Abstract
Annexins are a large family of intracellular phospholipid-binding proteins, yet several extracellular roles have been identified. Specifically, annexin A2, found in a heterotetrameric complex with S100A10, not only serves as a key extracellular binding partner for pathogens and host proteins alike, but also can be shed or secreted. We reported previously that soluble annexin A2 tetramer (A2t) activates human monocyte-derived macrophages (MDM), resulting in secretion of inflammatory mediators and enhanced phagocytosis. Although a receptor for A2t has been cloned from bone marrow stromal cells, data contained in this study demonstrate that it is dispensable for A2t-dependent activation of MDM. Furthermore, A2t activates wild-type murine bone marrow–derived macrophages, whereas macrophages from myeloid differentiation factor 88–deficient mice display a blunted response, suggesting a role for Toll-like receptor (TLR) signaling. Small interfering RNA knockdown of TLR4 in human MDM reduced the response to A2t, blocking antibodies against TLR4 (but not TLR2) blocked activation altogether, and bone marrow–derived macrophages from TLR4−/− mice were refractory to A2t. These data demonstrate that the modulation of macrophage function by A2t is mediated through TLR4, suggesting a previously unknown, but important role for this stress-sensitive protein in the detection of danger to the host, whether from injury or invasion.
Introduction
Annexins are calcium-dependent, anionic phospholipid-binding proteins, although most have important protein-binding partners as well.1,2 Like some other family members, annexin A2 is capable of forming a heterotetramer with a binding partner from the S100 family of phospholipid-binding proteins, most often S100A10. Annexin A2 tetramer (A2t) consists of 2 11-kD monomers of S100A10 (p11) that homodimerize, each making contact with both of the 36-kD annexin A2 (p36) monomers.3 The N terminus of annexin A2 contains the binding site for p11, which is in turn required for the targeting of A2t to the plasma membrane.4 Although other annexins are capable of forming heterotetramers with S100 family proteins,5 annexin A2 is unique in that a substantial subset of its functions requires tetramer formation.1,2,5 Although this may partially reflect the requirement for p11 association to target the plasma membrane, exogenously supplied p36 annexin A2 bypasses the need for externalization or secretion, but is often insufficient to rescue these functions.6,7
Although members of the annexin family are intracellular proteins with demonstrated roles in cytoplasmic membrane-associated processes, many perform well-documented extracellular functions,1 and several new reports delineate mechanisms of annexin secretion in the absence of a signal peptide.4,8-12 In a variety of settings, extracellular annexin A2 has been shown to be required for the initiation of inflammatory events that also require downstream nuclear factor (NF)-κB and/or mitogen-activated protein kinase (MAPK) activation. For example, annexin A2 found on the surface of endothelial cells is required for the activation of these cells by antiphospholipid antibodies that target the phospholipid-binding protein β2-glycoprotein I (β2GPI).13 It has been shown that β2GPI binds directly to annexin A2,14 that cross-linking of annexin A2 on the cell surface mimics this activation, and that monovalent F(ab′) fragments that block its availability prevent this activation from occurring.15 A2t has also been shown to be released from osteoclast-like cells, and acts as an autocrine/paracrine osteoclastogenic factor upon cells in bone marrow cultures,16 inducing MAPK and NF-κB signaling and inflammatory cytokine production.6 In a third example, endogenously produced17 or exogenously supplied18,19 plasmin induces the activation of monocytes and macrophages in a manner that requires the availability of annexin A2 on the monocyte/macrophage surface: blocking antibodies or small interfering RNAs (siRNAs) that target annexin A2 (or its binding partner S100A10) inhibit plasmin-dependent signaling.18,19 Finally, previous work from our laboratory demonstrated that exogenously supplied A2t directly activates human macrophages by inducing MAPK and NF-κB signaling and inflammatory cytokine and chemokine production.7
An A2t receptor (A2tR) shown to be involved in osteoclastogenesis has been cloned and confers A2t binding to transfected HEK293 cells.20 However, the predicted intracellular domain contains 4 amino acids, suggesting participation of a coreceptor(s). Plasmin and β2GPI are thought to signal through annexin A2 on the cell surface,15,19 although annexin A2 is a peripherally associated protein. Extracellular A2t plays a crucial role in several inflammatory cell activation decisions, but most likely requires other machinery to transmit these signals across the plasma membrane to activate NF-κB and the MAPK.
We previously reported that soluble A2t activates human monocyte-derived macrophages (MDMs), resulting in inflammatory cytokine secretion and an increase in bacterial phagocytic efficiency.7 Cloning of an A2tR from bone marrow stromal cells was recently reported and was shown to be required for nearly identical signaling and transcriptional events in those cells.20 We report that whereas the A2tR does not appear to play a role in A2t-dependent macrophage activation, Toll-like receptor (TLR) 4 is required for A2t-dependent inflammatory cytokine production by human and murine macrophages. Furthermore, A2t has different or additional requirements for TLR4 signaling compared with lipopolysaccharide (LPS), thus providing an opportunity for discrete regulation of TLR responses against host- and pathogen-derived signals.
Methods
Isolation and culture of human blood monocytes and culture of THP-1 cell line
Human peripheral blood monocytes were obtained from healthy volunteers by leukophoresis and further purified from mononuclear cells by Ficoll-Hypaque sedimentation, followed by countercurrent centrifugal elutriation, as described previously.21 Monocytes were plated in Dulbecco modified Eagle medium (DMEM; Invitrogen) containing 2mM l-glutamine and supplemented with 10 μg/mL gentamicin (Invitrogen) and at 6 × 106/2 mL/well in 6-well plates. After 4 to 6 hours, heat-inactivated fetal bovine serum (FBS; HyClone) was added to 10%, and monocytes were cultured for 7 days to enable differentiation into mature MDM.
THP-1 cells (a human acute monocytic leukemia cell line) were obtained from the ATCC, cultured in RPMI 1640 (Invitrogen) containing 2mM L-glutamine, and supplemented with 10% FBS, 1mM sodium pyruvate, and 10 μg/mL gentamicin. Cultures were maintained between 2 and 9 × 105 cells/mL, and were differentiated in the presence of 20nM phorbol 12-myristate 13-acetate (PMA; Sigma-Aldrich) at 6 × 106/2 mL/well in 6-well plates for 7 days.
Isolation and culture of murine bone marrow–derived macrophages
The generation and breeding of knockout mice used in this study have been reported previously.22,23 Age- and sex-matched C57BL/6J controls for the TLR4−/− mice were purchased from The Jackson Laboratory. Tibias and femurs from myeloid differentiation factor 88–deficient (MyD88−/−) mice were kindly provided by Dr Joao Pedras-Vasconcelos (Food and Drug Administration). Bone marrow–derived macrophages (BMDMs) were isolated according to a standard protocol.24 Macrophages at a density of 6 × 106/2 mL/well in 6-well plates were treated as indicated on the evening of the seventh day for 24 hours. All mouse experiments were approved by the Food and Drug Administration Institutional Animal Care and Use Committee.
Stimuli and blocking antibody reagents and conditions
Treatments were performed with Escherichia coli K12 LPS, S-(2,3-bispalmitoyloxypropyl)-N-palmitoyl-Cys-Lys-Lys-Lys-Lys (Pam3CSK4; both ultrapure preparations from InvivoGen), or annexin A2 tetramer (A2t) purified from bovine lung by an established protocol25 (Biodesign International) at indicated concentrations for indicated times. Rat polyclonal antibodies for neutralization of TLR2 and TLR4 (Invivogen) and rabbit anti–human A2tR20 (kindly provided by Dr G. David Roodman, University of Pittsburgh) were used as indicated.
RNA isolation and analysis with real-time polymerase chain reaction
Primer sequences, RNA isolation, reverse transcription, and real-time polymerase chain reaction conditions were described previously.7
Flow cytometry
For surface marker staining, cells were detached using Accutase (eBioscience), washed, and stained with the indicated concentration of rabbit anti–human A2tR, goat anti–human CD64 (Santa Cruz Biotechnology), or equal amounts of corresponding isotype control (Jackson ImmunoResearch Laboratories), then counterstained with a 1/200 dilution of Alexa 488 donkey anti–rabbit (for anti-A2tR) or Alexa 647 donkey anti–goat antibody (for CD64; both from Invitrogen) for 30 minutes at 4°C. Samples were resuspended in phosphate-buffered saline containing 1.0% paraformaldehyde, and analyzed on a FACSCalibur flow cytometer (BD Biosciences), using a gate set for single, intact macrophages.
For intracellular cytokine staining, cells were treated as indicated in the presence of a 1/500 dilution of GolgiPlug (containing brefeldin A; BD Biosciences), and then incubated at 37°C for the indicated time and detatched using Accutase. Intracellular staining was performed with the Cytofix/Cytoperm kit (BD Biosciences), as described in the protocol using phycoerythrin-conjugated anti–tumor necrosis factor α (TNF-α) (BD Biosciences) or isotype control.
Cytokine measurement by luminex assay
After indicated treatments, supernatants were clarified and diluted 10 to 20 times such that values obtained fit in the linear range of the standard curve for each cytokine reported. Assays were performed in duplicate using multiplex bead-based kits for the indicated human (BioSource International) or mouse (Millipore) cytokines, per manufacturers' instructions. Fluorescence of beads was measured using a Bioplex 200 analyzer (luminex; sold by Bio-Rad). Cytokine data analysis was performed using the BioPlex Manager software (Bio-Rad). Concentration was determined with a 5-parametric logistic nonlinear regression curve-fitting algorithm, and concentrations were entered into GraphPad Prism 5 software for plotting and statistical analysis.
Interferon regulatory factor 3 and NF-κB transcription factor enzyme-linked immunosorbent assays
TransAm p65, p52, and interferon regulatory factor 3 (IRF3) assays were performed per manufacturer's protocol (Active Motif), as described previously.7 To compare A2t- and LPS-induced signals of roughly equal magnitude, limited dose-response experiments were first performed with LPS and A2t to normalize for the amount of TNF-α RNA induced at 1 hour for these donors.
Transfection of HEK293 cells with plasmids that encode TLRs and coreceptors
HEK293 cells stably transfected with a NF-κB–driven green fluorescent protein (GFP) reporter construct (kindly provided by Dr Josh Leonard, National Cancer Institute, National Institutes of Health26 ) were grown in complete DMEM media (DMEM, 10% FBS, 1% penicillin/streptomycin, 1% l-glutamine, and 1% nonessential amino acids) and transiently transfected with plasmids encoding either TLR4 (pUNO-TLR4), TLR4 plus MD-2 (pDUO-hMD-2/TLR4a), or empty vector (pUNO; all vectors from InvivoGen) using a calcium phosphate transfection kit from Invitrogen. HEK293 NF-κB–GFP cells (106) were transfected in a 6-well plate format with 4 μg plasmid DNA per well. For the measurement of NF-κB activation, cells were stimulated 24 hours after transfection, as indicated, and harvested, and GFP fluorescence was measured by fluorescence-activated cell sorter (FACS).
Results
Inverse correlation between bone marrow stromal cell A2tR surface expression and monocyte/macrophage A2t responsiveness
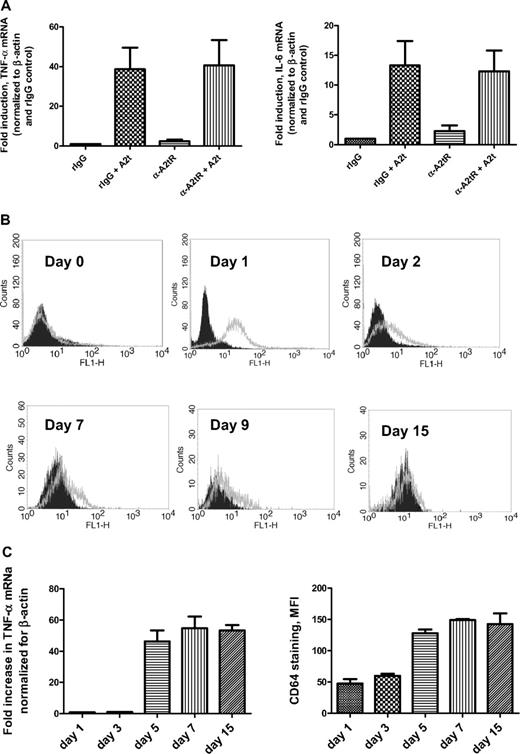
To determine whether the A2tR cloned from bone marrow stromal cells was also responsible for the activation of human macrophages by A2t, cells were treated with soluble A2t in the presence or absence of an antibody that had been demonstrated previously to block the binding site of the A2tR cloned from a bone marrow stromal cell line,20 and assessed for cytokine mRNA levels (Figure 1A). Although this antibody dramatically reduced osteoclast formation in the presence of A2t,20 pretreatment of macrophages with this blocking antibody failed to prevent inflammatory cytokine induction in human macrophages in response to A2t. Whereas both the anti-A2tR antibody and A2t were from the same sources as used in the previous study, anti-A2tR–blocking antibody had no significant effect on A2t-dependent macrophage activation between 0.1 μg/mL and 10 μg/mL (Figure 1A and data not shown), whereas 0.1 μg/mL was sufficient to significantly inhibit osteoclastogenesis.20 As mRNA expression for A2tR was previously found to be highest in monocytes, and decreased upon activation,20 it was possible that the A2tR recognized by this antibody was absent on the macrophage surface. Surface expression of this protein during monocyte-to-macrophage differentiation was characterized over a 15-day period (Figure 1B). Although no A2tR was observed on the surface of freshly elutriated monocytes in suspension, 1 day of adherence resulted in high surface expression as detected by the blocking antibody used above (Figure 1B). However, in agreement with the RNA data, it dropped precipitously during differentiation, and by day 7, little staining could be detected on the surface of mature macrophages from several donors.
Blocking antibody for A2tR fails to reduce A2t-dependent cytokine induction in human macrophages. (A) Seven-day-old macrophages were pretreated with either anti-A2tR–blocking antibody or rabbit polyclonal isotype control (10 μg/mL for 20 minutes at room temperature), followed by treatment with 2nM A2t for 1 hour at 37°C. RNA was isolated and reverse transcribed, and levels of the indicated species were analyzed by real-time polymerase chain reaction. (B) Monocytes were plated and aliquots were stained for A2tR expression at indicated times throughout macrophage differentiation. Histograms shown are representative of 4 donors. Black (filled) histograms = rabbit IgG isotype control, gray (open) = anti-A2tR–blocking antibody (both used at 1 μg/mL, then stained with 1:200 Alexa 488 donkey anti–rabbit). (C) Monocytes/macrophages were treated with 5nM A2t for 1 hour at 37°C at the indicated times (days after plating) during differentiation. mRNA levels were analyzed as in (B). Macrophages from the same donors were stained with goat anti–human CD64 antibody, followed by Alexa 488 donkey anti–goat secondary (values for isotype controls subtracted) at the same times to monitor macrophage differentiation. Results (A,C) are the mean ± SEM of 3 experiments with separate donors.
Blocking antibody for A2tR fails to reduce A2t-dependent cytokine induction in human macrophages. (A) Seven-day-old macrophages were pretreated with either anti-A2tR–blocking antibody or rabbit polyclonal isotype control (10 μg/mL for 20 minutes at room temperature), followed by treatment with 2nM A2t for 1 hour at 37°C. RNA was isolated and reverse transcribed, and levels of the indicated species were analyzed by real-time polymerase chain reaction. (B) Monocytes were plated and aliquots were stained for A2tR expression at indicated times throughout macrophage differentiation. Histograms shown are representative of 4 donors. Black (filled) histograms = rabbit IgG isotype control, gray (open) = anti-A2tR–blocking antibody (both used at 1 μg/mL, then stained with 1:200 Alexa 488 donkey anti–rabbit). (C) Monocytes/macrophages were treated with 5nM A2t for 1 hour at 37°C at the indicated times (days after plating) during differentiation. mRNA levels were analyzed as in (B). Macrophages from the same donors were stained with goat anti–human CD64 antibody, followed by Alexa 488 donkey anti–goat secondary (values for isotype controls subtracted) at the same times to monitor macrophage differentiation. Results (A,C) are the mean ± SEM of 3 experiments with separate donors.
As surface expression of A2tR is highest on 1-day-old monocytes (Figure 1B), they might be expected to be far more sensitive to A2t, and thus, the A2tR-blocking antibody, than macrophages. To this end, monocytes that had been plated for 24 hours were treated with A2t in the absence and presence of the A2tR-blocking antibody. Surprisingly, however, monocytes from 3 separate normal donors were refractory to soluble A2t (Figure 1C), although the presence of A2tR was simultaneously confirmed in these experiments by FACS (Figure 1B). Furthermore, treatment with A2t at distinct points along the differentiation pathway demonstrates that monocytes acquire sensitivity to A2t gradually, concomitant to surface expression changes consistent with macrophage differentiation (Figure 1C). Taken together, the inverse correlation of A2tR expression and A2t responsiveness, combined with the inability of the blocking antibody to prevent A2t-dependent cytokine production in macrophages, suggest that the A2tR derived from bone marrow is not involved in the activation of mature human MDM by A2t and imply the involvement of another receptor for A2t in these cells.
A2t-dependent macrophage activation attenuates their responsiveness to LPS
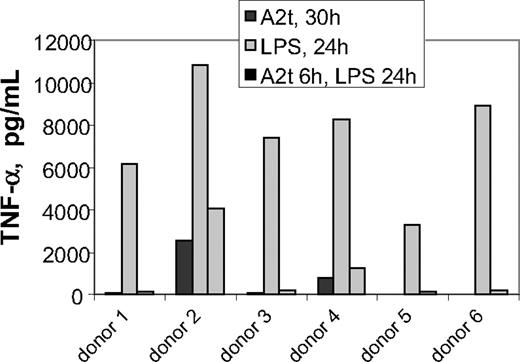
A2t-dependent macrophage activation has been demonstrated to occur via the MAPK and NF-κB pathways,7 which are activated by many different macrophage activation pathways. A valuable clue concerning potential receptors for A2t in this paradigm was provided by a set of studies in which macrophages were treated with A2t combinatorially with other macrophage stimuli in an effort to identify its possible roles in modulation of macrophage function during an immune response. It was observed that if A2t treatment of macrophages preceded LPS stimulation by 5 to 8 hours, TNF-α secretion was greatly reduced in most donors (Figure 2). This dramatic effect was readily observable because TNF-α induction in human MDM by 2nM A2t peaks early and is often unmeasurable at 24 hours.7 This phenomenon was highly reminiscent of endotoxin-induced tolerance,27 in which prior exposure to LPS can strongly attenuate certain subsequent cellular responses in response to higher doses of LPS after a period of time. The A2t used in these studies was not produced in E coli, but rather, purified from bovine lung by an established, multistep protocol.25 Furthermore, by the Limulus amebocyte lysate method, the A2t stock was found to contain less than 0.01 EU/mL. As a 1/5000 dilution of A2t was used in this experiment, the attribution of these effects to contaminating endotoxin is highly unlikely (refer also to Figure 6). Another possibility was that A2t itself invokes this tolerance by activating a common receptor pathway. LPS signals through TLR4, a partially MyD88-dependent receptor. Interestingly, antiphospholipid antibodies that react with β2GPI activate endothelium via surface annexin A215 through a pathway that is dependent upon MyD88.28
A2t attenuates macrophage TNF-α production in response to LPS. Macrophages were treated with 10 ng/mL LPS for 24 hours, with or without a 6-hour pretreatment with 2nM A2t. Controls were treated with 2nM A2t alone for 30 hours. Supernatants were collected at 24 hours after LPS treatment, and TNF-α was measured by luminex.
A2t attenuates macrophage TNF-α production in response to LPS. Macrophages were treated with 10 ng/mL LPS for 24 hours, with or without a 6-hour pretreatment with 2nM A2t. Controls were treated with 2nM A2t alone for 30 hours. Supernatants were collected at 24 hours after LPS treatment, and TNF-α was measured by luminex.
A2t stimulation of macrophages is partially MyD88-dependent
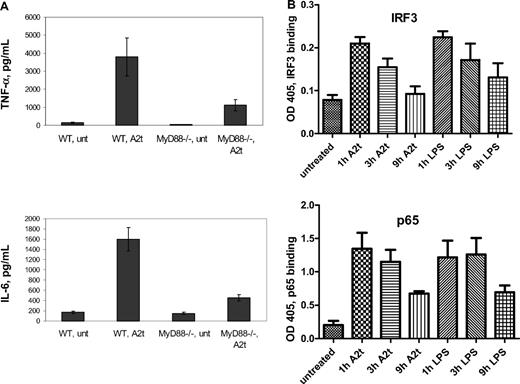
As TLR agonists have been shown to be capable of inducing tolerance not only to their cognate receptor, but also to other TLRs that share the same downstream signaling components (most notably, MyD8829 ), we next sought to determine whether A2t-dependent macrophage activation is MyD88 dependent. To this end, and to test the evolutionary conservation of this mechanism, cells derived from the bone marrow of both wild-type (WT) and MyD88−/− mice were treated with A2t, in addition to the TLR4 or TLR1/2 agonists, LPS and Pam3CSK4, respectively. WT BMDM were found to be responsive to A2t in much the same manner as primary human monocyte-derived macrophages (Figure 3A and Table 17 ), with the secretion of inflammatory cytokines and chemokines such as TNF-α, interleukin-6 (IL-6), IL-1α, and interferon-γ–inducible protein 10. This is perhaps not surprising: bovine annexin A2 is approximately 98% identical and 99% similar to both mouse and human sequences at the protein level, and S100A10 is 99% and 100% similar, respectively. However, BMDM from the MyD88-deficient mice displayed reduced secretion of TNF-α, IL-6, and IL-1α in response to A2t, although some chemokines (ie, those that are dependent upon MyD88-independent signaling, eg, interferon-γ–inducible protein 10 and regulated on activation normal T cell expressed and secreted; Table 1) were relatively unaffected. This was very similar to the pattern of inflammatory cytokine and chemokine reduction in MyD88−/− BMDM after LPS treatment compared with the WT LPS response (Table 1).
Macrophage activation in response to A2t involves both MyD88 and TRIF. (A) WT or MyD88−/− 7-day-old murine BMDM were treated with 5nM A2t for 24 hours, and levels of the indicated cytokines (in pg/mL) were measured in the supernatants by luminex. Macrophages were pooled from 4 males per genotype in each of 2 separate experiments run in duplicate. Results expressed as the mean ± SEM. (B) Nuclear extracts of 7-day-old human MDM treated with either LPS (2 ng/mL) or A2t (5nM) were tested for p65 or IRF3 activity by binding of plated DNA oligos bearing the specific NF-κB or IRF3 consensus site, followed by colorimetric detection of p65 or IRF3 by horseradish peroxidase–linked antibody.
Macrophage activation in response to A2t involves both MyD88 and TRIF. (A) WT or MyD88−/− 7-day-old murine BMDM were treated with 5nM A2t for 24 hours, and levels of the indicated cytokines (in pg/mL) were measured in the supernatants by luminex. Macrophages were pooled from 4 males per genotype in each of 2 separate experiments run in duplicate. Results expressed as the mean ± SEM. (B) Nuclear extracts of 7-day-old human MDM treated with either LPS (2 ng/mL) or A2t (5nM) were tested for p65 or IRF3 activity by binding of plated DNA oligos bearing the specific NF-κB or IRF3 consensus site, followed by colorimetric detection of p65 or IRF3 by horseradish peroxidase–linked antibody.
A2t-induced cytokine production by WT and MyD88-deficient BMDM
| . | WT, A2t . | MyD88−/−, A2t . | WT, LPS . | MyD88−/−, LPS . |
|---|---|---|---|---|
| IL-1α | 140 | 111 | 470 | 226 |
| IL-6 | 1418 | 402 | 20 189 | 7431 |
| TNF-α | 3874 | 1281 | 6253 | 2118 |
| MCP1 | 13 148 | 54 | 11 602 | 1285 |
| MIP-1α | 1982 | 76 | 8956 | 1301 |
| IP10 | 25 240 | 21 860 | 20 240 | 19 774 |
| RANTES | 12 540 | 8187 | 12 346 | 12 265 |
| . | WT, A2t . | MyD88−/−, A2t . | WT, LPS . | MyD88−/−, LPS . |
|---|---|---|---|---|
| IL-1α | 140 | 111 | 470 | 226 |
| IL-6 | 1418 | 402 | 20 189 | 7431 |
| TNF-α | 3874 | 1281 | 6253 | 2118 |
| MCP1 | 13 148 | 54 | 11 602 | 1285 |
| MIP-1α | 1982 | 76 | 8956 | 1301 |
| IP10 | 25 240 | 21 860 | 20 240 | 19 774 |
| RANTES | 12 540 | 8187 | 12 346 | 12 265 |
WT or MyD88−/− 7-day-old murine BMDM were treated with 5nM A2t or 10 ng/mL LPS for 24 hours, and levels of the indicated cytokines (in pg/mL) were measured in the supernatants by luminex. Macrophages were pooled from 3 males per genotype; supernatants were assayed in duplicate.
MCP1 indicates monocyte chemoattractant protein 1; MIP-1α, macrophage-inflammatory protein-1α; IP10, interferon-γ–inducible protein 10; and RANTES, regulated on activation normal T cell expressed and secreted.
As TLR4 is the only member of the TLR family that uses both MyD88-dependent and MyD88-independent pathways for signaling, it was of interest to determine whether A2t activates the second pathway of the TLR4 receptor complex, through TIR-domain containing adapter-inducing interferon β (TRIF) adaptor protein and the IRF3 transcription factor. To this end, nuclear lysates from A2t-treated human MDM were examined for IRF3 activation (Figure 3B). This was complemented by analysis of NF-κB activation from the same lysates, and comparison with LPS-stimulated nuclei, to examine the relative contributions of TRIF-dependent (IRF3) and MyD88-dependent (NF-κB) pathways. Under these conditions, comparison of nuclear lysates from A2t-treated cells with LPS-treated human MDM demonstrates similar activation of both IRF3 and p65 NF-κB (as well as p52 NF-κB; data not shown) by these 2 stimuli.
Decreased TLR4 expression renders macrophages less responsive to A2t
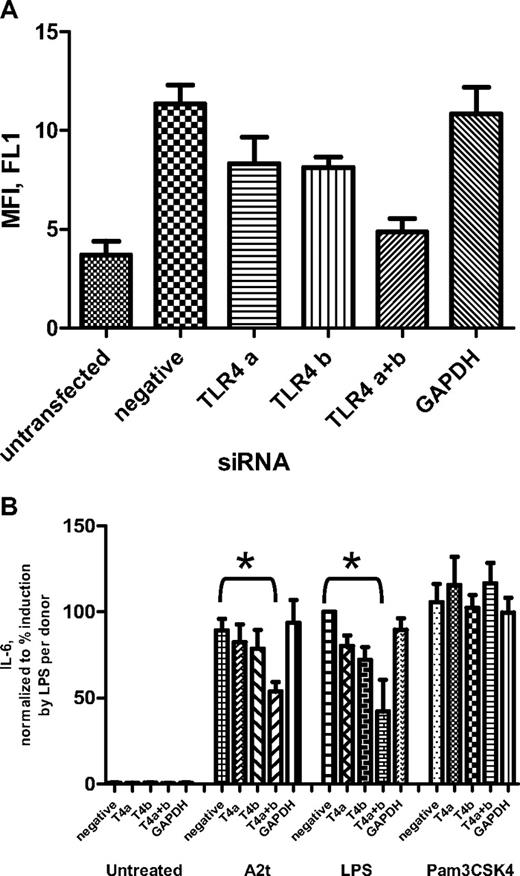
As the above data highly implicate TLR4 involvement in A2-dependent macrophage activation, TLR4-specific siRNAs were used to reduce its expression on the surface of human macrophages to test its effect on their A2t responsiveness. Transfection with siRNAs for TLR4 significantly decreased its surface expression on human macrophages (Figure 4A) and markedly reduced cytokine responses to LPS, a TLR4 ligand, but not Pam3CSK4, a TLR2 ligand, as measured 24 hours after treatment (Figure 4B). Their response to A2t was also diminished by siRNAs targeting TLR4, but not by those targeting an unrelated gene (glyceraldehyde-3-phosphate dehydrogenase) or a negative control sequence (Figure 4B).
Reduction of TLR4 on the macrophage surface inhibits A2t-driven cytokine production. (A) Seven-day macrophages from 3 donors were transfected with small interfering RNAs: 2 separate sequences specific for TLR4 were used, either alone or in combination (“TLR4 a” and “TLR4 b”), as well as negative control siRNA, and an siRNA for an unrelated gene (glyceraldehyde-3-phosphate dehydrogenase). Three days after transfection, cells were stained with fluorescein isothiocyanate–labeled mouse anti–human TLR4 antibody, and surface expression of TLR4 was analyzed by FACS. Results represent the mean ± SEM(after subtraction of fluorescence from the isotype control) of 3 experiments with separate donors. (B) Macrophages (transfected as in panel A) were treated 2 days posttransfectionwith A2t (5nM), LPS (10 ng/mL), or Pam3CSK4 (100 ng/mL) for 24 hours, and concentration of IL-6 was measured in the supernatants by luminex and expressed as the percentage of the value of IL-6 induced by LPS for that donor. Results are the mean ± SEM; *P < .05.
Reduction of TLR4 on the macrophage surface inhibits A2t-driven cytokine production. (A) Seven-day macrophages from 3 donors were transfected with small interfering RNAs: 2 separate sequences specific for TLR4 were used, either alone or in combination (“TLR4 a” and “TLR4 b”), as well as negative control siRNA, and an siRNA for an unrelated gene (glyceraldehyde-3-phosphate dehydrogenase). Three days after transfection, cells were stained with fluorescein isothiocyanate–labeled mouse anti–human TLR4 antibody, and surface expression of TLR4 was analyzed by FACS. Results represent the mean ± SEM(after subtraction of fluorescence from the isotype control) of 3 experiments with separate donors. (B) Macrophages (transfected as in panel A) were treated 2 days posttransfectionwith A2t (5nM), LPS (10 ng/mL), or Pam3CSK4 (100 ng/mL) for 24 hours, and concentration of IL-6 was measured in the supernatants by luminex and expressed as the percentage of the value of IL-6 induced by LPS for that donor. Results are the mean ± SEM; *P < .05.
Next, we confirmed the above results using independent methods to more thoroughly block or remove TLR4 availability and signaling. Blocking antibodies to either the TLR2 or TLR4 receptor were tested for their ability to prevent cytokine production in human MDM in response to A2t. TLR2-blocking antibody was used primarily as a negative control, as macrophages express TLR2 and are sensitive to the TLR2/1 heterodimer ligand, Pam3CSK4. However, as some endogenous TLR ligands have been reported to evoke a TLR2-dependent signal,30 the possibility existed that A2t could signal through TLR2 as well. The polyclonal antibody that antagonizes TLR4 completely abrogated TNF-α production in response to A2t, but the blocking antibody against TLR2 had no effect (Figure 5A-B). Specificity of the anti-TLR4 antibody was demonstrated by its lack of inhibition on Pam3CSK4 signaling (Figure 5A), whereas the efficacy of the TLR2-blocking antibody was confirmed by its ability to block this same TLR2-dependent signal (Figure 5A).
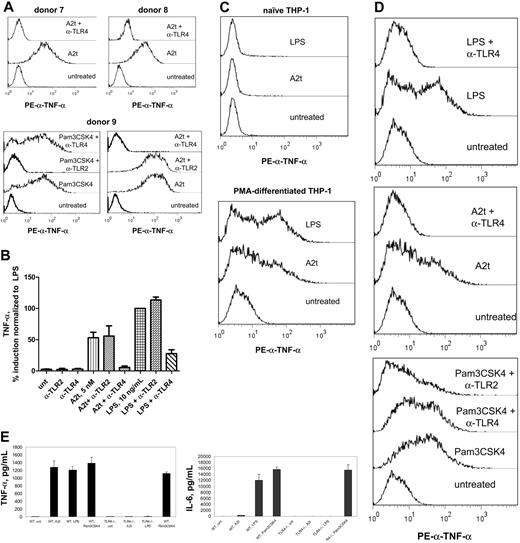
TLR4-blocking antibodies inhibit macrophage production of TNF-α in response to A2t and LPS, but not Pam3CSK4, a TLR2 ligand. Intracellular TNF-α staining of 7-day-old MDM after treatment with indicated stimuli for 6 hours in the presence of brefeldin A (A), or cytokine levels measured by luminex from 24 hours of culture with the same treatments in the absence of brefeldin A (B). Where indicated, cells were pretreated (15 minutes) with 10 μg/mL designated blocking antibody before stimulus addition. (C) Intracellular TNF-α staining of naive (left) or differentiated (right; 7 days with 15nM PMA) THP-1 cells after 6-hour incubation with indicated stimuli. (D) Seven-day PMA-differentiated THP-1 cells were treated with the indicated stimuli and blocking antibodies and stained for intracellular TNF-α, as in panel A. (E) WT or TLR-deficient (TLR4−/−) 7-day-old murine BMDM were treated with 5nM A2t, 10 ng/mL LPS, or 100 ng/mL Pam3CSK4 for 24 hours, and levels of the indicated cytokines (in pg/mL) were measured in the supernatants by luminex. Macrophages were pooled from 5 females per genotype; results are the mean ± SEM of 2 experiments performed in duplicate.
TLR4-blocking antibodies inhibit macrophage production of TNF-α in response to A2t and LPS, but not Pam3CSK4, a TLR2 ligand. Intracellular TNF-α staining of 7-day-old MDM after treatment with indicated stimuli for 6 hours in the presence of brefeldin A (A), or cytokine levels measured by luminex from 24 hours of culture with the same treatments in the absence of brefeldin A (B). Where indicated, cells were pretreated (15 minutes) with 10 μg/mL designated blocking antibody before stimulus addition. (C) Intracellular TNF-α staining of naive (left) or differentiated (right; 7 days with 15nM PMA) THP-1 cells after 6-hour incubation with indicated stimuli. (D) Seven-day PMA-differentiated THP-1 cells were treated with the indicated stimuli and blocking antibodies and stained for intracellular TNF-α, as in panel A. (E) WT or TLR-deficient (TLR4−/−) 7-day-old murine BMDM were treated with 5nM A2t, 10 ng/mL LPS, or 100 ng/mL Pam3CSK4 for 24 hours, and levels of the indicated cytokines (in pg/mL) were measured in the supernatants by luminex. Macrophages were pooled from 5 females per genotype; results are the mean ± SEM of 2 experiments performed in duplicate.
It was of interest to find a human-derived monocytic cell line that was A2t responsive in which to confirm these results as well as establish a reliable source of cells for future studies. To this end, THP-1 cells, derived from a human acute monocytic leukemia, were obtained from ATCC and tested for their ability to respond to A2t. Although unstimulated THP-1 cells were found to be unresponsive to A2t, differentiation with PMA for 7 days conferred A2t sensitivity upon THP-1 cells (Figure 5C). As this differential responsiveness of undifferentiated versus differentiated or activated THP-1 cells mirrors that seen in primary human monocytes versus monocyte-derived macrophages, these cells provide a reasonable surrogate for both. Furthermore, it was found that antagonism of TLR4 prevented A2t-dependent cytokine production in THP-1 cells, whereas blocking of TLR2 did not (Figure 5D).
Finally, inflammatory cytokine production by murine BMDM from TLR4-deficient animals was measured and compared with WT macrophages from the same background strain. The data demonstrate that BMDM from TLR4-deficient animals are completely unresponsive to A2t (Figure 5E and Table 2). Taken collectively, these data demonstrate that TLR4 is an absolute requirement for the ability of A2t to elicit an inflammatory response either from human monocyte-derived macrophages or from murine BMDM.
A2t-induced cytokine production by WT and TLR4-deficient BMDM
| . | WT, A2 . | TLR4−/−, A2t . | WT, LPS . | TLR4−/−, LPS . |
|---|---|---|---|---|
| IL-1α | 12 | <LL | 43 | <LL |
| IL-6 | 302 | <LL | 6012 | <LL |
| TNF-α | 1154 | 12 | 1312 | 9 |
| MCP1 | 653 | 78 | 1823 | 180 |
| MIP-1α | 84 | 50 | 260 | 50 |
| IP10 | 18 735 | 693 | 17 826 | 402 |
| RANTES | 805 | 12 | 1763 | 6 |
| . | WT, A2 . | TLR4−/−, A2t . | WT, LPS . | TLR4−/−, LPS . |
|---|---|---|---|---|
| IL-1α | 12 | <LL | 43 | <LL |
| IL-6 | 302 | <LL | 6012 | <LL |
| TNF-α | 1154 | 12 | 1312 | 9 |
| MCP1 | 653 | 78 | 1823 | 180 |
| MIP-1α | 84 | 50 | 260 | 50 |
| IP10 | 18 735 | 693 | 17 826 | 402 |
| RANTES | 805 | 12 | 1763 | 6 |
WT or TLR-deficient (TLR4−/−) 7-day-old murine BMDM were treated with 5nM A2t or 10 ng/mL LPS for 24 hours, and levels of the indicated cytokines (in pg/mL) were measured in the supernatants by luminex. Macrophages were pooled from 5 females per genotype; supernatants were assayed in duplicate.
LL indicates lower limit of quantitation; other abbreviations as in Table 1.
TLR4 is necessary but not sufficient for A2t-dependent inflammatory events
The data presented demonstrate that A2t uses the TLR4 receptor in both human and murine macrophage cell types, but they do not demonstrate sufficiency of TLR4 and its canonical coreceptors MD-2 and CD14, for recognition of the A2t ligand. To the contrary, cells known to be responsive to LPS displayed no measurable reaction to soluble A2t (Figure 1B and data not shown), suggesting distinct requirements for responses to these 2 different ligands. To investigate this further, responsiveness to A2t or LPS was examined during the process of differentiation from human monocyte to macrophage. Although human monocyte responses to LPS are low compared with differentiated MDM, by day 3, they have acquired increased LPS sensitivity, whereas they are completely unresponsive to A2t (Figure 6A). This effect was mirrored during the PMA-induced differentiation of monocyte-like THP-1 cells into a more adherent, macrophage-like state (Figure 6B). These phenomena were dissected in a more controlled manner with the use of HEK293 cells stably transfected with a NF-κB–responsive GFP expression plasmid,26 as macrophage activation by A2t is known to be NF-κB dependent.7 Although HEK293 cells express the intracellular signaling components to respond to the TLR family of receptors, they do not express appreciable levels of the TLRs themselves, and transfection of HEK293 cells with TLR4 alone does not confer responsiveness to LPS or A2t (Figure 6C). Transfection with both TLR4 and its coreceptor, MD-2, renders these cells responsive to LPS, as evidenced by the expression of GFP measured 24 hours after treatment (Figure 6C). However, TLR4 and MD-2 (and soluble CD14, which is supplied by the serum in the media) are not sufficient to confer A2t sensitivity to these cells, even at 20-fold the highest concentration used in these studies (100nM vs 5nM), and more than 200-fold the A2t concentration needed to activate human macrophages.7 Taken together with the differential responsiveness of human monocytes and early macrophages to LPS and A2t, the data point to the existence of another factor involved in A2t-dependent signaling through TLR4, one that is acquired relatively late in in vitro MDM differentiation.
TLR4 is necessary, but not sufficient to confer A2t responsiveness. (A-B) Intracellular TNF-α staining of 3-day-old human monocytes (A) or THP-1 cells differentiated with 15nM PMA for 3 days (B) after 6-hour treatment with indicated stimuli. (C) HEK293 cells stably transfected with a NF-κB–driven GFP reporter plasmid were transiently transfected with plasmids containing no insert (mock), or encoding TLR4 or TLR4 along with MD-2, then treated with the indicated stimuli (A2t = 100nM, LPS = 100 ng/mL, PMA = 5mM), and GFP fluorescence was measured by FACS 24 hours after treatment. Data are expressed as percentage of GFP induction compared with the PMA-induced fluorescence for the corresponding transfection and experiment. Results are the mean ± SEM of 3 experiments.
TLR4 is necessary, but not sufficient to confer A2t responsiveness. (A-B) Intracellular TNF-α staining of 3-day-old human monocytes (A) or THP-1 cells differentiated with 15nM PMA for 3 days (B) after 6-hour treatment with indicated stimuli. (C) HEK293 cells stably transfected with a NF-κB–driven GFP reporter plasmid were transiently transfected with plasmids containing no insert (mock), or encoding TLR4 or TLR4 along with MD-2, then treated with the indicated stimuli (A2t = 100nM, LPS = 100 ng/mL, PMA = 5mM), and GFP fluorescence was measured by FACS 24 hours after treatment. Data are expressed as percentage of GFP induction compared with the PMA-induced fluorescence for the corresponding transfection and experiment. Results are the mean ± SEM of 3 experiments.
Discussion
Although both infection and injury precipitate an immediate inflammatory reaction, the realization that the innate immune system responds to these 2 separate threats with common molecular machinery is relatively new. The TLRs have been demonstrated to provide a basis for this shared mechanism.31 Initially thought to function exclusively in early recognition of pathogenic invaders, it is now clear that some TLRs also respond to molecules that are host derived. To date, several endogenous ligands have been identified in mammals,32 many of which may require alteration of their structure or localization to induce TLR signaling. For example, glycosaminoglycan hyaluronate is normally present as an inert polymer. Upon injury, activated proteases degrade it into smaller fragments that trigger inflammation through activation of TLRs 2 and 4, promoting tissue repair.31 Recent findings suggest that activation or availability of these endogenous ligands is brought about not only by outright damage, but conditions of stress or tissue malfunction as well.31 One such class of endogenous activators of the innate immune system has been termed “alarmins” for their rapid secretion in response to danger, combined with their ability to recruit and activate antigen-presenting cells.33 Although A2t may not strictly belong to this class, as it has not been found to be chemotactic for monocytes,7 it rapidly induces chemokines and may prove to be directly chemotactic to other cell populations.
The finding that A2t activates macrophages through TLR4 is especially intriguing given the dramatic and well-documented modulations of several annexins, including annexin A2, in response to stress and damage.1 If endogenous proteins that induce inflammation are predominantly activated by desequestration,31 annexin A2, either alone or in complex with S100A10, could easily serve as this type of sentinel: induction or redistribution of annexin A2 has been demonstrated in response to heat shock,4 hypoxia,34 changes in cellular redox state,35 mechanical stress,1 and mild osmotic shock.36 It has recently become clear that the externalization of phosphatidylserine (a molecule to which all annexins bind at the plasma membrane) is not only a hallmark of apoptosis, but also is a common and reversible sign of activation or stress on many cell types.37
Although the mechanism whereby annexin A2 is externalized and/or secreted has long been a mystery, recent reports have provided some potential answers.4,8-12 Perhaps the most striking, in light of the above findings, is that secretion of annexin A2 and S100A10 from macrophages and keratinocytes was found to be highly dependent upon the noncanonical, caspase-1 (and thus, inflammasome)–dependent secretory pathway.38 As this requires adenosine triphosphate, it cannot simply be an artifact of cell death. The proteins whose secretion proved most dependent upon this pathway were found to be involved in inflammation or tissue repair, although the authors made mention of the lack of an identified extracellular role for annexin A2. The data reported above suggest that A2t release by a cell, by any of the potential mechanisms,4,8-12 may provide a juxtacrine or localized signal for neighboring cells bearing both TLR4 and the necessary cofactors, as the annexins are known to have high affinities for membrane phospholipids and may only experience limited diffusion. However, as some mechanisms of annexin A2 release result from cleavage events that reduce affinity for membrane phospholipids4,8-12 and detectable levels of annexin A2 in the plasma have been reported,1 the spatial constraints of this signal warrant further investigation. The concentration of A2t used in these studies reflects a value near the middle of the dose-response curve for most donors7 and is orders of magnitude lower than that which is secreted by macrophages in the 24 hours after exposure to certain stimuli (J.F.A.S., unpublished data, December 2009).
Indeed, annexin A2 externalization and secretion by macrophages have likewise been found to be augmented in response to multiple bacterial and viral pathogenic motifs and conditions of stress (J.F.A.S., unpublished data, December 2009), and its proinflammatory effects on multiple cell types have now been well documented.6,7,18,19 It may be that TLR4 is involved, either directly or cooperatively, in all of these paradigms. For example, it has been observed that plasmin-dependent monocyte activation through annexin A2 is sensitive to pertussis toxin,39 yet no calcium flux has been demonstrated (T. Simmet, oral communication, October 2007). Activation of macrophages by A2t is similarly inhibitable by pertussis toxin in the absence of an observable calcium flux, in monocytes or macrophages that demonstrate robust calcium mobilization in response to f-Met-Leu-Phe or chemokine stimulation (J.F.A.S., unpublished results, January 2008). These seemingly contradictory qualities are consistent with the involvement of TLR4, which is sensitive to pertussis toxin due to signaling requirements for the Gαi subunit,40 but does not induce gross mobilization of calcium.41 Another case for TLR4 involvement in annexin A2-dependent signaling has been made in numerous studies concerning the events leading to antiphospholipid syndrome, in which a requirement for surface annexin A2 has been demonstrated in the endothelial activation brought about by antibodies recognizing phospholipid-binding protein β2GPI.15 Evidence for participation of the TLR-adapter protein MyD88 in this phenomenon was supplied by the reduced ability of an endothelial cell line, HMEC-1, to respond to anti-β2GPI antibodies when cells were transfected with dominant-negative MyD88 and TNF receptor-associated factor 6 constructs.28 In vivo evidence for the involvement of TLR4 was provided by a study in which C3H/HeJ mice, which express a missense mutation in the Tlr4 gene, demonstrated reduced thrombus formation in response to treatment with anti-β2GPI antibodies from lupus antiphospholipid syndrome patients.42 Recently in this journal, these results were validated by a report in which the same reduction in thrombus size in response to these antibodies was also demonstrated in annexin A2−/− mice.43 In addition to its interaction with annexin A2,14 β2GPI has also been recently reported to interact with TLR4.44 In our studies, exogenous human β2GPI was not sufficient to confer A2t sensitivity to immature monocytic cells, suggesting that β2GPI alone does not represent the coreceptor or cofactor that lends A2t responsiveness to macrophages (data not shown), although A2t was able to augment monocyte activation by β2GPI/anti-β2GPI antibodies, confirming the well-established role of annexin A2 in that paradigm.13
The identity of the additional factor(s) necessary to confer A2t sensitivity to TLR4-bearing cells remains a mystery. Although MD-2, CD14, and even β2GPI may all be necessary, the presence of all 3 still does not allow for A2t sensitivity on immature monocytic cells. In addition, according to our experiments, the A2tR protein is neither necessary nor sufficient for macrophage responsiveness to A2t. Although this novel protein is clearly important for A2t-dependent events in the bone marrow environment,45 it may be that it works in concert with other molecules that are absent on monocytes that have entered the circulation. Alternatively, immature monocytic cells that lack A2t responsiveness in vitro may express inhibitory molecules required for keeping signaling quiet under normal circumstances from either A2tR or TLR4, and in vitro culture and differentiation of human MDM may lead to a scenario that is artificially permissible, whereas in vivo, TLR4 would only respond to A2t in situations of stress, injury, or invasion. For instance, protease activation might be required for A2t activation of TLR4, as is the case for creation of TLR4-reactive epitopes from other host proteins.31 This is particularly attractive in the case of A2t, as it is already known to be pivotal in the activation of plasmin46 and is also known to be a substrate of protease cleavage.12,19
Although initial investigations into the usage of TRIF- versus MyD88-mediated pathways (Figure 3B), or the noncanonical pathway of NF-κB (data not shown) reveal no significant differences between LPS- and A2t-induced activation of TLR4, distinctions in some of the downstream transcriptome and proteome changes in response to these different stimuli have been identified and will be more closely examined. Whether they reflect broader distinctions between reactions to endogenous versus exogenous TLR4 ligands remains to be seen. To date, simultaneous addition of these 2 agonists has failed to show reproducible synergistic or interfering effects, although these investigations are ongoing as well.
Just as it remains unclear whether the TLR family first evolved to recognize damaged self or nonself, the responsiveness of A2t to both pathogenic motifs and cellular stress and injury provides little clue as to which will prove more physiologically relevant. By either activating or inducing tolerance in TLR4, A2t may play important roles in modulation of inflammatory events that contribute either to maintenance or disease.31 For example, annexin A2 and its S100-binding partners are known to be overexpressed and highly implicated in the development of more aggressive cancers;47,48 in addition to (or perhaps as a result of) their role in activating plasmin, it may be that TLR4 activation by these proteins plays a role in the carcinogenesis and/or metastasis for which the importance of the TLRs is now becoming clear,49 but the ligands are yet unknown. It may be that deficiencies in annexin A2−/− mice with regard to immune competency or stress responses will only be uncovered using particular conditions of stress or immune challenge, as is often the case. Alternatively, such a defect could be obscured by redundancy. It is interesting to note that levels of other annexins, especially annexin A2, are increased in annexin A1−/− mice,50 suggesting coregulation of some of these family members. Indeed, the role of other annexins in regard to danger signaling warrants further investigation. The discovery that A2t activation of macrophages requires TLR4 sheds new light on the significance of annexin externalization and secretion, the possibility of A2t responsiveness in other cell types, and the potential cooperation of these players in homeostasis and disease.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Dr G. David Roodman for the α-A2tR antibody, Dr Joao Pedras-Vasconcelos for animal tissues, Dr Josh Leonard for NF-κB–driven GFP-transfected HEK293 cells, and Drs Wendy Weinberg and Francisco Borrego for critical evaluation of this manuscript.
This work was supported in part by National Institutes of Health grant AI-18797 (S.N.V.).
National Institutes of Health
Authorship
Contribution: J.F.A.S., N.B., and S.B. performed experiments; J.F.A.S. designed experiments, analyzed results, wrote the manuscript, and made the figures; and G.M.F. and S.N.V. helped design experiments and write the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Gerald M. Feldman, Laboratory of Molecular and Developmental Immunology, Division of Monoclonal Antibodies, Office of Biotechnology Products, Center for Drug Evaluation and Research, Food and Drug Administration, 29 Lincoln Dr, Bldg 29A, Rm 3C24, HFD-123, Bethesda, MD 20892; e-mail: gerald.feldman@fda.hhs.gov.