Abstract
B cells currently are not viewed as being capable of producing granzyme B or being cytotoxic. We found that B-chronic lymphocytic leukemia (B-CLL) cells treated with interleukin-21 (IL-21) produce low levels of granzyme B. The addition of either CpG oligodeoxynucleotide (ODN) or anti-B-cell-receptor antibody (anti-BCR) to IL-21 results in enhanced production of functional granzyme B by B-CLL cells. B-CLL cells treated with IL-21 and CpG ODN undergo apoptosis and are able to induce apoptosis of untreated bystander B-CLL cells. This effect can be inhibited by anti-granzyme B antibody. Benign human B cells, Epstein-Barr virus (EBV)-transformed lymphoblasts, and many standard lymphoma cell lines produce high levels of granzyme B in response to IL-21 and anti-BCR. Our results suggest that the ability to induce production of functional granzyme B by B cells could open new approaches to the therapy of B-CLL and other B-cell malignancies. Our findings also have significant implications for our understanding of the role of B cells for immune regulation and for a variety of immune phenomena, including cancer immunity, autoimmunity, and infectious immunity.
Introduction
A key killing mechanism used by cytotoxic T lymphocytes (CTLs) and natural killer (NK) cells is the release of cytotoxic granules into the secretory synapse between effector and target cell.1 A major constituent of these granules is the 32-kDa serine protease granzyme B.2,3 Granzyme B is activated by cathepsin C after its release into the secretory synapse, taken up into the target cell via fluid phase and mannose-6-phosphate receptor-mediated endocytosis,2 and released from the endosome in response to a second signal, such as perforin, or microbial factors such as adenovirus or certain bacterial toxins.4-7 The transduction of the death signal mediated by granzyme B in the target cell occurs via 2 pathways, 1 by direct signaling to the mitochondria via BH3-interacting death agonist and 1 by activation of the classical caspase cascade. Granzyme B is one of the most effective and fastest executioners of apoptosis known. To date, CTLs and NK cells are the cell populations known to express and secrete granzyme B.
B-chronic lymphocytic leukemia (B-CLL) is the most prevalent leukemia in the Western world and is characterized by the progressive accumulation of malignant B cells in peripheral blood, secondary lymphoid tissue, and bone marrow. In the majority of cases, B-CLL cells are positive for the T-cell marker CD5 and are therefore easily detectable by flow cytometry and immunohistochemistry.8,9 Despite the recent development of a number of effective agents, B-CLL is generally considered incurable, and there is a need for targeted agents or combinations.
Therapy of B-CLL with a variety of cytokines and other immunostimulatory agents is under evaluation based on the understanding that signals that induce proliferation of benign B cells can sometimes induce apoptosis of malignant B cells. For example, interleukin-21 (IL-21) is a novel IL-2 family cytokine produced by activated CD4+ T cells. IL-21 has pleiotropic effects on B, T, and NK cells.10-12 In primary human B cells, IL-21 enhances anti-CD40-mediated proliferation. A recent report indicates that the combination of IL-21 plus anti-CD40 induces apoptosis of B-CLL cells.13 We have evaluated the effect of the Toll-like receptor 9 (TLR9) ligand CpG oligodeoxynucleotide (ODN) on B-CLL cells, and found it activates and induces proliferation of benign B cells, but is capable of inducing apoptosis in most B-CLL samples.14-18 While caspase activation is a common pathway observed in the apoptosis of B-CLL induced by both IL-21 and CpG ODN, the precise apoptotic signals that lead to caspase activation for these agents is not known.
We therefore evaluated the combination of IL-21 and CpG ODN for their effect on B-CLL cells, and assessed how such treatment might lead to death of the B-CLL cells. As outlined below, we found these agents were synergistic in their ability to induce apoptosis of B-CLL cells. Unexpectedly, this therapy induced production of granzyme B by the B-CLL cells. The granzyme B could induce apoptosis of both the treated and untreated bystander B-CLL cells. Further studies demonstrated that the combination of IL-21 and anti-B-cell receptor (BCR) also induced granzyme B production by B-CLL cells. This combination also induced granzyme B production by other B cells. These findings suggest a novel treatment strategy for B-CLL and possibly other B-cell malignancies. They also raise important questions about the role of cytotoxic B lymphocytes in the immune system in general, and in the interactions between B cells and IL-21-producing CD4+ T cells in particular.
Patients, materials, and methods
Human subjects and cell culture
Peripheral blood from a total of 17 different patients with B-CLL and 16 different healthy volunteers was acquired after obtaining informed consent from each individual. Use of blood from healthy human subjects and patients with B-CLL for the study described in this paper was approved by the institutional review board at the University of Iowa. Patients with B-CLL were heterogeneous, but consisted mostly of newly diagnosed subjects with early-phase disease (n = 14). Three patients with more advanced disease who had previously been treated with fludarabine and cytoxan were also tested. None were under treatment at the time the samples were obtained. Use of blood from healthy human subjects and patients with B-CLL for the study described in this article was approved by the Institutional Review Board at the University of Iowa. Mononuclear cells were immediately isolated, and red blood cells were removed by resuspending the cells in 5 mL red cell lysis buffer according to standard procedures. In some experiments CD19+ B-CLL cells or benign B cells were magnetically purified using the B-cell isolation kit I for B-CLL cells and the B cell isolation kit II for benign B cells according to the manufacturer's instructions (Miltenyi Biotec, Auburn, CA). During in vitro culture, peripheral blood cells were suspended in AIM-V medium (Gibco BRL, Grand Island, NY) without supplements. Cells were incubated on 96-well plates (1 × 106 cells/mL, 200 μL/well, if not stated otherwise) in the presence of different reagents as indicated.
Reagents for functional assays
The phosphorothioate-modified CpG motif-containing ODN 2006 (henceforth referred to as CpG ODN) and the phosphorothioate phosphodiester-modified control ODN 2243 (henceforth referred to as control ODN) were purchased from Coley Pharmaceutical Group (Wellesley, MA). The control ODN is the same sequence we used in prior studies, and was selected based on its lack of immunostimulatory effects on B cells.15 Endotoxin levels in all ODN were less than 0.075 EU/mL by limulus amebocyte lysate assay. Specific ODN sequences were as follows: CpG ODN, 5′-TCG TCG TTT TGT CGT TTT GTC GTT-3′; control ODN, 5′-GGG GGA GCA TGC TGG GGG GG-3′. ODN were diluted in TE (10 mM Tris-HCl,1 mM EDTA [pH 8]) using pyrogen-free reagents, and used at a final concentration (fc) of 2.5 μg/mL. Human IL-21 (fc, 100 ng/mL) was purchased from BioSource (Camarillo, CA), IL-2 (fc, 100 U/mL) was purchased from Peprotech (Rocky Hill, NJ). B-cell-receptor stimulation was performed using affinity purified rabbit F(ab′)2 fragments against human IgA+IgG+IgM (H+L; Jackson ImmunoResearch Laboratories, West Grove, PA; fc, 10 μg/mL). For CD40 stimulation, mouse monoclonal antibody (mAb) against human CD40 (clone B-B20) was used (Diaclone Research, Tepnel Lifecode, Stamford, CT; fc, 10 μg/mL). For granzyme B inhibition experiments, carrier- and preservative-free rabbit anti-human granzyme B polyclonal antibody (IgG) from USBiological (Swampscott, MA) was used at the concentrations indicated. ImmunoPure Rabbit IgG from Pierce (Rockford, IL) served as control IgG.
Flow cytometry
For staining of surface markers, cells were harvested at the indicated time points and stained as described previously.19 Antibodies to CD5, CD19, and CD107a were purchased from BD Biosciences (San Diego, CA). IL-21 receptor protein expression was detected using a mAb (clone 152512) from R&D Systems (Minneapolis, MN). PKH26, a red fluorescent dye used for general cell membrane labeling, was used to identify untreated cells in bystander assays. B-CLL cells were resuspended at a density of 1 × 107 cells/mL in diluent C containing 2 μM PKH26 (both from Sigma, St Louis, MO). After 3 minutes, the reaction was stopped by adding an equal volume of FBS (HyClone, Ogden, UT). Cells were washed 3 times and finally resuspended in AIM-V medium. For flow cytometric granzyme detection, cells were incubated at 1 × 106/mL for 13 hours, Brefeldin A (Epicentre Technologies, Madison, WI) was added to a final concentration of 1 μg/mL, and cells were cultured for 5 more hours. Intracellular staining was performed using a Fix and Perm kit (Caltag Laboratories, Burlingame, CA) according to the manufacturer's instructions. Briefly, cells were washed once and resuspended in fixation buffer, incubated for 15 minutes at room temperature, and washed with PBS. Cells were then resuspended in permeabilization buffer and PE- or FITC-labeled antibodies to granzyme B (clone GB12; Caltag Laboratories), granzyme A (clone CB9; BD Pharmingen), or suitable control antibodies were added. After another 15 minutes of incubation at room temperature, cells were washed with PBS. Flow cytometric analyses were performed on a FACScan (Becton Dickinson Immunocytometry Systems, San Jose, CA), and data analyzed using FlowJo software (version 6.4.1; Tree Star, Stanford, CA).
Flow cytometric apoptosis assays
Cells were stained with annexin V (BD Biosciences), or cell-permeable fluorogenic substrates specific for granzyme B or caspase 6 for 1 hour at room temperature according to the manufacturer's instructions (OncoImmunin, Gaithersburg. MD). A predetermined number of calibration beads (CaliBRITE Beads; BD Biosciences) were added to each sample to allow for normalization of cell counts at different time points. Propidium iodide at 1 μg/mL was added just prior to flow cytometric analysis. The count of viable cells rather than the count of apoptotic cells was used for the calculation of cell survival because of concerns that some nonviable cells may have undergone lysis and would not have been available for counting. Absolute cell survival was expressed as percentage of viable cell counts relative to initial plating counts.
ELISPOT assays for human granzyme B
Human granzyme B enzyme-linked immunospot (ELISPOT) kits were purchased from Cell Sciences, (Canton, MA). PVDF-bottomed 96-well plates were purchased from Millipore (Bedford, MA). The assays were performed according to the manufacturer's protocol. Briefly, plates were prepared by adding the capture antibody and blocking with 2% skim milk in PBS, then cells were resuspended in AIM-V medium, plated (100 μL/well), and CpG ODN (2.5 μg/mL), IL-21 (100 ng/mL), anti-BCR (10 μg/mL), or combinations of these agents were added and cultured for 16 hours. Freshly isolated peripheral blood mononuclear cells (PBMCs) stimulated with PHA (10 μg/mL) served as a positive control. After culture, the detection antibody was added and plates were incubated for 1.5 hours. Streptavidinalkaline phosphatase was distributed, and plates were incubated for 1 hour. Finally, BCIP/NBT buffer was added and color was allowed to develop for 10 minutes at room temperature, followed by rinsing with distilled water. Plates were dried completely, and spots read on an Immunospot Series 1 Analyzer and counted using Immunospot 3 software, both from CTL Cellular Technology (Cleveland, OH).
TUNEL assay
B-CLL cells were isolated and purified to a percentage of more than 99.9% using magnetic cell separation. Cells were then suspended at 2 × 106/mL and plated on a 24-well cell-culture plate at 1 mL/well in the presence of different agents as indicated. After 12 hours cells were harvested, fixed in 1% (wt/vol) paraformaldehyde, suspended at 2 × 106/mL in 70% (vol/vol) ethanol and stored for at least 1 hour at -20°C. Fixed cells were stained using the APO-DIRECT Kit from BD Biosciences according to the manufacturer's instructions. Briefly, cells were washed to remove the ethanol, incubated at 37°C for 60 minutes in the presence of reaction buffer, TdT enzyme, and FITC-dUTP, rinsed, suspended in PI/RNAse staining buffer, and analyzed within 3 hours of staining by flow cytometry.
Microarray profiling
B-CLL cells were isolated from other cells by magnetic beads as outlined in “Human subjects and cell culture,” and cultured in media or 2.5 μg/mL CpG ODN for 2 hours. RNA was isolated using Trizol (Invitrogen, Grand Island, NY) followed by the RNeasy Kit (Qiagen, Valencia, CA) used according to the manufacturer's specifications. Subsequently, the RNA sample was concentrated to 7 mg/mL by centrifugation on a filtration concentrator to clean up the RNA. The RNA concentration was determined by spectrophotometry and RNA was stored at -80°C. Gene expression profiling was conducted in the University of Iowa DNA Core. Gene profiles were generated according to manufacturer guidelines for the U133A chip (Affymetrix, Santa Clara, CA). Quality control test arrays include an Affymetrix test chip containing housekeeping genes (eg, GAPDH, β-actin) with targets corresponding to various regions of the gene from 3′ to 5′. The signal was assessed for discrepancies from 3′ to 5′, and if the signal was more than 3-fold different, the sample failed quality control and was prepared again. Intensity values were normalized using Affymetrix MAS 5. After normalization, data analysis was carried out using R software (R Development Core 2005, Vienna, Austria).
General statistical analysis
Data are expressed as means ± SEM. To determine statistical differences between the means of 2 data columns, the paired Student t test was used. A P value of less than .05 was considered significant; a P value of less than .005 was considered to be highly significant. Isobolographic analysis was performed using FlashCalc version 20.5 (FlashCalc Pharmacologic calculations; M. H. Ossipov, Tucson, AZ).
Results
CpG ODN induces up-regulation of the IL-21 receptor by B-CLL cells
We and others have found that CpG ODN can induce apoptosis and alter the phenotype of B-CLL cells.15-18,20,21 B-CLL cells also have receptors for, and respond to, a variety of IL-2-related cytokines, including IL-2, IL-15, and IL-21.13,22,23 The ability of CpG ODN to alter expression of these receptors had not been studied previously. We therefore evaluated how CpG ODN affects the expression of a number of cytokine receptors by B-CLL cells, using fluorescence-activated cell-sorting (FACS) analysis and gene array. Among the observed changes was a 4- to 16-fold increase over baseline of the gene for the IL-21 receptor (IL21R). Changes in expression of other receptors involved in apoptosis (IL4, TACI, VEGFR) were limited. We also observed a consistent up-regulation of IL-21 receptor protein after 4 and 7 days of incubation of the B-CLL cells with CpG ODN. Control ODN had no detectable effect on protein levels of IL-21 receptor (Figure S1, found on the Blood website; see the Supplemental Figures link at the top of the online article).
IL-21 and CpG ODN are synergistic in their ability to enhance apoptosis of B-CLL cells
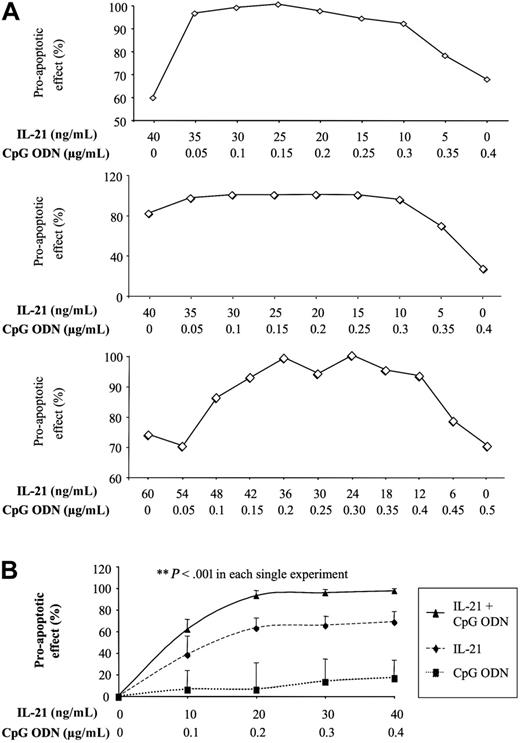
These results prompted us to study the effects on B-CLL cells of IL-21 alone and in combination with CpG ODN. B-CLL cells were isolated and incubated for 4 days in the presence or absence of IL-21 or IL-2. Apoptosis was detected using annexin V and PI. IL-21 alone induced some apoptosis in B-CLL cells. This effect was strongly enhanced in all samples studied when B-CLL cells were simultaneously treated with CpG ODN (Figure 1). Measurement of apoptosis after 12 hours using DNA fragmentation as measured by flow cytometric transferase-mediated dUTP nick-end labeling (TUNEL) analysis gave similar results (Figure S2). In contrast to IL-21, IL-2 did not induce apoptosis of B-CLL when combined with CpG ODN. The ability of varying doses of IL-21 and CpG ODN to induce apoptosis was also assessed. The effective dose causing apoptosis in 50% of cells for each agent alone was identified (IL-21, 40 ng/mL; CpG ODN, 0.4 μg/mL), and the interaction between these agents determined as described by Tallarida.24 Isobolographic analysis demonstrated that the combined proapoptotic effect of IL-21 and CpG ODN on B-CLL cells is synergistic (Figure 2A-B).
IL-21 and CpG ODN induce apoptosis of highly purified B-CLL cells
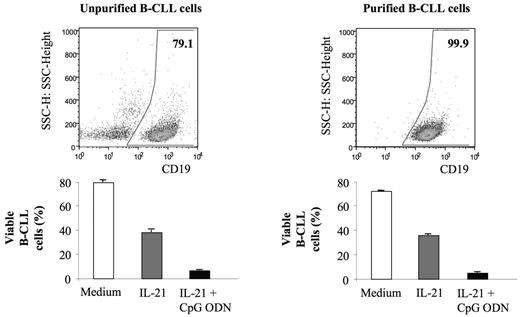
One possible explanation for the observed effects on B-CLL cells is that CpG ODN stimulates plasmacytoid dendritic cells (pDCs) or other non-B-CLL cells to produce cytokines such as IFN-α that then affect the B-CLL cells. To assess this possibility, B cells were purified from B-CLL samples by magnetic bead cell sorting to a purity of more than 99.9%, with less than 0.005% of the remaining cells being pDCs. Essentially, all sorted cells were CD19+, CD5+, suggesting that the number of benign B cells in the preparations were very small. As shown in Figure 3, the proapoptotic effect of CpG ODN and IL-21 on B cells was similar in unpurified and purified samples, suggesting therapy affects the B-CLL cells directly, and not secondarily through activation of benign mononuclear cells that are also in blood.
IL-21 plus CpG ODN induces apoptosis of B-CLL cells. PBMCs from 9 patients with B-CLL were cultured for 4 days in the presence of CpG ODN (2.5 μg/mL) and IL-21 (100 ng/mL) or IL-2 (100 U/mL). Cell survival of B-CLL cells was determined using annexin V and PI staining and counterstaining with antibodies to CD19. (A) Shown are annexin V/PI dot plots from 1 representative experiment. Gated are CD19+ B-CLL cells. Numbers in the gates represent percentages of displayed events. (B) The mean B-CLL cell-survival rates from 9 independent experiments are plotted. Error bars indicate SEM.
IL-21 plus CpG ODN induces apoptosis of B-CLL cells. PBMCs from 9 patients with B-CLL were cultured for 4 days in the presence of CpG ODN (2.5 μg/mL) and IL-21 (100 ng/mL) or IL-2 (100 U/mL). Cell survival of B-CLL cells was determined using annexin V and PI staining and counterstaining with antibodies to CD19. (A) Shown are annexin V/PI dot plots from 1 representative experiment. Gated are CD19+ B-CLL cells. Numbers in the gates represent percentages of displayed events. (B) The mean B-CLL cell-survival rates from 9 independent experiments are plotted. Error bars indicate SEM.
The proapoptotic effect of IL-21 and CpG ODN on B-CLL cells is synergistic. PBMCs from 3 patients with B-CLL were cultured with CpG ODN and IL-21 at different concentration ratios for 4 days, and the percentage of apoptotic cells was determined. (A) Three individual experiments demonstrating a synergistic interaction between CpG ODN and IL-21 are shown. A horizontal line indicates an additive interaction, while the observed convex curve demonstrates synergy. (B) The effect of varying concentrations of CpG ODN and IL-21 demonstrated that both agents induced greater apoptosis than either agent alone, even at concentrations that give peak effects for each individual agent. Plotted are the mean cell-survival rates (n = 3). Error bars indicate SEM.
The proapoptotic effect of IL-21 and CpG ODN on B-CLL cells is synergistic. PBMCs from 3 patients with B-CLL were cultured with CpG ODN and IL-21 at different concentration ratios for 4 days, and the percentage of apoptotic cells was determined. (A) Three individual experiments demonstrating a synergistic interaction between CpG ODN and IL-21 are shown. A horizontal line indicates an additive interaction, while the observed convex curve demonstrates synergy. (B) The effect of varying concentrations of CpG ODN and IL-21 demonstrated that both agents induced greater apoptosis than either agent alone, even at concentrations that give peak effects for each individual agent. Plotted are the mean cell-survival rates (n = 3). Error bars indicate SEM.
CpG ODN and IL-21 induce expression of CD107a and functional granzyme B secretion by B-CLL cells
Further studies were done to explore the mechanisms behind the observed synergy between IL-21 and CpG ODN. Multicolor flow cytometric analysis demonstrated IL-21 and CpG ODN induced an increase in B-CLL cell granularity as indicated by enhanced side scatter (Figure 4A) and enhanced surface expression of lysosomal-associated membrane protein-1 (LAMP-1, CD107a) on B-CLL cells (Figure 4A-B). Since CD107a is known to be a degranulation marker,25-27 we evaluated the treated B-CLL cells for expression of granzyme B. Flow cytometric analysis gating on CD19+ cells revealed rare granzyme B+ B cells in samples treated with IL-21 alone. The number of granzyme B+ B cells increased significantly in samples treated with the combination of IL-21 and CpG ODN (Figure 4C). Multicolor analysis demonstrated the granzyme B cells were CD19+, CD5+ (data not shown).
To assess whether granzyme B secreted by B-CLL cells is functionally active, and to confirm it is the B-CLL cells that are producing the granzyme B, cells were treated with a granzyme B-sensitive cell-permeable substrate. This substrate fluoresces when cleaved by active granzyme B. B-CLL cells were cultured in the presence of IL-21, CpG ODN, or both for 4 days (Figure 4D and samples analyzed by flow cytometry after gating on the CD19+ cells). As shown in Figure 4D, granzyme B activity in B-CLL cells was approximately 8-fold higher than baseline after treatment with IL-21 plus CpG ODN. The activity patterns for granzyme B function were similar to those seen with staining of granzyme B protein. Cells were also treated with a caspase 6-sensitive substrate. As shown in Figure S4, the activity patterns for caspase 6 were similar to the granzyme B, with the greatest caspase 6 activity being seen after treatment of cells with IL-21 and CpG ODN.
Similar assays demonstrated no detectable granzyme A (data not shown). An ELISPOT assay for granzyme B was used to confirm this finding, and demonstrated that the granzyme B produced by B-CLL cells was secreted. As with the flow cytometric assay, the ELISPOT assay demonstrated that IL-21 plus CpG ODN induced granzyme B production to a greater degree than either agent alone (Figure S3A). As an additional control, samples of purified B cells were treated with PHA, which would be expected to induce granzyme B secretion by any contaminating T or NK cells. The number of granzyme B-producing cells in these samples was close to 0, providing further evidence that it is the B-CLL cells, and not contaminating T or NK cells, producing the granzyme B (Figure S3B).
Effect of IL-21 and other B-cell activators on B-CLL cells
Combinations of other B-cell-stimulatory agents were also tested for their ability to induce secretion of granzyme B by B-CLL cells. A stimulating anti-BCR antibody, but not a stimulating anti-CD40 antibody, enhanced IL-21-induced granzyme B secretion by B-CLL cells (Figure 5A-B). While anti-BCR alone protected B-CLL cells from spontaneous apoptosis, IL-21 plus anti-BCR had a proapoptotic effect that was similar to IL-21 plus CpG ODN (Figure 5C). IL-2 did not induce granzyme B, nor did it have a proapoptotic effect when it was used instead of IL-21 in these combinations (data not shown).
B-CLL cells treated with CpG ODN and IL-21 can kill untreated autologous cells: bystander killing is blocked by anti-granzyme B antibody
We next evaluated whether B-CLL cells treated with IL-21 and CpG ODN have the potential to kill. To avoid the complexity of dealing with an allogenic interaction, this was done using autologous B-CLL cells. Purified B-CLL cells were split into 2 fractions. One fraction was stained with the membrane dye PKH26 to enable us to differentiate treated from untreated cells, and was incubated for 24 hours with IL-21 and CpG ODN. These stained, treated cells were washed 3 times to remove the agents and mixed with the unstained, untreated fraction for 2 days. The survival of the unstained, untreated cells was then evaluated by flow cytometry. As illustrated in Figure 6A-B, the cells treated with IL-21 and CpG ODN induced apoptosis of the untreated cells, indicating B-CLL cells treated with IL-21 and CpG ODN were capable of killing untreated B-CLL cells. The addition of anti-human granzyme B antibody inhibited this bystander killing in a dose-dependent manner (Figure 6C), providing further evidence that granzyme B was involved in the observed cell death.
Proapoptotic effect of IL-21 and CpG ODN on B-CLL cells is direct. PBMCs from 3 patients with B-CLL were isolated and divided into 2 fractions. One fraction was purified to a percentage of more than 99% CD5+, CD19+ B-CLL cells. Both fractions were incubated for 3 days with IL-21, CpG ODN, or both agents, and apoptosis was determined flow cytometrically. Data are from 1 representative experiment of 3. Dot plots demonstrate the purity of B-cell populations based on CD19 expression. Numbers in the gates represent percentages of displayed events. Bar graphs illustrate the mean B-CLL cell-survival rates in response to treatment. Error bars indicate SEM from experimental replicates.
Proapoptotic effect of IL-21 and CpG ODN on B-CLL cells is direct. PBMCs from 3 patients with B-CLL were isolated and divided into 2 fractions. One fraction was purified to a percentage of more than 99% CD5+, CD19+ B-CLL cells. Both fractions were incubated for 3 days with IL-21, CpG ODN, or both agents, and apoptosis was determined flow cytometrically. Data are from 1 representative experiment of 3. Dot plots demonstrate the purity of B-cell populations based on CD19 expression. Numbers in the gates represent percentages of displayed events. Bar graphs illustrate the mean B-CLL cell-survival rates in response to treatment. Error bars indicate SEM from experimental replicates.
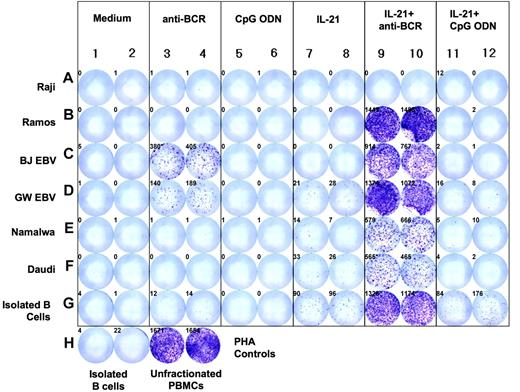
IL-21 and anti-BCR can induce granzyme B production by other B-cell populations, including benign B cells, EBV-transformed B cells, and B-cell lines
Isolated peripheral blood B cells from healthy donors, Epstein-Barr virus (EBV)-transformed lymphoblasts, and standard human lymphoma cell lines were evaluated for their response to IL-21 and CpG ODN or anti-BCR using ELISPOT (Figure 7). The combination of IL-21 and anti-BCR had no effect on Raji cells, but induced a significant degree of granzyme B production by Ramos, Namalwa, and Daudi cell lines as well as benign B cells and EBV-transformed B cells from 2 donors. Anti-BCR antibody alone induced a low level of granzyme B in the EBV-transformed cells, while IL-21 alone induced a low level of granzyme B production by B cells. In contrast to our findings with B-CLL, CpG ODN induced no granzyme B induction alone or in combination with IL-21. Granzyme B-specific polymerase chain reaction (PCR) after 12 hours incubation of purified B cells with IL-21 and anti-BCR showed the presence of mRNA for granzyme B, suggesting that the secretion of granzyme B by B cells was due to de novo synthesis rather than the release of preformed granules (data not shown).
Discussion
IL-21 is an IL-2 family cytokine, produced mainly by activated CD4+ T cells. It has pleiotropic effects on T, B, and NK cells.10,11,28 One effect of IL-21 is to up-regulate the granzyme A and B genes in cytotoxic CD8+ T cells.12,29 Apart from CTLs and NK cells, no other human lymphocyte populations are known to produce and secrete granzymes at biologically active levels. In the present study we demonstrate de novo synthesis and secretion of granzyme B by activated human B cells in response to IL-21. This effect can be enhanced in select B-cell populations by BCR cross-linking, as well as the TLR9 agonist CpG ODN.
The combination of IL-21 plus CpG ODN is cytotoxic to B-CLL cells, and the granzyme B produced by treated B-CLL cells can kill untreated autologous bystander B-CLL cells. This effect is blocked in part by anti-granzyme B, confirming that at least some of the observed effect results from the cytotoxic effects of secreted granzyme B. Our observation of bystander killing being inhibited by anti-granzyme B antibody indicates the granzyme B is functional and can have cytotoxic effects. We reported previously that CpG ODN as a single agent can induce up-regulation by B-CLL cells of a number of death receptors as well as their ligands,18 and we are currently exploring whether such receptors may be contributing to the synergistic proapoptotic effects we are seeing with IL-21 plus CpG ODN.
IL-21 and CpG ODN induce B-CLL cell expression of lysosome-associated molecular protein 1 (LAMP-1, CD107a) and granzyme B. PBMCs from 7 patients with B-CLL were cultured in the presence of CpG ODN, IL-21, or both. Expression of CD107a (LAMP-1) on CD19+ B-CLL cells was determined using FACS analysis. (A-B) Dot plots from 1 representative experiment of 7 show percentages of CD19+ B-CLL cells with increased side scatter and CD107a expression. The bar graph illustrates relative median fluorescence intensities (MFI) for CD107a expression compared with unstimulated cells. Error bars indicate SEM. (C) PBMCs from 5 patients with B-CLL were isolated and cultured in the presence of IL-21, CpG ODN, or both. For the last 4 hours, 1 μg/mL brefeldin A was added to the cells. Cells were then fixed, permeabilized, and stained with antibodies to anti-CD19 and granzyme B. The boldfaced number in Figure 4C represents the percentage of granzyme B-positive cells when treated with IL-21 and CpG ODN. One representative experiment of 5 is shown. (D) PBMCs from 5 patients with B-CLL were stained with a granzyme B colorimetric substrate, then cultured as outlined for panel C. The bar graph shows the percentage of CD19+ cells with substrate that was activated by granzyme B. Error bars indicate SEM.
IL-21 and CpG ODN induce B-CLL cell expression of lysosome-associated molecular protein 1 (LAMP-1, CD107a) and granzyme B. PBMCs from 7 patients with B-CLL were cultured in the presence of CpG ODN, IL-21, or both. Expression of CD107a (LAMP-1) on CD19+ B-CLL cells was determined using FACS analysis. (A-B) Dot plots from 1 representative experiment of 7 show percentages of CD19+ B-CLL cells with increased side scatter and CD107a expression. The bar graph illustrates relative median fluorescence intensities (MFI) for CD107a expression compared with unstimulated cells. Error bars indicate SEM. (C) PBMCs from 5 patients with B-CLL were isolated and cultured in the presence of IL-21, CpG ODN, or both. For the last 4 hours, 1 μg/mL brefeldin A was added to the cells. Cells were then fixed, permeabilized, and stained with antibodies to anti-CD19 and granzyme B. The boldfaced number in Figure 4C represents the percentage of granzyme B-positive cells when treated with IL-21 and CpG ODN. One representative experiment of 5 is shown. (D) PBMCs from 5 patients with B-CLL were stained with a granzyme B colorimetric substrate, then cultured as outlined for panel C. The bar graph shows the percentage of CD19+ cells with substrate that was activated by granzyme B. Error bars indicate SEM.
IL-21 induces granzyme B secretion by B-CLL cells, which is synergistically enhanced by CpG ODN and anti-BCR. B-CLL cells from 3 patients were isolated and purified to a percentage of at least 99.9% based on CD19 expression. The cells were cultured at 37°C on 96-well ELISPOT plates for granzyme B detection at 100 000 cells/well and in the presence of different B-cell activators alone or with IL-21. After 16 hours, plates were developed and dots counted. Every condition was run in triplicate. (A) Individual ELISPOT plate from 1 representative experiment of 3 is shown. (B) Average spot numbers from 1 representative experiment of 3 are depicted. Error bars indicate SD. (C) B-CLL cell survival after 4 days of incubation with anti-BCR or CpG ODN alone ( ) or with IL-21 (▪) was flow cytometrically detected using annexin V, anti-CD19, and PI staining. Averages from 3 independent experiments are shown. Error bars indicate SEM.
) or with IL-21 (▪) was flow cytometrically detected using annexin V, anti-CD19, and PI staining. Averages from 3 independent experiments are shown. Error bars indicate SEM.
IL-21 induces granzyme B secretion by B-CLL cells, which is synergistically enhanced by CpG ODN and anti-BCR. B-CLL cells from 3 patients were isolated and purified to a percentage of at least 99.9% based on CD19 expression. The cells were cultured at 37°C on 96-well ELISPOT plates for granzyme B detection at 100 000 cells/well and in the presence of different B-cell activators alone or with IL-21. After 16 hours, plates were developed and dots counted. Every condition was run in triplicate. (A) Individual ELISPOT plate from 1 representative experiment of 3 is shown. (B) Average spot numbers from 1 representative experiment of 3 are depicted. Error bars indicate SD. (C) B-CLL cell survival after 4 days of incubation with anti-BCR or CpG ODN alone ( ) or with IL-21 (▪) was flow cytometrically detected using annexin V, anti-CD19, and PI staining. Averages from 3 independent experiments are shown. Error bars indicate SEM.
) or with IL-21 (▪) was flow cytometrically detected using annexin V, anti-CD19, and PI staining. Averages from 3 independent experiments are shown. Error bars indicate SEM.
Many factors can affect whether granzyme B production by a cell induces autolysis or results in cytotoxic potential against bystander cells. These need further evaluation in various populations of B cells that produce granzyme in response to IL-21-based therapy. Effector and target cells need to get into close contact to each other and form a secretory synapse involving a series of receptors and ligands on both the effector and the target cell side.1 Killing mediated by granzyme B is generally associated with perforin, which allows release of granzyme B from endosomes into the cytosol after uptake into the target cell. Preliminary studies in our laboratory suggest that stimulation of B-CLL cells and other B cells can lead to production of perforin. This finding is currently under investigation in our laboratory. Cells that produce granzyme B are known to express proteins, such as proteinase inhibitor 9 (PI-9), which protects the cells from the proapoptotic effects of granzyme B.30 Malignant B cells that express PI-9 can still be sensitive to cytotoxic granule-mediated apoptosis,31 suggesting that expression of such proteinases is not a guarantee against apoptosis. B-CLL cells were obtained from a variety of patients at different stages of disease who had previously received a variety of treatments. Sensitivity to anti-BCR cross-linking has been reported to be dependent on mutational status and zap70 expression by the B-CLL cells.32 Further studies are clearly needed to determine how these factors impact on response to the combination of anti-BCR and IL-21. For these initial mechanistic studies, benign B cells were obtained largely from young healthy adults. These factors may all have contributed to the heterogeneity in the cell death we observed related to apoptosis of granzyme B-expressing B-CLL cells and other B-cell populations after IL-21-based therapy.
B-CLL cells treated with IL-21 plus CpG ODN can induce apoptosis of untreated, bystander B-CLL cells. Anti-granzyme B antibodies inhibit bystander B-CLL cell killing. (A) Purified B-CLL cells were split into 2 fractions. One fraction was stained with PKH-26, then incubated for 24 hours in IL-21 with or without CpG ODN. Unstained cells were maintained in culture without stimulus. The stained, treated cells were washed, added to the untreated cells, and cocultured for 2 days. Survival of the untreated cells (as indicated by lack of membrane dye) was analyzed by flow cytometry. Plotted are the B-CLL cell-survival rates for the untreated (ie, bystander) cells cultured at different ratios with treated cells. One representative experiment of 3 with similar results is shown. (B) Average B-CLL cell-survival rates for the untreated (ie, bystander) B-CLL cells cultured at a ratio of 1:2 with treated cells. Results are from 3 independent experiments. Error bars indicate SEM. (C) B-CLL cells from 3 patients were cultured for 4 days in the presence of IL-21 (100 ng/mL), CpG ODN (2.5 μg/mL), and anti-human granzyme B antibody ( )ora control antibody (□). B-CLL cell survival was determined by FACS analysis using annexin V/PI staining and counterstaining with antibodies to CD19. Plotted are the mean B-CLL cell-survival rates in percent from 1 representative experiment of 3 with similar results. Error bars indicate SD.
)ora control antibody (□). B-CLL cell survival was determined by FACS analysis using annexin V/PI staining and counterstaining with antibodies to CD19. Plotted are the mean B-CLL cell-survival rates in percent from 1 representative experiment of 3 with similar results. Error bars indicate SD.
B-CLL cells treated with IL-21 plus CpG ODN can induce apoptosis of untreated, bystander B-CLL cells. Anti-granzyme B antibodies inhibit bystander B-CLL cell killing. (A) Purified B-CLL cells were split into 2 fractions. One fraction was stained with PKH-26, then incubated for 24 hours in IL-21 with or without CpG ODN. Unstained cells were maintained in culture without stimulus. The stained, treated cells were washed, added to the untreated cells, and cocultured for 2 days. Survival of the untreated cells (as indicated by lack of membrane dye) was analyzed by flow cytometry. Plotted are the B-CLL cell-survival rates for the untreated (ie, bystander) cells cultured at different ratios with treated cells. One representative experiment of 3 with similar results is shown. (B) Average B-CLL cell-survival rates for the untreated (ie, bystander) B-CLL cells cultured at a ratio of 1:2 with treated cells. Results are from 3 independent experiments. Error bars indicate SEM. (C) B-CLL cells from 3 patients were cultured for 4 days in the presence of IL-21 (100 ng/mL), CpG ODN (2.5 μg/mL), and anti-human granzyme B antibody ( )ora control antibody (□). B-CLL cell survival was determined by FACS analysis using annexin V/PI staining and counterstaining with antibodies to CD19. Plotted are the mean B-CLL cell-survival rates in percent from 1 representative experiment of 3 with similar results. Error bars indicate SD.
)ora control antibody (□). B-CLL cell survival was determined by FACS analysis using annexin V/PI staining and counterstaining with antibodies to CD19. Plotted are the mean B-CLL cell-survival rates in percent from 1 representative experiment of 3 with similar results. Error bars indicate SD.
IL-21 induces de novo granzyme B synthesis and secretion by benign peripheral B cells and different B-cell lines. Standard cell lines, EBV transformed lymphoblasts, and B cells from healthy subjects (> 99.5% CD19+) were cultured in medium alone, anti-BCR, CpG ODN, IL-21, or combinations of these agents for 16 hours at a concentration of 100 000 cells/well. ELISPOT analysis for granzyme B was then performed. Controls included isolated B cells and unfractionated PBMCs treated with PHA.
IL-21 induces de novo granzyme B synthesis and secretion by benign peripheral B cells and different B-cell lines. Standard cell lines, EBV transformed lymphoblasts, and B cells from healthy subjects (> 99.5% CD19+) were cultured in medium alone, anti-BCR, CpG ODN, IL-21, or combinations of these agents for 16 hours at a concentration of 100 000 cells/well. ELISPOT analysis for granzyme B was then performed. Controls included isolated B cells and unfractionated PBMCs treated with PHA.
Irrespective of whether granzyme B has autocrine or paracrine effects, the finding that IL-21 plus CpG ODN, and IL-21 plus anti-BCR are potent inducers of apoptosis in B-CLL cells provides rationale for evaluating these combinations as treatment for B-cell malignancies and possibly other cancers. Each of these agents is being independently evaluated in early-phase clinical trials as treatments for B-CLL. The potential role of B-cell granzyme B production needs to be considered as the results of these clinical trials are interpreted. Our results also suggest that the combinations of these agents should be considered once the single-agent studies are complete.
Most of the studies outlined focused on the effect of CpG ODN on B-CLL cells. As illustrated in Figure 7, the ability of IL-21-based therapy to induce granzyme B production is not limited to B-CLL cells, as it was also observed with benign B cells from healthy donors, EBV-transformed lymphoblasts, and many standard lymphoma cell lines. Extensive additional studies are required before we can understand the effects such therapy has on benign B cells and other malignant B-cell populations, and the immunologic and therapeutic implications of such findings. Ongoing studies are exploring the effect of IL-21-based therapy on benign and a variety of malignant B cells with respect to gene expression, activation of apoptotic pathways, the relative potency of IL-21 plus CpG ODN versus IL-21 plus anti-BCR, and assessment of whether such B cells acquire cytotoxic potential.
If, indeed, we find benign B cells can be cytotoxic, additional studies are also needed to help us understand the impact granzyme B-producing B cells have on immunity. A number of possibilities need to be considered. First, B cells activated by IL-21 and other B-cell stimuli could undergo apoptosis after producing granzyme B, thus providing a negative feedback loop that limits excessive B-cell activation. This could be particularly interesting for the understanding of B-cell selection processes in the bone marrow. Negative feedback could also result if granzyme B produced by B cells induces apoptosis of the CD4+ T cell that produced the IL-21. On the other hand, cytotoxic B cells could be part of a positive feedback response to significant infection when multiple activation signals are present in a local environment. More specifically, local bacterial infection could lead to B-cell activation by BCR cross-linking or TLR9 activation by microbial DNA, in the same microenvironment where IL-21 is being produced by activated CD4+ T cells. B cells stimulated under such conditions would become cytotoxic toward cells expressing target antigen, independent of major histocompatibility complex (MHC), thereby resulting in an accelerated cytotoxic response against microbial intruders. In contrast to CpG ODN treatment or BCR ligation, CD40 ligation did not enhance granzyme B production by IL-21-treated B cells. A recent report suggests that IL-21 plus engagement of CD40 can induce B cells to terminally differentiate into plasma cells, while IL-21 plus BCR cross-linking does not.33 This finding, combined with our results, suggests that CD40 ligation plus IL-21 could provide the environment for naive B-cell differentiation and a longer-term, adaptive, and systemic immune response. In contrast, a microenvironment that results in exposure of the naive B cells to IL-21 plus BCR cross-linking (or CpG ODN) might signal for a more rapid, local, and undirected immune response and development of B cells with cytotoxic potential. Irrespective of the actual role of granzyme B secretion by naive B cells, the implications of our results for the understanding of a variety of immune phenomena including autoimmunity, infectious immunity, and cancer immunity could be substantial and clearly warrant further study.
In conclusion, we have demonstrated that B-CLL cells treated with CpG ODN and IL-21 produce granzyme B and can be cytotoxic, suggesting this combination may be a particularly potent treatment for B-CLL and perhaps other cancers. The combination of anti-BCR and IL-21 also induces granzyme B production from a variety of human B cells. The amount of granzyme B secreted by B cells can reach levels of the same order of magnitude as those secreted by CTLs. Our results therefore also raise important questions about the role of granzyme B secretion by naive B cells in immune regulation.
Prepublished online as Blood First Edition Paper, June 29, 2006; DOI 10.1182/blood-2006-03-014001.
Supported in part by American Cancer Society Grant IRG-77-004-25 administered through the Holden Comprehensive Cancer Center, and National Institutes of Health grants R01 CA77764 and P50 CA97274.
B.J. generated the concept, conducted experiments, analyzed experimental results, discussed and interpreted experimental results, and prepared the manuscript; S.E.B. conducted experiments, analyzed experimental results, discussed and interpreted experimental results; J.E.W. obtained informed consent, analyzed experimental results, and discussed and interpreted experimental results; J.H. analyzed experimental results; M.W.A., L.S.J., and C.M.T. obtained informed consent and acquired and processed samples; and G.J.W. generated the concept, analyzed experimental results, discussed and interpreted experimental results, and prepared the manuscript.
The online version of this manuscript contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors thank Justin Fishbaugh and Gene Hess for excellent technical assistance with flow cytometry, Gerald F. Gebhart and Michael Ossipov for assistance with the isobolographic analyses, Todd Rouse for technical assistance with the Immunospot Analyzer, and Dorit Fabricius and Emil Racila for helpful discussion.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal