activity of IL-21 against DLBCL cell lines and primary tumors suggests that the cytokine could be useful for treatment of patients. To evaluate IL-21's effects in vivo, groups of nonobese diabetic/severe combined immunodeficient mice were inoculated subcutaneously with RC-K8 or MC116 cells and, after tumor development, IL-21 (10 μg) or PBS was injected in situ daily for 7 consecutive days. IL-21 treatment resulted in complete tumor regression within 10 to 15 days in a majority of mice, whereas mice injected with PBS showed continued tumor growth (Figure 4A-B). This effect was highly reproducible in repeated experiments. Although mice treated with the cytokine did eventually succumb to relapsed tumors, the single 7-day IL-21 treatment cycle significantly prolonged survival compared with control animals (Figure 4C-D).
In situ injections of IL-21 induce tumor remission and prolong survival of mice bearing DLBCL xenograft tumors. Mice bearing subcutaneous RC-K8 or MC116 xenograft tumors were treated with 10 μL in situ injections of either PBS (3 mice per experiment) or IL-21 (1 mg/mL; 5 mice per experiment). (A-B) Tumor size (area) in IL-21–treated and PBS-treated mice bearing RC-K8 (A) or MC116 (B) tumors. Dashed lines represent tumor size in individual PBS-treated mice, whereas solid lines represent individual IL-21–treated mice. (C-D) Overall survival of mice bearing RC-K8 (C) or MC116 (D) tumors treated with PBS (dashed line; n = 3) or IL-21 (solid line; n = 5). Similar results to those shown in panels A-D were observed in an additional independent experiment performed with each cell line.
In situ injections of IL-21 induce tumor remission and prolong survival of mice bearing DLBCL xenograft tumors. Mice bearing subcutaneous RC-K8 or MC116 xenograft tumors were treated with 10 μL in situ injections of either PBS (3 mice per experiment) or IL-21 (1 mg/mL; 5 mice per experiment). (A-B) Tumor size (area) in IL-21–treated and PBS-treated mice bearing RC-K8 (A) or MC116 (B) tumors. Dashed lines represent tumor size in individual PBS-treated mice, whereas solid lines represent individual IL-21–treated mice. (C-D) Overall survival of mice bearing RC-K8 (C) or MC116 (D) tumors treated with PBS (dashed line; n = 3) or IL-21 (solid line; n = 5). Similar results to those shown in panels A-D were observed in an additional independent experiment performed with each cell line.
IL-21–induced apoptosis of DLBCL cells proceeds through the intrinsic apoptotic pathway
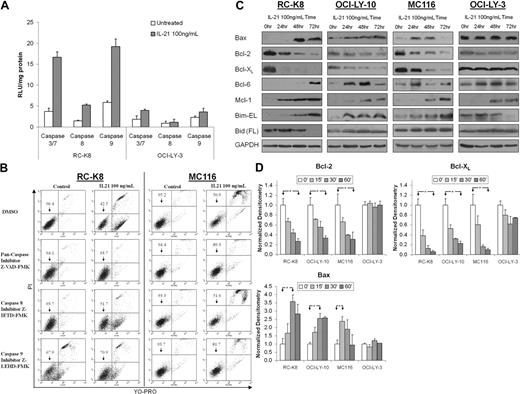
Previous studies reported that IL-21–induced apoptosis of CLL is associated with activation of caspase-8 leading to activation of caspases-3/7 and apoptosis.14 To determine which apoptotic pathways are activated by IL-21 in DLBCL, we monitored the activation of caspases-8, -9, and -3/7 during treatment. IL-21–induced apoptosis of RC-K8 DLBCL cells was associated with activation of caspases-8 and -9 as well as caspases-3/7, whereas no significant activation of caspases was observed in the IL-21–resistant cell line OCI-LY-3 (Figure 5A).
IL-21–induced apoptosis is caspase dependent and is associated with changes in expression of Bcl-2 family members. (A) RC-K8 and OCI-LY-3 cells were treated with IL-21 (100 ng/mL) for 24 hours, lysed, and assayed for caspase activation as described in “Proliferation and apoptosis studies.” Relative luciferase units (RLUs) readings were normalized to total protein content. Results are shown as the means ± SD and are representative of 3 independent experiments. (B) RC-K-8 and MC116 cells were pretreated with either dimethyl sulfoxide (DMSO) or the pan-caspase inhibitor Z-VAD-FMK, the selective caspase-9 inhibitor Z-LEHD-FMK, or caspase-8 inhibitor Z-IETD-FMK at 50 μM for 30 minutes and then treated with IL-21 (100 ng/mL). Cell viability was assayed after 48 hours by YO-PRO/PI staining. (C) Cells were treated with IL-21 (100 ng/mL) for the specified time period and protein expression was assayed by immunoblotting with specific antibodies. Immunoblotting for GAPDH served as a loading control. The results in panels B-C are representative of 3 independent experiments. (D) Densitometry analysis of Western blots from 3 independent experiments. The values in specimens at time point 0 were arbitrarily defined as 1. Error bars represent SE. *A statistically significant difference (P < .05) between experimental conditions marked by arrowheads.
IL-21–induced apoptosis is caspase dependent and is associated with changes in expression of Bcl-2 family members. (A) RC-K8 and OCI-LY-3 cells were treated with IL-21 (100 ng/mL) for 24 hours, lysed, and assayed for caspase activation as described in “Proliferation and apoptosis studies.” Relative luciferase units (RLUs) readings were normalized to total protein content. Results are shown as the means ± SD and are representative of 3 independent experiments. (B) RC-K-8 and MC116 cells were pretreated with either dimethyl sulfoxide (DMSO) or the pan-caspase inhibitor Z-VAD-FMK, the selective caspase-9 inhibitor Z-LEHD-FMK, or caspase-8 inhibitor Z-IETD-FMK at 50 μM for 30 minutes and then treated with IL-21 (100 ng/mL). Cell viability was assayed after 48 hours by YO-PRO/PI staining. (C) Cells were treated with IL-21 (100 ng/mL) for the specified time period and protein expression was assayed by immunoblotting with specific antibodies. Immunoblotting for GAPDH served as a loading control. The results in panels B-C are representative of 3 independent experiments. (D) Densitometry analysis of Western blots from 3 independent experiments. The values in specimens at time point 0 were arbitrarily defined as 1. Error bars represent SE. *A statistically significant difference (P < .05) between experimental conditions marked by arrowheads.
To further elucidate the apoptotic pathway induced by IL-21, we pretreated RC-K8 and MC116 cells with the pan-caspase inhibitor Z-VAD-FMK before IL-21 stimulation. Z-VAD-FMK almost completely abrogated IL-21–induced apoptosis in both cell lines (Figure 5B). Furthermore, the selective caspase-9 inhibitor Z-LEHD-FMK also prevented IL-21–induced apoptosis in both cell lines, although this inhibitor exhibited some inherent toxicity to RC-K8 cells, as previously reported in some lymphoma cells.21 In contrast, the selective caspase-8 inhibitor Z-IETD-FMK did not prevent IL-21–induced apoptosis. Because caspase-9 activation can lead to downstream caspase-8 activation,22 these overall observations suggest that IL-21 induced apoptosis of DLBCL cells by activating the intrinsic apoptotic pathway.
We then investigated whether IL-21–induced activation of the intrinsic apoptotic pathway involves changes in expression of the apoptosis-regulating proteins of the Bcl-2 family. Previous studies have shown that IL-21 can alter the expression of apoptosis-regulating proteins Bcl-2, Bcl-XL, Bim, and Bax in mouse lymphocytes and CLL cells.5,15,20 We observed marked differences in the effects of IL-21 on some members of the Bcl-2 family between DLBCL cell lines exhibiting either apoptosis or resistance to IL-21 treatment. The expression of Bim-EL, a proapoptotic member of the Bcl-2 family, was increased at 24, 48, and 72 hours after IL-21 treatment in sensitive cell lines (RC-K8, MC116, OCI-LY-10), whereas its expression in IL-21–resistant OCI-LY-3 cell line was decreased (Figure 5C). The expression levels of the antiapoptotic proteins Bcl-2 and Bcl-XL were reduced upon IL-21 treatment in the IL-21–sensitive cell lines (RC-K8, MC116, OCI-LY-10) compared with the minimal change observed in the IL-21–resistant OCI-LY-3 cell line (Figure 5C-D). The expression of Bax, a cell death effector, increased in the IL-21–sensitive DLBCL cell lines, but not in the IL-21–resistant OCI-LY-3 cell line. Protein levels of Mcl-1, a known target of STAT3,23 and Bcl-6, a known target of IL-21 signaling,24 were increased in all cell lines treated with IL-21. Finally, no consistent changes in the expression of full-length Bid, a protein previously implicated in proapoptotic IL-21 signaling,14 were observed in the sensitive cell lines, although Bid cleavage was observed at 72 hours after IL-21 treatment in the RC-K8 cell line.
Previous studies suggested that Bim-EL may mediate IL-21–induced apoptosis in mouse lymphocytes and CLL.5,15 Because we observed an increase in Bim-EL expression in sensitive cell lines, we tested whether this protein is necessary for IL-21–induced apoptosis. Although transfecting cells with siRNA targeting Bim prevented the induction of this protein by IL-21, it did not affect levels of apoptosis seen in treated cells (supplemental Figure 2).
IL-21–induced apoptosis of DLBCL cells requires activation of the STAT3–c-Myc signaling pathway
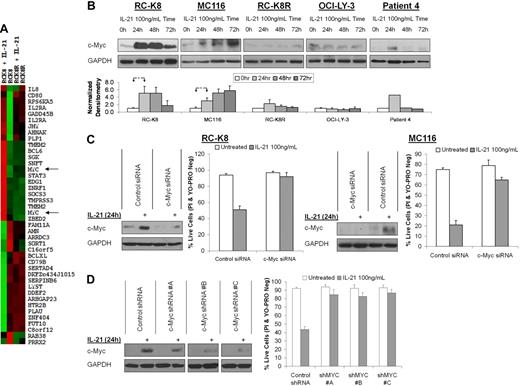
To further examine the mechanism of IL-21–induced apoptosis of DLBCL cells and to interrogate upstream signaling pathways that led to the observed decrease in the expression of Bcl-2 and Bcl-XL proteins, we performed gene expression profiling of untreated and IL-21–treated sensitive and resistant RC-K8 cells (the latter were generated by continuous exposure of cells to increasing concentrations of IL-21 and will be referred to as RC-K8R cells hereafter). mRNA levels of 375 genes and 402 genes were up-regulated and down-regulated, respectively, at least 2-fold in RC-K8 cells (supplemental Figure 3). A subset of these genes was not modulated by IL-21 treatment in RC-K8R cells. C-MYC, GADD45b, BCL-XL, IL-16, IL-2Rα, and SOCS3 genes were among those that exhibited the most prominent differences in mRNA expression upon IL-21 stimulation between RC-K8 and RC-K8R cell lines (Figure 6A). Further microarray studies showed similar changes in C-MYC mRNA expression in additional IL-21–sensitive DLBCL cell lines (not shown). Changes in c-Myc protein expression corresponded to gene expression data as IL-21 stimulation strongly up-regulated c-Myc protein levels in the RC-K8 and MC116 cells, whereas no increase was observed in the RC-K8R or OCI-LY-3 cells (Figure 6B). IL-21 also induced c-Myc protein expression in cells from a de novo DLBCL primary tumor that exhibited apoptosis upon in vitro treatment with IL-21 (Figure 6B patient 4).
Apoptosis induced by IL-21 is dependent on c-Myc. (A) Wild-type RC-K8 and IL-21–resistant RC-K8R cells were treated with IL-21 (100 ng/mL) for 6 hours and RNA was collected as specified in “Microarray hybridization and analysis.” Microarray gene expression analysis was performed. Presented is a heat map of 42 genes with the most dramatic changes between RC-K8 and RC-K8R upon IL-21 treatment. (B) RC-K8, MC116, RC-K8R, and OCI-LY-3 cell lines and cells from a primary DLBCL tumor from patient 4 were treated with IL-21 (100 ng/mL). At 24, 48, and 72 hours after treatment, cellular proteins were resolved by SDS–polyacrylamide gel electrophoresis (PAGE) and immunoblotted for c-Myc. Immunoblotting for GAPDH served as a loading control. The cell line results are representative of 3 independent experiments. Normalized densitometry of c-Myc/GAPDH ratio is shown. The values in specimens at time point 0 were arbitrarily defined as 1. *A statistically significant difference (P < .05) between experimental conditions marked by arrowheads. (C) RC-K8 and MC-116 cells were transfected with siRNA targeting c-Myc or control siRNA. Twenty-four hours after transfection, cells were treated with IL-21 (100 ng/mL) for 24 hours. Cellular proteins from untreated and IL-21–treated cells were resolved by SDS-PAGE and immunoblotted for c-Myc or GAPDH. (D) RC-K8 cells were transfected with shRNA targeting c-Myc or control shRNA. Twenty-four hours after transfection, cells were treated with IL-21 (100 ng/mL) for 24 hours. Cellular proteins from untreated and IL-21–treated cells were resolved by SDS-PAGE and immunoblotted for c-Myc or GAPDH. All immunoblots shown in panel D originated from the same membrane and identical exposure; superfluous lanes were removed. Cell viability in panels C-D was assayed by YO-PRO/PI staining after 48 hours of IL-21 treatment. The results in panels C-D are representative of 3 independent experiments.
Apoptosis induced by IL-21 is dependent on c-Myc. (A) Wild-type RC-K8 and IL-21–resistant RC-K8R cells were treated with IL-21 (100 ng/mL) for 6 hours and RNA was collected as specified in “Microarray hybridization and analysis.” Microarray gene expression analysis was performed. Presented is a heat map of 42 genes with the most dramatic changes between RC-K8 and RC-K8R upon IL-21 treatment. (B) RC-K8, MC116, RC-K8R, and OCI-LY-3 cell lines and cells from a primary DLBCL tumor from patient 4 were treated with IL-21 (100 ng/mL). At 24, 48, and 72 hours after treatment, cellular proteins were resolved by SDS–polyacrylamide gel electrophoresis (PAGE) and immunoblotted for c-Myc. Immunoblotting for GAPDH served as a loading control. The cell line results are representative of 3 independent experiments. Normalized densitometry of c-Myc/GAPDH ratio is shown. The values in specimens at time point 0 were arbitrarily defined as 1. *A statistically significant difference (P < .05) between experimental conditions marked by arrowheads. (C) RC-K8 and MC-116 cells were transfected with siRNA targeting c-Myc or control siRNA. Twenty-four hours after transfection, cells were treated with IL-21 (100 ng/mL) for 24 hours. Cellular proteins from untreated and IL-21–treated cells were resolved by SDS-PAGE and immunoblotted for c-Myc or GAPDH. (D) RC-K8 cells were transfected with shRNA targeting c-Myc or control shRNA. Twenty-four hours after transfection, cells were treated with IL-21 (100 ng/mL) for 24 hours. Cellular proteins from untreated and IL-21–treated cells were resolved by SDS-PAGE and immunoblotted for c-Myc or GAPDH. All immunoblots shown in panel D originated from the same membrane and identical exposure; superfluous lanes were removed. Cell viability in panels C-D was assayed by YO-PRO/PI staining after 48 hours of IL-21 treatment. The results in panels C-D are representative of 3 independent experiments.
Although c-Myc has been studied extensively for its oncogenic properties, it may also be proapoptotic, especially when the level of up-regulation is higher than what is typically observed after mitogenic stimulation.25-28 To determine whether apoptosis is dependent on c-Myc up-regulation, we used specific siRNAs (Figure 6C) and shRNAs (Figure 6D) to block the up-regulation of c-Myc that is evident in the IL-21–sensitive cell lines. Prevention of the c-Myc up-regulation after IL-21 treatment with either siRNA or shRNA effectively blocked IL-21–induced apoptosis of DLBCL cells, whereas nontargeting control siRNA or shRNA did not. These observations demonstrate that IL-21–induced apoptosis is dependent on up-regulation of c-Myc.
c-Myc has been shown to induce apoptosis via up-regulation of proapoptotic Bax and down-regulation of antiapoptotic Bcl-2 and Bcl-XL.26,29,30 IL-21 stimulation of sensitive DLBCL cell lines showed similar changes in these apoptosis-regulating proteins (Figure 5C-D). To demonstrate that the observed changes in the expression of these c-Myc target proteins may contribute to the apoptosis induced by IL-21, we transiently overexpressed Bcl-XL and/or Bcl-2 in RC-K8 cells before stimulation with IL-21. As expected, ectopic expression of either Bcl-XL or Bcl-2 partially prevented IL-21–induced apoptosis (Figure 7A). This effect was specific because similar levels of overexpression of the antiapoptotic protein Mcl-1, which is not a known c-Myc target, did not affect apoptosis. Furthermore, knockdown of Bax, which is known to be required for c-Myc–induced apoptosis,26,31,32 completely blocked apoptosis after IL-21 stimulation (Figure 7B).
IL-21–induced apoptosis is dependent on STAT-3 and Bax and rescued by Bcl-2 and Bcl-XL. (A) RC-K8 cells were transfected with PCDNA3.1–BCL-XL, –BCL-2, –MCL-1, or empty vector. Seventy-two hours after transfection, cells were treated with IL-21 (100 ng/mL). After 24 hours, cellular proteins from untreated and IL-21–treated cells were resolved by SDS-PAGE and immunoblotted for Bcl-XL, Bcl-2, Mcl-1, or GAPDH. Cell viability was assayed by YO-PRO/PI staining after 48 hours of IL-21 treatment. (B) RC-K8 cells were transfected with siRNA targeting Bax or control siRNA. Twenty-four hours after transfection, cells were treated with IL-21 (100 ng/mL). Cellular proteins from untreated and IL-21–treated cells were resolved by SDS-PAGE and immunoblotted for Bax and c-Myc at 24 hours after treatment. Immunoblotting for GAPDH served as a loading control. Cell viability was assayed by YO-PRO/PI staining after 48 hours of IL-21 treatment. (C-D) RC-K8 cells were transfected with siRNA targeting STAT3 or control siRNA. Seventy-two hours after transfection, cells were treated with IL-21 (100 ng/mL). Cellular proteins from untreated and IL-21–treated cells were resolved by SDS-PAGE and immunoblotted for p-STAT3 and STAT3 at 15 minutes after treatment (C) and for c-Myc, Bcl-XL, Bcl-2, and STAT3 at 24 hours after treatment (D). Cell viability was assayed by YO-PRO/PI staining after 48 hours of IL-21 treatment (C). The results in panels A through D are representative of 3 independent experiments. (E) A model of IL-21–induced apoptosis in DLBCL. IL-21 binding to IL-21Rα results in Jak activation and subsequent phosphorylation and activation of STAT3. Homodimerized STAT3 enters the nucleus and activates transcription of c-Myc. c-Myc protein promotes the transcription of Bax and suppresses Bcl-2 and Bcl-XL, thus disrupting the Bcl-2 rheostat within the cells and triggering mitochondrial outer membrane permeabilization, caspase-9 activation, and apoptosis.
IL-21–induced apoptosis is dependent on STAT-3 and Bax and rescued by Bcl-2 and Bcl-XL. (A) RC-K8 cells were transfected with PCDNA3.1–BCL-XL, –BCL-2, –MCL-1, or empty vector. Seventy-two hours after transfection, cells were treated with IL-21 (100 ng/mL). After 24 hours, cellular proteins from untreated and IL-21–treated cells were resolved by SDS-PAGE and immunoblotted for Bcl-XL, Bcl-2, Mcl-1, or GAPDH. Cell viability was assayed by YO-PRO/PI staining after 48 hours of IL-21 treatment. (B) RC-K8 cells were transfected with siRNA targeting Bax or control siRNA. Twenty-four hours after transfection, cells were treated with IL-21 (100 ng/mL). Cellular proteins from untreated and IL-21–treated cells were resolved by SDS-PAGE and immunoblotted for Bax and c-Myc at 24 hours after treatment. Immunoblotting for GAPDH served as a loading control. Cell viability was assayed by YO-PRO/PI staining after 48 hours of IL-21 treatment. (C-D) RC-K8 cells were transfected with siRNA targeting STAT3 or control siRNA. Seventy-two hours after transfection, cells were treated with IL-21 (100 ng/mL). Cellular proteins from untreated and IL-21–treated cells were resolved by SDS-PAGE and immunoblotted for p-STAT3 and STAT3 at 15 minutes after treatment (C) and for c-Myc, Bcl-XL, Bcl-2, and STAT3 at 24 hours after treatment (D). Cell viability was assayed by YO-PRO/PI staining after 48 hours of IL-21 treatment (C). The results in panels A through D are representative of 3 independent experiments. (E) A model of IL-21–induced apoptosis in DLBCL. IL-21 binding to IL-21Rα results in Jak activation and subsequent phosphorylation and activation of STAT3. Homodimerized STAT3 enters the nucleus and activates transcription of c-Myc. c-Myc protein promotes the transcription of Bax and suppresses Bcl-2 and Bcl-XL, thus disrupting the Bcl-2 rheostat within the cells and triggering mitochondrial outer membrane permeabilization, caspase-9 activation, and apoptosis.
C-MYC is a known target of the STAT3 transcription factor,33-35 which we showed to be activated by IL-21 stimulation (Figure 1B). To determine whether STAT3 was responsible for c-Myc up-regulation, we knocked down levels of STAT3 expression with specific siRNAs that prevented STAT3 activation by IL-21 and blocked apoptosis. STAT3 knockdown also prevented the up-regulation of c-Myc and down-regulation of Bcl-2 and Bcl-XL, confirming the role of STAT3 in mediating IL-21–induced apoptosis upstream of these proteins (Figure 7C-D). Knockdown of STAT1 expression by specific siRNAs neither prevented c-Myc up-regulation nor blocked IL-21–induced apoptosis of the DLBCL cells (not shown). Taken together, these findings demonstrate that activation of STAT3 by IL-21 causes a prohibitively high induction in c-Myc expression leading to the down-regulation of Bcl-2 as well as Bcl-XL and up-regulation of Bax. These changes shift the balance of the apoptosis-regulating proteins within the DLBCL cells and trigger apoptosis.
Discussion
IL-21 is a recently discovered member of the IL-2 family of cytokines that is capable of promoting not only immunostimulatory and prosurvival effects but also death of certain B lymphocytes. The effects of IL-21 on B-cell fate depend on their activation status and developmental stage. In addition, the effects of IL-21 differ among lymphocytes derived from humans and various strains of mice. In the present study, we investigated the effects of IL-21 in human DLBCL and showed that it potently induced apoptosis of both germinal center–like and activated B cell–like DLBCL cell lines and primary tumors and prolonged survival of mice harboring human DLBCL xenograft tumors. We demonstrated that IL-21–induced apoptosis was mediated by activation of the STAT3–c-Myc signaling pathway that down-regulated expression of the antiapo-ptotic Bcl-2 and Bcl-XL proteins and up-regulated Bax, resulting in subsequent activation of the intrinsic apoptotic pathway (Figure 7E). This is a novel mechanism of IL-21–induced apoptosis and is one of the few examples in which the STAT3–c-Myc pathway promotes cell death.
Previous studies showed that IL-21 treatment can induce a low level of apoptosis in CLLs that express relatively low levels of surface IL-21R. This cell death was augmented by up-regulating IL-21R levels via anti-CD40 before stimulation. IL-21–induced apoptosis of CLL B cells was associated with activation of caspases-8 and -3, up-regulation of Bim, cleavage of p27, Bid, and PARP, and secretion of granzyme B in the presence of CPG-DNA.15,36 Herein, we confirmed that IL-21 can induce a low level of apoptosis in CLL. However, in contrast to the relatively mild apoptosis observed in CLL tumors that were not manipulated to increase IL-21R expression, we demonstrated that IL-21 induced pronounced apoptosis of DLBCL cell lines and de novo primary tumors that inherently express high levels of IL-21R. Our studies of xenograft DLBCL tumors in mice further demonstrated the potent in vivo activity of IL-21.
Intriguingly, our studies provide evidence that the observed mechanism of IL-21–induced apoptosis in DLBCL cells is different from the mechanism reported in CLL cells, which involved the up-regulation of Bim and granzyme B.15,36 IL-21 did not induce expression of granzyme B in DLBCL alone or when used in combination with CPG or lipopolysaccharide (not shown) and knockdown of Bim did not prevent IL-21–induced apoptosis of DLBCL cells. Herein we demonstrated a novel mechanism of IL-21–induced apoptosis of DLBCL that is based on activation of the STAT3–c-Myc pathway. The differences in apoptotic pathways activated by IL-21 in different B-cell neoplasms may be due to the pleiotropic effects of IL-21 seen in nonneoplastic B cells of different activation statuses and developmental stages and require further investigation.
The STAT family of transcription factors regulates diverse cellular events such as proliferation, differentiation, and cell survival.37 Previous research has cast STAT1 and STAT3 in opposing roles with the former promoting cell death and the latter most commonly promoting cell survival and proliferation.22,38,39 However, several reports have shown that STAT3 can induce cell death. For instance, STAT3 can be proapoptotic when triggering terminal differentiation and apoptosis in myeloid leukemia M1 cells40 or during involution of mammary glands via induction of c-Myc.41 In the present study, we demonstrated that activation of STAT3, and not STAT1, is necessary for IL-21–induced apoptosis of DLBCL. To our knowledge, this is the first example in which the STAT3–c-Myc pathway is used to mediate tumor cell death by a therapeutic agent. The mechanism determining whether STAT3 activation promotes cell survival or induction of cell death is not completely understood. A previous report showed that STAT3 can be converted into a proapoptotic transcription factor in cells that lose expression of SOCS3. This loss of SOCS3 promotes a more intense and prolonged activation of STAT3, leading to up-regulation of c-Myc and increased cell apoptosis.41 We similarly observed a more intense and longer STAT3 phosphorylation in cells sensitive to IL-21 treatment, although in our studies SOCS3 likely did not contribute to the observed apoptosis because its expression was induced upon IL-21 treatment in both resistant and sensitive DLBCL cell lines.
In the present study, we showed that apoptosis is dependent on STAT3-induced c-Myc up-regulation. c-Myc is normally expressed only transiently and at low levels in response to mitogenic signaling and is a key regulator of cellular proliferation and differentiation. Recent studies demonstrated that c-Myc effects may depend on levels of its expression, with low-level induction promoting cell growth and high-level induction stimulating apoptosis.28,42 In our study, the high levels of c-Myc induced by IL-21 stimulation would favor its proapoptic effects, as was confirmed by prevention of apoptosis through siRNA-mediated impairment of c-Myc up-regulation. In contrast, IL-21–induced induction of c-Myc was markedly diminished in the resistant cell lines. As in previous studies, Bcl-2 or Bcl-XL rescue partially prevented apoptosis, confirming the importance of their down-regulation in mediation of c-Myc–induced apoptosis.29,30,43,44 Taken together, these data are in agreement with previous reports that a prohibitively high induction of c-Myc can trigger apoptosis via modulation of the Bcl-2 family of proteins.
The heterogeneous nature of DLBCL with varied mechanisms of transformation may contribute to different sensitivity to therapeutic interventions. Despite having high levels of IL-21R, the viability of the OCI-LY-3 cell line was unaffected by IL-21 treatment, which contrasts with the effects of IL-21 on the remaining DLBCL cell lines as well as primary tumors. We demonstrated that although IL-21 activated STAT3 in OCI-LY-3, there was no subsequent up-regulation of c-Myc, which is required for IL-21–induced apoptosis. Although the levels of pSTAT3 achieved after IL-21 activation were similar across all DLBCL cell lines, the fold-change increase in pSTAT3 levels was markedly smaller in OCI-LY-3 due to prior constitutive activation of STAT3. This might contribute to the absence of IL-21–induced up-regulation of c-Myc in this cell line. No constitutive activation of STAT3 was observed in the RC-K8R cell line after development of resistance. Further studies to elucidate mechanisms of resistance to IL-21 are needed.
Our results represent the first evidence that the STAT3–c-Myc signaling pathway may be exploited for treatment of cancer. IL-21 was recently evaluated in clinical trials as a therapeutic approach to stimulate the immune system to reject solid tumors such as melanoma and renal cell carcinoma.45 These trials demonstrated some clinical activity and showed favorable pharmacodynamic and pharmacokinetic profiles. Our studies of xenograft DLBCL tumors in mice demonstrated the potent in vivo antilymphoma activity of this cytokine because IL-21 treatment promoted tumor regression and improved overall survival. IL-21 injections were well tolerated by mice as evidenced by maintenance of body weight and lack of behavioral changes, consistent with findings from clinical trials in humans showing good patient tolerability and limited side effects.45,46 Our preclinical studies demonstrate that IL-21 may be used for direct targeting of DLBCL tumors that express high levels of IL-21R and may benefit patients when used as a single agent or in conjunction with standard chemotherapies. These studies, combined with further investigation of mechanisms of IL-21 resistance and facilitation of c-Myc–induced apoptosis, will not only elucidate the biologic functions of IL-21 but also potentially improve the outcome of DLBCL patients.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
I.S.L. is supported by National Institutes of Health (NIH) grants NIH CA109335 and NIH CA122105, and the Dwoskin Family, Bankhead-Coley, and Fidelity Foundations. The work of R.M. was supported by a fellowship from Fundación Caja Madrid. The work of A.J.G. is supported by NIH U56CA112973.
National Institutes of Health
Authorship
Contribution: K.A.S. conceptualized the idea of the study, performed experiments, analyzed the data, and wrote the paper; R.M. performed experiments and analyzed the data; H.N. conceptualized the idea of the study; A.J.G. analyzed the data; E.A. contributed valuable materials; I.S.L. conceptualized the idea of the study, supervised the experiments, analyzed the data, and wrote the paper; and all authors approved the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Izidore S. Lossos, Department of Hematology and Oncology, University of Miami, Sylvester Comprehensive Cancer Center, 1475 NW 12th Ave (D8-4), Miami, FL; e-mail: ilossos@med.miami.edu.