Abstract
Proper thymocyte development is required to establish T-cell central tolerance and to generate naive T cells, both of which are essential for T-cell homeostasis and a functional immune system. Here we demonstrate that the loss of transcription factor Foxp1 results in the abnormal development of T cells. Instead of generating naive T cells, Foxp1-deficient single-positive thymocytes acquire an activated phenotype prematurely in the thymus and lead to the generation of peripheral CD4+ T and CD8+ T cells that exhibit an activated phenotype and increased apoptosis and readily produce cytokines upon T-cell receptor engagement. These results identify Foxp1 as an essential transcriptional regulator for thymocyte development and the generation of quiescent naive T cells.
Introduction
In the thymus, by recognizing major histocompatibility complex (MHC)–self peptide complexes, CD4+CD8+ double-positive (DP) thymocytes with functional T-cell receptors (TCRs) will undergo positive and negative selection and differentiate into CD4 single-positive (SP) or CD8 SP thymocytes.1,2 SP thymocytes egress from the thymus and become mature naive T cells in the periphery. Both SP thymocytes and naive T cells are considered quiescent cells without effector functions.3 T-lymphocyte quiescence was thought to be due to the lack of activation signals. Recent advances, however, have shown that T-cell quiescence is not a default state, but an actively maintained balance of stimulatory and inhibitory signals including active transcriptional regulation, and both extrinsic and intrinsic mechanisms exist to control this complex quiescence program.4-7 How the quiescent state of SP thymocytes is achieved during thymocyte development is not understood.
Low- or high-affinity self-ligands induce distinct TCR binding kinetics leading to differential downstream signaling events during thymocyte differentiation.8-10 How these differences in TCR signaling lead to different functional outcomes in thymocyte differentiation is still unclear. In particular, not much is known about transcriptional regulation downstream of TCR signaling in thymocytes. NFAT, NF-κB, and several other transcription factor families, including Egr-1, Id3, E2A and Schnurri-2 (Shn-2) have been studied in thymocyte development.11-17 Yet to a large extent, their downstream targets in thymocytes remain unknown. Krupple-like factor 2 (KLF2), a zinc-finger transcription factor induced during DP to SP thymocyte differentiation, has been reported to regulate SP T-cell quiescence and survival.18 Later studies, however, showed that KLF2 directly regulates the expression of the sphingosine-1-phosphate (S1P) receptor S1P1, the adhesion molecule L-selectin (CD62L),19 and several chemokine receptors.20 Therefore, it seems as if KLF2 controls the migration rather than the quiescence of thymocytes.
Forkhead box (FOX) proteins are a large transcription factor family with diverse functions in development, metabolism, organogenesis, and cancer.21 In the immune system, several forkhead proteins have been shown to be critically involved in the development and function of B and T lymphocytes.22-30 Foxp1, a member of the “Foxp” subfamily, was originally cloned from a mouse B-cell leukemia cell line and later demonstrated to be an essential transcriptional regulator of B lymphopoiesis via direct regulation of the B cell–specific Erag enhancer.31,32 However, Foxp1 is expressed in many other cell types including monocytes,31,33,34 where it also appears to have an essential role in differentiation and macrophage function.35
In this study, we address the role of Foxp1 in T cells. We show that its conditional deletion at the DP stage results in SP thymocytes that abnormally acquire an activated phenotype in the thymus, demonstrating that Foxp1 is essential for the generation of quiescent naive T cells during thymocyte development.
Methods
Mice
All animals were maintained in specific pathogen-free barrier facilities and were used in accordance with protocols approved by Institutional Animal Care and User Committee at The Wistar Institute. C57BL/6J mice and B6 CD45.1 congenic mice were purchased from The Jackson Laboratory. Cd4Cre transgenic mice were purchased from Taconic.
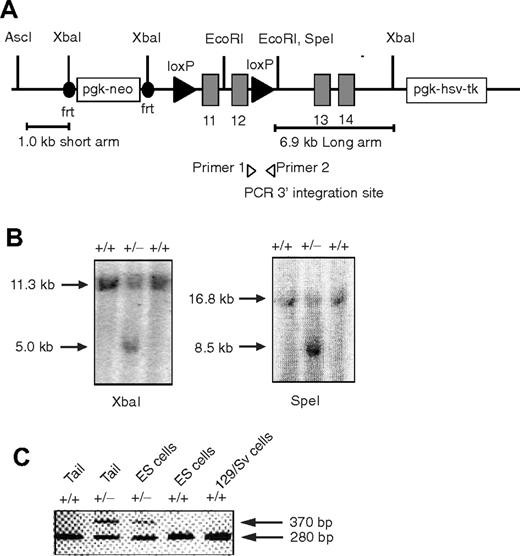
The Foxp1 conditional targeting construct was designed to have short (1.0 kb) and long (6.9 kb) arms of homology. The targeting strategy was designed to use 2 site-specific recombinations in vivo: (1) Flp recombinase to delete the neo marker from the mouse germline, and (2) Cre recombinase to conditionally delete Foxp1 exons 11 and 12 flanked by 2 loxP sites. Generation of the conditionally targeted mouse used similar procedures as described.34 Foxp1f/+ mice (129 background) were backcrossed with C57BL/6 mice for at least 5 generations. Genotypes of Foxp1f/+, Foxp1f/f mice were determined by polymerase chain reaction (PCR) amplification. The wild-type allele was identified by the production of a 370–base pair PCR product and mutated Foxp1 allele was identified by the production of a 280–base pair PCR product with primer 1 (5′-CTCCTAGTCACCTTCCCCAGTGC-3′) and primer 2 (5′-GAACACTGTCGAATGACCCTGC-3′). Foxp1f/f mice were crossed with Cd4Cre transgenic mice to generate Foxp1f/fCd4Cre and control Foxp1f/+Cd4Cre mice. In some experiments Foxp1+/+Cd4Cre mice were used as control mice.
Flow cytometry, cell-surface staining, and cell sorting
Single-cell suspensions of thymuses, lymph nodes, spleens, and bone marrow (2 femurs) were prepared. For each staining, at least 5000 events were collected for analysis. The following antibodies were obtained from eBioscience, Biolegend, or BD PharMingen: allophycocyanin–anti-CD4 (GK1.5), phycoerythrin (PE)–anti-CD44 (IM7), PE–anti-CD5 (53-7.3), PE–anti-CD25 (PC61), PE–anti-CD127 (A7R43), PE–anti-CD122 (TM-b1), PE–anti-FasL (FML3), PE–cyanine 7 (cy7)–anti-Fas (Jo2), PE–anti-TCRβ (H57-597), fluorescein isothiocyanate (FITC)–anti-TCRVα2 (B20.1), FITC–anti–TCR-Vα3.2 (RR3-16), PE–anti-TCR-Vα8.3 (B21.14), FITC–anti-TCR-Vα11.1.2 (RR8-1), FITC–TCR-Vβ5 (MH3-2), PE–anti–TCRVβ8.1.2 (MR5-2), PE–anti-TCRVβ8.3 (CT-8C1), PE–anti-TCRVβ10 (B21.5), peridinin-chlorophyll-protein complex (Percep)–cy5.5–anti-CD62L (MEL-14), Percep–cy5.5–anti-CD8 (53-6.7), FITC–anti-CD3 (145-2C11), FITC–anti-CD69 (H1.2F3), FITC–anti-CD8β (H35-17.2), FITC–anti-Qa2 (69H1-9-9), biotin–anti–heat stable antigen (HSA) (M1/69), and Percep-cy5.5–conjugated streptavidin. Cells were counted by trypan blue staining. Nonspecific antibody binding was blocked with anti-CD16/CD32 antibodies (2.4G2) before staining. Cell staining samples were analyzed by either FACSCalibur or LSRII (BD PharMingen). Data were analyzed by FlowJo software (TreeStar). A FACSVantage (BD PharMingen) was used for cell sorting with a combination of various antibodies. The sorted populations were more than 98% pure.
Peripheral T-cell purification, CFSE labeling, and cell proliferation
Peripheral CD4+ T and CD8+ T cells from pooled spleens and lymph nodes were isolated with the Dynabeads Mouse CD4 kit or FlowComp Mouse CD8 kit (Invitrogen Dynal AS). For carboxyfluorescein succinimidyl ester (CFSE) labeling of peripheral T cells, purified CD4+ T or CD8+ T cells were incubated at 107/mL with 1 to 1.5μM CFSE (Invitrogen/Molecular Probes) for 8 minutes at room temperature, then washed twice in medium with 10% fetal bovine serum. To examine the proliferation of purified peripheral T cells to TCR stimulation, CFSE-labeled CD4+ T or CD8+ T cells were stimulated with 0.1 to 1 μg/mL anti-CD3 antibodies (145.2-C11) with or without 2 μg/mL anti-CD28 antibodies (37.51) in plates precoated with 0.3 mg/mL goat anti–hamster immunoglobulin G (ICN Pharmaceuticals). The cells were cultured in Dulbecco modified Eagle medium supplemented with 10% heat-inactivated fetal calf serum, 2.5mM l-glutamine, 50 U/mL penicillin, 50 μg/mL streptomycin, 10mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid, and 5 × 10−5M 2-mercaptoethanol. CFSE profiles were examined at 24 or 48 hours of culture.
Cytokine intracellular staining
For peripheral T cells, freshly isolated spleen cells were stimulated for 4 hours with 5 ng/mL phorbol 12-myristate 13-acetate (PMA) plus 1 μg/mLionomycin, or 2 μg/mL anti-CD3 plus 2.5 μg/mL anti-CD28 antibodies. For fluorescence-activated cell sorting (FACS)–sorted CD8 SP thymocytes, the cells were stimulated with 50 ng/mL PMA plus 500 ng/mL ionomycin for 4 hours. Brefeldin A (BD PharMingen) was added for the last 2 hours of the cultures for stimulation. Intracellular staining for interferon γ (IFN-γ), interleukin-2 (IL-2), and IL-4 was performed using the Cytofix/Cytoperm Kit (BD Pharmingen).
Apoptosis and in vitro regulatory T-cell suppression assays
Immediately after isolation from the mice, thymocytes, spleen, and lymph node cells were first stained with various cell-surface markers, washed, then stained with FITC–anti–annexin V (eBioscience). The cells were washed and incubated on ice with 0.5 μg/mL propidium iodide (PI; Invitrogen) for 15 minutes right before analysis.
To examine T-cell apoptosis in vitro, purified peripheral CD4+ T or CD8+ T cells were cultured in medium with or without 10 μg/mL anti-FasL antibody (MFL3; eBioscience) for 24 hours, and analyzed for annexin V and PI staining. To induce activation-induced cell death, purified T cells were activated with 1 μg/mL anti-CD3 and 2 μg/mL anti-CD28 for 2 days and then cultured in the presence of recombinant human IL-2 for 3 additional days. On day 5 the cells were restimulated with 2 μg/mL anti-CD3 for 24 hours with or without 10 μg/mL anti-FasL antibody, then analyzed for annexin V and PI staining.
For in vitro regulatory T-cell suppression assay, responder CD4+CD25− T cells were FACS-sorted from 10-week-old Foxp1f/+Cd4Cre mice and labeled with CFSE. CFSE-labeled responder T cells (7 × 105) were cultured in medium alone or with FACS-sorted CD4+CD25+ T cells (regulatory T [Treg] cells) from Foxp1f/+Cd4Cre or Foxp1f/fCd4Cre mice at a variety of Treg to responder ratios (1:2, 1:4, 1:8) in the presence of 0.05 μg/mL anti-CD3 and irradiated wild-type splenocytes (4 × 106) in 24-well plates. After 4 days of culture, cells were prepared for flow cytometric analysis of responder cell division and cytokine production. Cells were stimulated for 4 hours in the presence of PMA (50 ng/mL), ionomycin (1μM), and brefeldin A. Cells were then stained with anti-CD4 and anti-CD25 before being fixed and permeabilized and stained with anti–IFN-γ.
Real-time reverse-transcription PCR
CD5low DP thymocytes from 4- to 5-week-old Foxp1+/+Cd4Cre or Foxp1f/fCd4Cre mice were FACS-sorted. RNA was purified and real-time reverse-transcription PCR was performed as described.32 The primers used to detect the expression of Rag1 and Rag2 genes are as follows: forward, 5′-CTGTGGCATCGAGTGTTAACAAC-3′ and reverse, 5′-GGAGGCAGCCATGTTGG-3′ for Rag1; forward, 5′-CTGGCCTTCAGTGCCAAAATAAG-3′ and reverse, 5′-GGCTATGTTATGACCCACTGTTAC-3′ for Rag2.
Immunoblot analysis
Thymocyte subsets as well as peripheral CD4+ T and CD8+ T cells were purified from C57BL/6, Foxp1+/+Cd4Cre, Foxp1f/+Cd4Cre, or Foxp1f/fCd4Cre mice by FACS-sorting or Dynabeads purification. Lysates of 1.5 × 106 cells of each cell population were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis. Foxp1-specific monoclonal antibody 1G1, goat anti-Foxp1 polyclonal antibody (P20; Santa Cruz Biotechnology), and anti–β-actin (Santa Cruz Biotechnology) were used for immunoblotting.
Intrathymic transfer
FACS-sorted CD5low DP thymocytes (9-10 × 106) from 4-week-old Foxp1f/+Cd4Cre or Foxp1f/fCd4Cre mice were transferred in 20 μL of sterile phosphate-buffered saline into the thymuses of wild-type B6 CD45.1 recipient mice that were sublethally irradiated (5 Gy). Thymocytes of recipient mice were analyzed at days 3, 5, and 6 after transfer.
Mixed bone marrow chimeras
Bone marrow cells from Foxp1+/+Cd4Cre (CD45.1+CD45.2+) and Foxp1f/fCd4Cre (CD45.1− CD45.2+) mice were depleted of CD4+, CD8+, CD3+, B220+, and NK1.1+ cells by FACS-sorting using a combination of PE-conjugated antibodies, and were mixed at an approximately 1:1 ratio before being transferred intravenously into the lethally irradiated (9 Gray) B6 CD45.1 recipient mice. The mixed chimeras were analyzed 6 to 10 weeks after reconstitution.
Histopathology
Lungs, livers, and kidneys were removed from 24-week-old mice. Needle perfusion was performed on each organ using 10% formalin. Samples were formalin-fixed, paraffin-embedded, and stained with routine hematoxylin and eosin staining before tissue histology. Photomicrographs were taken at both ×10 and ×40 magnifications.
Statistics
Two-tailed Student t tests were used to calculate P values for all the relevant figures.
Results
Reduced cell number and activated phenotype of peripheral Foxp1-deficient T cells
In T lymphopoiesis, we found that Foxp1 was expressed at all developmental stages from double-negative (DN) cells in the thymus to mature naive T and regulatory T (Treg) cells in the periphery, and the full-length Foxp1A was found to be the most highly expressed isoform (supplemental Figure 1A, available on the Blood website; see the Supplemental Materials link at the top of the online article).
Foxp1-null mice die at embryonic day 14.5 due to heart valve and outflow tract anomalies.34 To determine the function of Foxp1 in T cells, we generated a conditionally targeted strain (Figure 1 and supplemental Figure 1B). Floxed Foxp1 (Foxp1f/f) mice were crossed with Cd4Cre transgenic mice in which the Cre recombinase is under the control of a Cd4 promoter and starts to be expressed at the CD4+CD8+ (DP) stage of thymocyte development.36 Accordingly, we found that DP thymocytes and peripheral CD4+ T and CD8+ T cells from Foxp1f/fCd4Cre mice were devoid of Foxp1 expression (supplemental Figure 1C-D), demonstrating efficient deletion of Foxp1 in both CD4 and CD8 T cells.
Generation of the conditional Foxp1 allele. (A) Diagram of the targeting vector containing Foxp1 exons 11 to 14. (B) Southern blot analysis to identify embryonic cell lines carrying the targeted Foxp1 locus. XbaI-digested (left) and SpeI-digested (right) genomic DNA from embryonic stem cells, hybridized to the 2 probes in the short and long arms, respectively. (C) PCR analysis of heterozygous mice confirms germline transmission. Shown is a PCR result indicating the cointegration of the 3′ downstream loxP site (370 bp) and wild-type allele product (280 bp) using primers 1 and 2.
Generation of the conditional Foxp1 allele. (A) Diagram of the targeting vector containing Foxp1 exons 11 to 14. (B) Southern blot analysis to identify embryonic cell lines carrying the targeted Foxp1 locus. XbaI-digested (left) and SpeI-digested (right) genomic DNA from embryonic stem cells, hybridized to the 2 probes in the short and long arms, respectively. (C) PCR analysis of heterozygous mice confirms germline transmission. Shown is a PCR result indicating the cointegration of the 3′ downstream loxP site (370 bp) and wild-type allele product (280 bp) using primers 1 and 2.
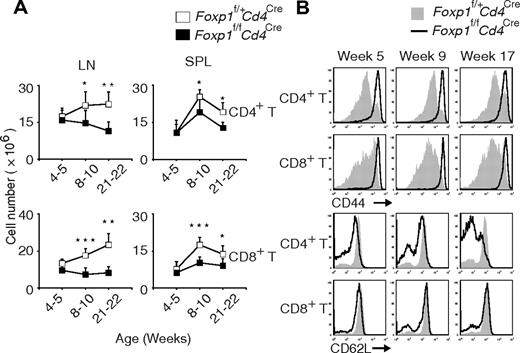
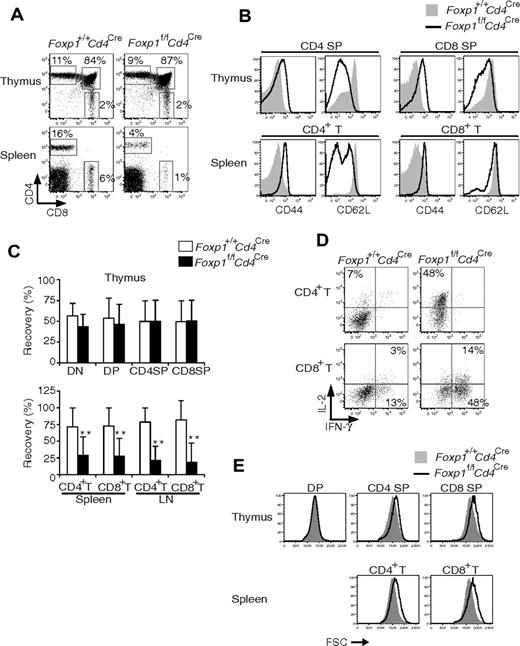
Compared with those in the control Foxp1f/+Cd4Cre mice, the total numbers of CD4+ T and CD8+ T cells in Foxp1f/fCd4Cre mice were normal at weeks 4 to 5, but became significantly lower with age (Figure 2A). And we found that almost all T cells from Foxp1f/fCd4Cre mice had an activated phenotype with uniformly high expression of CD44 (Figure 2B). Also with age, the percentage of CD62Llow CD4+ T cells increased in Foxp1f/fCd4Cre mice (Figure 2B). Although CD62L expression on CD8+ T cells was comparable between Foxp1f/fCd4Cre and the control mice at all ages (Figure 2B), we found that the percentages of CD122high CD8+ T cells and the percentages of CD69positive CD4+ T as well as CD8+ T cells increased in Foxp1f/fCd4Cre mice with age (supplemental Figure 2A-B). These results demonstrate that in the absence of overt antigen challenge, peripheral Foxp1-deficient T cells exhibit an activated phenotype and have reduced cellularity.
Peripheral Foxp1-deficient T cells have reduced cell numbers and an activated phenotype. (A) Total cell numbers of CD4+ T and CD8+ T cells in the lymph nodes and spleens of Foxp1f/+Cd4Cre and Foxp1f/fCd4Cre mice at different ages. Data represent average ± SD, weeks 4 to 5: n = 5, weeks 8-10: n = 7, and weeks 21-22: n = 5. *P < .05; **P < .01; ***P < .001. (B) Expression of CD44 or CD62L in splenic CD4+ T or CD8+ T cells from Foxp1f/+Cd4Cre and Foxp1f/fCd4Cre mice at different ages. Data are representative of at least 3 independent experiments. The histogram with solid gray represents Foxp1f/+Cd4Cre mice, and the histogram with dark line represents Foxp1f/fCd4Cre mice.
Peripheral Foxp1-deficient T cells have reduced cell numbers and an activated phenotype. (A) Total cell numbers of CD4+ T and CD8+ T cells in the lymph nodes and spleens of Foxp1f/+Cd4Cre and Foxp1f/fCd4Cre mice at different ages. Data represent average ± SD, weeks 4 to 5: n = 5, weeks 8-10: n = 7, and weeks 21-22: n = 5. *P < .05; **P < .01; ***P < .001. (B) Expression of CD44 or CD62L in splenic CD4+ T or CD8+ T cells from Foxp1f/+Cd4Cre and Foxp1f/fCd4Cre mice at different ages. Data are representative of at least 3 independent experiments. The histogram with solid gray represents Foxp1f/+Cd4Cre mice, and the histogram with dark line represents Foxp1f/fCd4Cre mice.
Peripheral Foxp1-deficient T cells respond like activated T cells and have increased apoptosis
To determine whether Foxp1-deficient T cells are functional, we examined their capacity to produce cytokines ex vivo. Without TCR stimulation, neither Foxp1-deficient nor the control T cells produced any cytokines examined (data not shown). However, upon transient ex vivo stimulation by anti-CD3 plus anti-CD28 antibodies, or by phorbol 12-myristate 13-acetate (PMA) plus ionomycin, higher percentages of Foxp1-deficient CD4+ T cells produced IL-2 (Figure 3A), but not IFN-γ or IL-4 (data not shown). Higher percentages of Foxp1-deficient CD8+ T cells were found to produce IL-2 and IFN-γ under both stimulation conditions (Figure 3A).
Peripheral Foxp1-deficient T cells respond like activated T cells and have increased apoptosis. (A) Total spleen cells from 4-week-old Foxp1f/+Cd4Cre and Foxp1f/fCd4Cre mice were stimulated with anti-CD3 plus anti-CD28 antibodies or PMA plus ionomycin for 4 hours and analyzed for IL-2 and IFN-γ production by intracellular staining. The data are gated on CD4+ T or CD8+ T cells, and are representative of various time points (weeks 4, 8, and 21) in at least 3 independent experiments. (B) Purified CD4+ T or CD8+ T cells from 4-week-old Foxp1f/+Cd4Cre and Foxp1f/fCd4Cre mice were labeled with CFSE and stimulated with 1 μg/mL anti-CD3 antibodies for 2 days. Cell proliferation was analyzed by measuring the CFSE profile. Data are representative of 2 independent experiments. (C) Thymic and lymph node cells from 8-week-old Foxp1f/+Cd4Cre and Foxp1f/fCd4Cre mice were analyzed for apoptosis by annexin V and PI staining. The bars represent the percentages of annexin V+ PI− cells in gated CD4+ or CD8+ T cells. Data represent average ± SD of the data of 8-week-old mice, n = 4. *P < .05; **P < .01.
Peripheral Foxp1-deficient T cells respond like activated T cells and have increased apoptosis. (A) Total spleen cells from 4-week-old Foxp1f/+Cd4Cre and Foxp1f/fCd4Cre mice were stimulated with anti-CD3 plus anti-CD28 antibodies or PMA plus ionomycin for 4 hours and analyzed for IL-2 and IFN-γ production by intracellular staining. The data are gated on CD4+ T or CD8+ T cells, and are representative of various time points (weeks 4, 8, and 21) in at least 3 independent experiments. (B) Purified CD4+ T or CD8+ T cells from 4-week-old Foxp1f/+Cd4Cre and Foxp1f/fCd4Cre mice were labeled with CFSE and stimulated with 1 μg/mL anti-CD3 antibodies for 2 days. Cell proliferation was analyzed by measuring the CFSE profile. Data are representative of 2 independent experiments. (C) Thymic and lymph node cells from 8-week-old Foxp1f/+Cd4Cre and Foxp1f/fCd4Cre mice were analyzed for apoptosis by annexin V and PI staining. The bars represent the percentages of annexin V+ PI− cells in gated CD4+ or CD8+ T cells. Data represent average ± SD of the data of 8-week-old mice, n = 4. *P < .05; **P < .01.
Studies have shown that activated T cells are more responsive than naive T cells upon TCR stimulation.37 We found that higher percentages of Foxp1-deficient T cells proliferated and up-regulated CD69 and CD25 expression than did the control cells to suboptimal stimulation by anti-CD3 antibodies without costimulation (Figure 3B and supplemental Figure 3A). These results suggest that Foxp1-deficient T cells in the periphery are not naive cells masked with an activated phenotype. They produce cytokines rapidly and respond like activated T cells.
To understand the mechanism underlying the reduced numbers of Foxp1-deficient T cells in the periphery, we examined apoptosis of Foxp1-deficient T cells by annexin V staining. We found that in the periphery but not in the thymus, the percentages of annexin V–positive but propidium iodide (PI)–negative CD4+ T and CD8+ T cells were significantly higher in Foxp1f/fCd4Cre mice than in the control mice (Figure 3C), indicating that Foxp1-deficient T cells have increased apoptosis in the periphery. We examined Fas and FasL expression and found that only Fas expression was slightly increased in Foxp1-deficient T cells (supplemental Figure 3B). To determine whether the increased apoptosis of Foxp1-deficient T cells is mediated by Fas-FasL interaction, we used a blocking anti-FasL antibody that prevented activation-induced cell death of both control and Foxp1-deficient T cells in vitro (supplemental Figure 3C). When cultured in vitro in medium alone, higher percentages of both Foxp1-deficient CD4+ T and CD8+ T cells underwent apoptosis (supplemental Figure 3D). However, apoptosis could not be prevented by adding the blocking anti-FasL antibodies (supplemental Figure 3D).
Our previous study showed that Foxp1 regulates expression of Rag genes in B- but not in T-lineage cells.32 In accordance, we found no differences in the expression of Rag genes between Foxp1-deficient and control DP thymocytes (supplemental Figure 4A). We also examined the distribution of the TCR repertoire and found that the percentages of all the TCR Vα and Vβ families examined were almost identical in the periphery of Foxp1f/fCd4Cre and the control mice (supplemental Figure 4B). These results suggest that although Foxp1-deficient T cells have an activated phenotype and increased apoptosis, it is unlikely due to biased generation or selective survival of a particular subpopulation of T cells during T-cell development in the absence of Foxp1.
Despite the overtly activated Foxp1-deficient T cells, we did not find any significant infiltration of lymphocytes in multiple organs of 24-week-old Foxp1f/fCd4Cre mice examined by histopathologic analysis, and the structure of those organs remained normal (supplemental Figure 5).
Foxp1-deficient Treg cells are generated normally and are functional
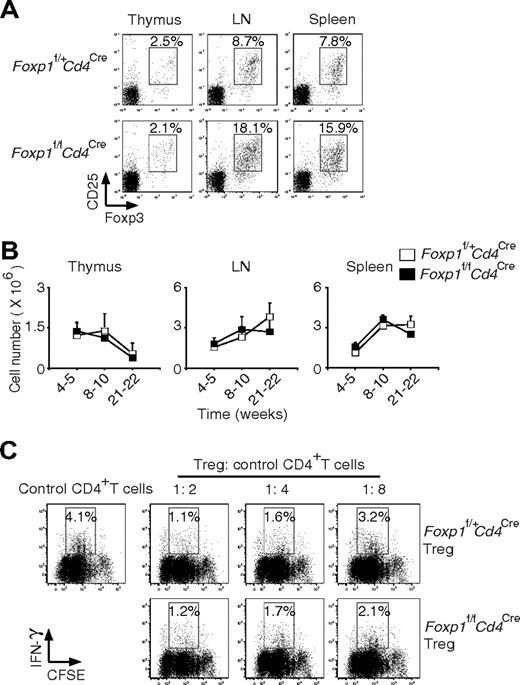
Treg cells have been shown to be essential for maintaining the homeostasis of peripheral T cells.25,26 We asked whether the activated phenotype of Foxp1-deficient T cells could be due to the abnormal development of Treg cells. By surface staining of CD4 and CD25 and intracellular staining of Foxp3, we found normal Treg cell generation in the thymus of Foxp1f/fCd4Cre mice (Figure 4A). Although the percentages of Treg cells were higher in the periphery of Foxp1f/fCd4Cre mice, the absolute numbers of Treg cells were the same as those in the control mice at all ages due to the reduced cellularity of CD4+ T cells in Foxp1f/fCd4Cre mice (Figure 4B). In the suppression coculture to examine Treg cell function, we found that Foxp1-deficient Treg cells inhibited IFN-γ production in the responder CD4+ T cells as well as the control Treg cells, indicating that Foxp1-deficient Treg cells are functional (Figure 4C).
Foxp1-deficient Treg cells develop normally and are functional. (A) Staining of cell-surface CD4 and CD25 and intracellular Foxp3 in the thymuses, lymph nodes, and spleens of 10-week-old Foxp1f/+Cd4Cre and Foxp1f/fCd4Cre mice. The percentages of CD25+Foxp3+ cells in CD4+ T cells were shown. Data are representative of 2 independent experiments. (B) Total cell numbers of Treg cells in the thymuses, lymph nodes, and spleens of Foxp1f/+Cd4Cre and Foxp1f/fCd4Cre mice at different ages. Bars represent average ± SD, weeks 4-5: n = 5, weeks 8-10: n = 7, and weeks 21-22: n = 5. (C) In Treg cell suppression cocultures, CFSE-labeled control CD4+ T cells were incubated with various ratios of Treg cells from Foxp1f/+Cd4Cre or Foxp1f/fCd4Cre mice. The IFN-γ production by control CD4+ T cells was analyzed by intracellular staining. Data are representative of 2 independent experiments.
Foxp1-deficient Treg cells develop normally and are functional. (A) Staining of cell-surface CD4 and CD25 and intracellular Foxp3 in the thymuses, lymph nodes, and spleens of 10-week-old Foxp1f/+Cd4Cre and Foxp1f/fCd4Cre mice. The percentages of CD25+Foxp3+ cells in CD4+ T cells were shown. Data are representative of 2 independent experiments. (B) Total cell numbers of Treg cells in the thymuses, lymph nodes, and spleens of Foxp1f/+Cd4Cre and Foxp1f/fCd4Cre mice at different ages. Bars represent average ± SD, weeks 4-5: n = 5, weeks 8-10: n = 7, and weeks 21-22: n = 5. (C) In Treg cell suppression cocultures, CFSE-labeled control CD4+ T cells were incubated with various ratios of Treg cells from Foxp1f/+Cd4Cre or Foxp1f/fCd4Cre mice. The IFN-γ production by control CD4+ T cells was analyzed by intracellular staining. Data are representative of 2 independent experiments.
Foxp1-deficient T cells acquire the activated phenotype during DP to SP thymocyte transition
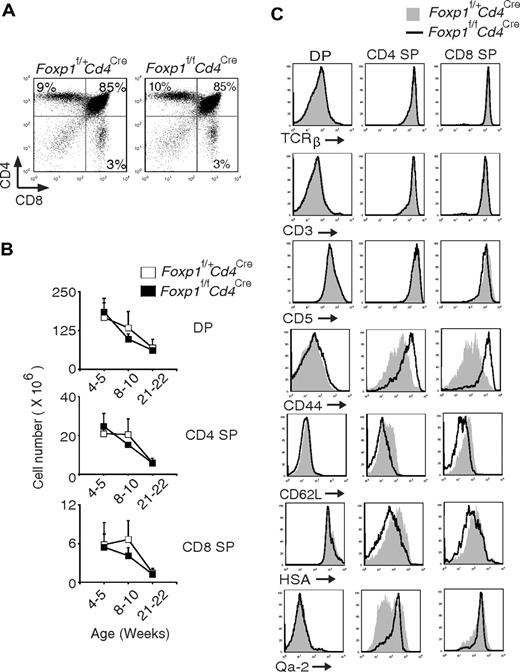
We examined peripheral T cells in 2-week-old Foxp1f/fCd4Cre mice and found that Foxp1-deficient T cells already had an activated phenotype (data not shown), suggesting that Foxp1-deficient T cells probably start to acquire the activated phenotype during early T-cell development in the thymus. Therefore, we examined the thymocytes in Foxp1f/fCd4Cre mice. The CD4/CD8 profile and total cell numbers of DP, CD4 SP, and CD8 SP thymocytes in Foxp1f/fCd4Cre mice were normal compared with those in control mice (Figure 5A-B). We also examined several cell surface markers that reflect thymocyte activation and maturation.38 Regardless of their age, we found that the expression of TCRβ, CD3, and CD5 in DP and SP thymocytes was comparable between Foxp1f/fCd4Cre mice and the control mice (Figure 5C and data not shown). However, CD44 levels were higher, whereas CD62L levels were lower in Foxp1-deficient SP thymocytes than in the control SP thymocytes (Figure 5C). In addition, we found that both Foxp1-deficient CD4 SP and CD8 SP thymocytes expressed a slightly lower level of HSA but a higher level of Qa-2 (more obvious in Foxp1-deficient CD4 SP thymocytes) (Figure 5C). These results indicate that Foxp1-deficient CD4 SP and CD8 SP thymocytes have an activated phenotype and they are mature thymocytes.
Foxp1-deficient CD4 SP and CD8 SP thymocytes have an activated phenotype. (A) Thymic CD4 and CD8 staining profile of 4-week-old Foxp1f/+Cd4Cre and Foxp1f/fCd4Cre mice. Data are representative of various time points (weeks 4, 5, 8, 10, 21, 22) in at least 4 independent experiments. (B) Total cell numbers of DP, CD4 SP, and CD8 SP thymocytes in Foxp1f/+Cd4Cre and Foxp1f/fCd4Cre mice at different ages. Data represent average ± SD, weeks 4-5: n = 7, weeks 8-10: n = 7, and weeks 21-22: n = 5. (C) Expression of TCRβ, CD3, CD5, CD44, CD62L, HSA, and Qa-2 in DP, CD4 SP, and CD8 SP thymocytes of 4- to 5-week-old Foxp1f/+Cd4Cre and Foxp1f/fCd4Cre mice. Data are representative of at least 2 independent experiments.
Foxp1-deficient CD4 SP and CD8 SP thymocytes have an activated phenotype. (A) Thymic CD4 and CD8 staining profile of 4-week-old Foxp1f/+Cd4Cre and Foxp1f/fCd4Cre mice. Data are representative of various time points (weeks 4, 5, 8, 10, 21, 22) in at least 4 independent experiments. (B) Total cell numbers of DP, CD4 SP, and CD8 SP thymocytes in Foxp1f/+Cd4Cre and Foxp1f/fCd4Cre mice at different ages. Data represent average ± SD, weeks 4-5: n = 7, weeks 8-10: n = 7, and weeks 21-22: n = 5. (C) Expression of TCRβ, CD3, CD5, CD44, CD62L, HSA, and Qa-2 in DP, CD4 SP, and CD8 SP thymocytes of 4- to 5-week-old Foxp1f/+Cd4Cre and Foxp1f/fCd4Cre mice. Data are representative of at least 2 independent experiments.
It has been shown that SP cells in the thymus are a mix of thymocytes and recirculating activated peripheral T cells that are CD44high.39 To distinguish whether the high CD44 expression in Foxp1-deficient SP thymocytes is acquired during thymocyte differentiation or is due to recirculating CD44high T cells from the periphery, we performed intrathymic transfers of CD5low DP thymocytes into congenic hosts and examined the phenotype of the newly generated SP thymocytes (Figure 6A). Three days after intrathymic transfer, some of the transferred DP thymocytes had differentiated into SP thymocytes (Figure 6B). We found that the CD44 expression was increased in both Foxp1-deficient CD4 SP and CD8 SP thymocytes compared with control SP thymocytes (Figure 6B). It has been reported that newly generated SP thymocytes reside in the thymus for 4 to 5 days before they egress.39 Therefore our results clearly define that the activated phenotype of Foxp1-deficient T cells is first acquired during the transition from DP stage to SP stage. We also examined IFN-γ production in FACS-sorted CD8 SP thymocytes ex vivo and found that a higher percentage of Foxp1-deficient CD8 SP thymocytes produced IFN-γ than did the control cells (Figure 6C).
Foxp1-deficient T cells acquire the activated phenotype during the differentiation from DP stage to SP stage. (A) Diagram of intrathymic adoptive transfer. (B) CD44 expression of donor thymocytes 3 days after the intrathymic transfer by staining. Data are representative of various time points (days 3, 5, and 6) in at least 2 independent experiments. The histogram with solid gray represents Foxp1f/+Cd4Cre mice, the histogram with dark line represents Foxp1f/fCd4Cre mice. (C) Foxp1-deficient CD8 SP thymocytes readily produce IFN-γ ex vivo. FACS-sorted CD8 SP thymocytes from 6-week-old Foxp1f/+Cd4Cre and Foxp1f/fCd4Cre mice were stimulated with PMA and ionomycin for 4 hours and analyzed for IFN-γ production by intracellular staining. Data are representative of 2 independent experiments.
Foxp1-deficient T cells acquire the activated phenotype during the differentiation from DP stage to SP stage. (A) Diagram of intrathymic adoptive transfer. (B) CD44 expression of donor thymocytes 3 days after the intrathymic transfer by staining. Data are representative of various time points (days 3, 5, and 6) in at least 2 independent experiments. The histogram with solid gray represents Foxp1f/+Cd4Cre mice, the histogram with dark line represents Foxp1f/fCd4Cre mice. (C) Foxp1-deficient CD8 SP thymocytes readily produce IFN-γ ex vivo. FACS-sorted CD8 SP thymocytes from 6-week-old Foxp1f/+Cd4Cre and Foxp1f/fCd4Cre mice were stimulated with PMA and ionomycin for 4 hours and analyzed for IFN-γ production by intracellular staining. Data are representative of 2 independent experiments.
Foxp1-deficient T cells acquire an activated phenotype by a cell-autonomous mechanism
To determine whether the abnormal development of Foxp1-deficient T cells is by a cell-intrinsic or -extrinsic mechanism, we reconstituted lethally irradiated wild-type congenic mice with mixed bone marrow cells from Foxp1f/fCd4Cre and control Foxp1+/+Cd4Cre mice. At 6 to 10 weeks after reconstitution, we analyzed the generation and phenotype of donor T cells in the mixed chimeras. In the thymus, the generation and CD4/CD8 profile of Foxp1-deficient thymocytes was normal (Figure 7A,C).
Foxp1-deficient T cells acquire an activated phenotype by a cell-autonomous mechanism. At 6 to 10 weeks after mixed bone marrow reconstitution and gating on congenic markers, the donor thymocytes and peripheral T cells in mixed chimeras were analyzed for (A) CD4 and CD8 profile; (B) expression of CD44 and CD62L; and (C) percentages of Foxp1+/+Cd4Cre and Foxp1f/fCd4Cre T cells recovered in the thymuses, spleens, and lymph nodes of the mixed chimeras. The thymic data are normalized to the ratios of Foxp1+/+Cd4Cre and Foxp1f/fCd4Cre B220+ cells in the bone marrow; the peripheral T-cell data are normalized to thymic T cells. Bars represent average ± SD. n = 10. **P < .01. (D) IL-2 and IFN-γ production in splenic CD4+ T and CD8+ T cells by intracellular staining after PMA plus ionomycin stimulation for 4 hours. (E) Cell size. All data are representative of 2 independent experiments.
Foxp1-deficient T cells acquire an activated phenotype by a cell-autonomous mechanism. At 6 to 10 weeks after mixed bone marrow reconstitution and gating on congenic markers, the donor thymocytes and peripheral T cells in mixed chimeras were analyzed for (A) CD4 and CD8 profile; (B) expression of CD44 and CD62L; and (C) percentages of Foxp1+/+Cd4Cre and Foxp1f/fCd4Cre T cells recovered in the thymuses, spleens, and lymph nodes of the mixed chimeras. The thymic data are normalized to the ratios of Foxp1+/+Cd4Cre and Foxp1f/fCd4Cre B220+ cells in the bone marrow; the peripheral T-cell data are normalized to thymic T cells. Bars represent average ± SD. n = 10. **P < .01. (D) IL-2 and IFN-γ production in splenic CD4+ T and CD8+ T cells by intracellular staining after PMA plus ionomycin stimulation for 4 hours. (E) Cell size. All data are representative of 2 independent experiments.
Expression of CD44 and CD62L in both Foxp1-deficient and control DP thymocytes was low/negative (data not shown). However, both Foxp1-deficient CD4 SP and CD8 SP thymocytes had increased expression of CD44 and decreased expression of CD62L (Figure 7B), suggesting that Foxp1-deficient thymocytes acquire an activated phenotype by a cell-intrinsic mechanism independent of their environment.
In the periphery of mixed chimeras, Foxp1-deficient T cells exhibited an activated phenotype (Figure 7B). In agreement with the results from Foxp1f/fCd4Cre mice (Figures 2–3), the percentages and cell numbers of peripheral Foxp1-deficient CD4+ T and CD8+ T cells in mixed chimeras were reduced (Figures 7A,C and data not shown), and they produced cytokines rapidly (Figure 7D). In both the thymus and the periphery, Foxp1-deficient T cells (except DP thymocytes) were larger than the control cells (Figure 7E).
Discussion
In this study, we found that instead of generating normal quiescent naive T cells, Foxp1-deficient SP thymocytes became prematurely activated in the thymus and acquired effector functions. High Qa-2 expression and low HSA expression in Foxp1-deficient SP thymocytes indicated that such cells were mature SP thymocytes. However, the down-regulation of CD62L and higher expression of CD44 in such cells, their larger cell size, and the capacity of Foxp1-deficient CD8 SP thymocytes to produce IFN-γ all indicate that these cells were hyperactivated. The results suggest that there is an abnormal activation or differentiation of the thymocytes in the absence of Foxp1. The mixed bone marrow chimera experiments demonstrate that this abnormal thymocyte activation/differentiation is cell intrinsic.
Stable expression of high levels of CD44 is induced only after naive T-cell activation.40 One potential explanation for high levels of CD44 in Foxp1-deficient SP thymocytes is that Foxp1, which can function as a repressor,41 may directly regulate CD44 transcription. On the other hand, it is also possible that the high levels of CD44 expression in Foxp1-deficient T cells are due to an indirect alteration of transcriptional regulation as a consequence of abnormal thymocyte activation/differentiation. Further studies are needed to understand the mechanism underlying this phenotypic change in Foxp1-deficient SP thymocytes.
The down-regulation of CD62L in Foxp1-deficient SP thymocytes seems to be transient. In the periphery of Foxp1f/fCd4Cre mice, the percentage of CD62Llow cells increased mainly in Foxp1-deficient CD4+ T cells but not CD8+ T cells with age. Bromodeoxyuridine experiments showed that peripheral Foxp1-deficient T cells proliferated at a greater rate than control naive T cells (data not shown). These results, together with the finding that higher percentages of peripheral Foxp1-deficient T cells expressed CD69, suggest that there is an ongoing homeostatic-driven T-cell proliferation in Foxp1f/fCd4Cre mice, which may further contribute to the activated phenotype and the effector functions of Foxp1-deficient T cells. The results also indicate that the down-regulation of CD62L in the absence of Foxp1 is most likely secondary.
The number of SP thymocytes is usually used as an indicator for aberrations in thymic positive or negative selection. In our study, despite an overt activated phenotype of Foxp1-deficient SP thymocytes, the total cell numbers of Foxp1-deficient SP thymocytes were comparable with the controls. In addition, we did not detect obvious differences in apoptosis between control and Foxp1-deficient thymocytes ex vivo, although it should be noted that in the thymus there is usually a rapid phagocytosis and clearance of apoptotic cells that could obscure any differences in apoptosis. Nevertheless, because our study was conducted in a polyclonal T-cell background, we cannot rule out the possibility of altered positive or negative selection. TCR ligation by low-affinity self-ligands promotes positive selection, whereas TCR ligation by high-affinity self-ligands induces negative selection.42-44 It has been reported that expression levels of CD5 on SP thymocytes directly parallel the avidity of the selecting TCR–self-ligand interaction in the thymus.45 In our study, we did not observe any change in the CD5 expression levels in Foxp1-deficient SP thymocytes, suggesting that selection of TCRs with biased avidities is unlikely. Our study suggests that in normal thymocyte differentiation, after TCR signals initiate the differentiation program in DP thymocytes, downstream transcriptional control by Foxp1 is likely negatively regulating thymocyte activation/differentiation, which would lead to the proper generation of quiescent SP thymocytes and naive T cells.
In our study, we have largely used heterozygous Foxp1f/+Cd4Cre mice as controls. However, we also compared Foxp1+/+Cd4Cre and Foxp1f/+Cd4Cre mice to determine whether Foxp1 may exert a gene dosage effect on T-cell development. We found that only CD8+ T cells from Foxp1f/+Cd4Cre mice had a slightly increased CD44 expression in the periphery with higher percentages of the cells producing IFN-γ upon ex vivo stimulation (supplemental Figure 6), indicating that CD8+ T-lineage cells are more sensitive to the regulation of Foxp1 gene dosage.
Recently 2 elegant studies reported that T cell–specific deletion of another forkhead protein, Foxo1, leads to impaired T-cell survival and spontaneous T-cell activation.29,30 Although both Foxp1-deficient and Foxo1-deficient T cells have a similar activated phenotype, the underlying mechanisms seem to be different. Foxo1 has been shown to regulate the expression of CD62L and IL-7Rα directly.29,30,46 In Foxp1-deficient T cells, we did not detect any down-regulation of IL-7Rα (data not shown). As demonstrated by intrathymic transfer experiments, the abnormal activation/differentiation of Foxp1-deficient T cells initiates as early as during the transition from the DP to the SP stage in the thymus. However, Foxo1-deficient thymocytes seem to develop quite normally.29,30 Because TCR transgenic Foxo1-deficient T cells have a typical naive phenotype, the slight increase of Foxo1-deficient TCRβhi CD4 SP and CD8 SP thymocytes in a polyclonal T-cell background more likely results from the recirculation of activated peripheral T cells.29,30 IL-7Rα transgenic expression has been shown to rescue peripheral T-cell numbers in TCR transgenic (OT-II) Foxo1-deficient mice, but it does not correct the T-cell activation phenotype on a polyclonal background.30 Thus, although the mechanisms remain unclear, there are clear functional differences between these 2 transcription factors in T cells.
Several thymocyte-derived populations, namely nonclassical MHC class I T cells, CD8αα+ T cells, natural killer T cells, and CD4+CD25+ Treg cells, display an activated or partially activated phenotype in wild-type animals.47-49 However, the phenotype of Foxp1-deficient T cells does not seem to reflect an expansion of any of these cell populations. We found that Foxp1-deficient CD8+ T cells were CD8αβ+ T cells (data not shown). The fact that Foxp1-deficient CD8+ T cells did not express CD122 in the young mice, or NK1.1 in mice of any age (supplemental Figure 2A and data not shown), suggests they are unlikely to be nonclassical MHC class I T cells.
Interestingly, even though both Foxp1-deficient CD4+ T and CD8+ T cells exhibited an activated phenotype and were functional, we did not find obvious infiltration of such activated cells in the nonlymphoid organs, and the mice did not show any obvious signs of autoimmune diseases (supplemental Figure 5 and data not shown). In the absence of Foxp1, Treg cells seemed to develop and function normally. Because the total cell number of Foxp1-deficient Treg cells was normal, whereas the total cell numbers of Foxp1-deficient CD4+ T cells and CD8+ T cells decreased in the periphery, the ratios between Treg cells and non-Treg T cells in the periphery were increased. Because the repression function of Treg cells is dependent on the Treg cell/non-Treg T-cell ratio, this may be one reason why the mice have not developed autoimmune disease in the absence of Foxp1. Foxp1 and Foxp3 belong to the same Foxp subfamily with a highly conserved DNA binding domain and it is possible that the 2 transcription factors share some target genes. At this stage we do not know whether normal numbers of Foxp1-deficient Treg cells in Foxp1f/fCd4Cre mice are due to Foxp3 compensating for the loss of Foxp1, or because of the changed environment resulting from the abnormal development of Foxp1-deficient non-Treg T cells.
In summary, our results demonstrate that during thymocyte development, after TCR signals initiate the differentiation of DP thymocytes, transcriptional regulation is involved in the generation of quiescent SP thymocytes. Specifically, we have identified Foxp1 as a novel participant in the transcriptional network that is important for thymocyte development and the proper generation of quiescent naive T cells.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank E. Pure for critical reading of the paper and discussions; J. S. Faust, D. Ambrose, and D. Hussey for excellent technical help with flow cytometry; R. Delgiacco for excellent technical help with histopathology; and A. Kossenkov for statistical analysis.
This work was supported by National Institutes of Health (NIH) grant 1K22AI070317-01A1 and Wistar starting package (H.H.), NIH AI59166 (A.J.C.), and NIH CA031534 and Marie Betzner Morrow Centennial endowment (P.W.T.).
National Institutes of Health
Authorship
Contribution: H.H. and P.W.T. initiated the collaboration on analyzing T cells in Foxp1 conditionally targeted mice, which were generated by G.C.I., S.D.M., and J.V.H. under the supervision of P.W.T.; H.H. initiated the collaboration with A.J.C. and A.B.; X.F. and L.T. performed most of the phenotypic and functional analysis of Foxp1-deficient T cells with help from J.W.; K.W. performed ex vivo cytokine production and apoptosis analysis; A.S. performed intrathymic transfer experiment and K.W. performed the analysis; S.O. performed the Treg cell phenotypic and functional analysis; R.M.B. and J.W. performed the histopathology analysis; A.B. and A.J.C. helped with supervision and discussions; H.H. provided overall supervision; and X.F., L.T., and H.H. prepared the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Hui Hu, The Wistar Institute, 3601 Spruce St, Philadelphia, PA 19104; e-mail: hhu@wistar.org. Specific mouse requests should be addressed to Philip W. Tucker, Department of Molecular Genetics and The Institute for Cellular and Molecular Biology, The University of Texas at Austin, Austin, TX 78712; e-mail: philtucker@mail.utexas.edu.
References
Author notes
X.F., G.C.I., and L.T. contributed equally to this work.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal