Abstract
The generation of natural regulatory T cells (nTregs) is crucial for the establishment of immunologic self-tolerance and the prevention of autoimmunity. Still, the origin of nTregs and the mechanisms governing their differentiation within the thymus are poorly understood, particularly in humans. It was recently shown that conventional dendritic cells (cDCs) in human thymus were capable of inducing nTreg differentiation. However, the function of plasmacytoid DCs (pDCs), the other major subset of thymic DCs, remains unknown. Here we report that pDCs resident in the human thymus, when activated with CD40 ligand (CD40L) plus interleukin-3, efficiently promoted the generation of CD4+CD25+Foxp3+ nTregs from autologous thymocytes. The progenitors of these nTregs were selectively found within CD4+CD8+ thymocytes that had accomplished positive selection, as judged by their CD69hiTCRhi phenotype. Supporting the involvement of the CD40-CD40L pathway in pDC-induced nTreg generation, we show that positively selected CD4+CD8+ progenitors specifically transcribed CD40L in vivo and up-regulated CD40L expression on T-cell receptor engagement, thereby promoting the activation of pDCs. Finally, evidence is provided that nTregs primed by pDCs displayed reciprocal interleukin-10/transforming growth factor-β cytokine expression profiles compared with nTregs primed by cDCs. This functional diversity further supports a nonredundant tolerogenic role for thymic pDCs in the human thymus.
Introduction
Dendritic cells (DCs) are highly specialized antigen-presenting cells (APCs) that are crucial in the regulation of innate and adaptive immunity. DCs respond to danger signals by antigen uptake, maturation, and induction of antigen-specific immune responses in secondary lymphoid organs.1,2 In addition, tissue DCs are able to present antigen in a tolerogenic fashion, leading to anergy or deletion of potentially self-reactive T cells, or inducing the generation of regulatory T cells (Tregs).3-6 Tregs play a key role in the control of immune homeostasis and immunologic tolerance and are crucial to protect against fatal autoimmunity throughout life. Tregs also regulate or suppress other classes of immune responses, such as allograft rejection, allergy, tumor immunity, and responses to microbes.7,8 Thus, there is great interest in understanding how do Tregs develop.
During thymopoiesis, nearly all positively selected CD4+CD8+ double-positive (DP) thymocytes carrying high-affinity T-cell receptors (TCRs) for self-peptide-major histocompatibility complexes are eliminated by a mechanism of clonal deletion that ensures central tolerance.6 Self-reactive thymocytes that escape clonal deletion undergo an alternative process of nondeletional tolerance that involves their positive selection and further differentiation into immunosuppressive CD4+ T cells, termed natural Tregs (nTregs).8-11 In addition, Tregs can be induced in the periphery (iTregs) from CD4+ T lymphocytes.4 Generation of thymic nTregs critically involves the interleukin-2 receptor-α chain (CD25), the transcription factor Foxp3, and the costimulatory molecule CD28,12-14 as genetic defects in these molecules impair nTreg generation, leading to systemic autoimmunity.15-17
Despite the crucial function of nTregs, the exact nature of the nTreg progenitors and the mechanisms by which self-reactive thymocytes are diverted into the Treg lineage remain unclear. Several studies in mice suggest that thymic epithelial cells (TECs) regulate positive selection of nTreg progenitors with TCRs recognizing self-antigens with moderate to high avidity below the threshold of negative selection.9,11,18 DCs can also function as efficient tolerogenic APCs in the mouse thymus.19 In humans, Watanabe et al20 found that thymic DCs were able to induce the generation of nTregs from allogeneic CD4+CD25− single-positive (SP) thymocytes in response to thymic stromal-derived lymphopoietin (TSLP) produced by Hassal corpuscle TECs. Of the 2 major subsets of thymic DCs, ie, plasmacytoid DCs (pDCs) and conventional DCs (cDCs),21-23 TSLP-responsive DCs belong to the latter.20 However, no function has been established for thymic pDCs, although the tolerogenic role of pDCs as inducers of Tregs in the periphery has been illustrated.24-26 Therefore, the contribution of thymic pDCs to the development of nTregs remains to be determined in both mouse and human. In addition, it is important to establish the cellular origin of nTreg progenitors and the nature of signals involved in their development.
The present work was aimed at investigating the possible tolerogenic function of human thymic pDCs, the molecular mechanisms underlying the generation of human nTregs, and the identity of the physiologic progenitors of nTregs in the human thymus. Our results provide evidence that pDCs resident in the human thymus are efficient tolerogenic DCs, able to induce the differentiation of positively selected CD69hiTCRhiCD4+CD8+ DP autologous thymocytes into nTregs. Moreover, our data suggest that CD40 ligand (CD40L)-dependent crosstalk between pDCs and nTreg precursors may be involved in that process.
Methods
DC isolation, flow cytometry, and culture
Experiments were performed in accordance with procedures approved by the Spanish Research Council Bioethics Committee. Human postnatal thymocytes were isolated from thymus fragments removed during corrective cardiac surgery of patients 1 month to 4 years of age, after informed consent was provided in accordance with the Declaration of Helsinki. Thymocyte suspensions enriched in non-T cells by sheep erythrocyte rosetting27 were depleted of monocyte/macrophages by anti-CD14 bead magnetic sorting (autoMACS, Miltenyi Biotec). Flow cytometry was performed in a FACSCalibur (BD Biosciences) on electronically gated CD123+CD13−, CD123loCD13−, and CD123loCD13+ cells using phycoerythrin (PE)–conjugated anti-CD123 and PE-Cy5–coupled anti-CD13 monoclonal antibodies (mAbs; Beckman Coulter) in combination with either fluorescein isothiocyanate (FITC)–conjugated or unlabeled mAbs against BDCA2 (Miltenyi Biotec), CD11c, CD45RA, and CD45RO (Caltag), and CD86, CD80, and HLA-DR (BD Biosciences). Secondary reagents were FITC-coupled goat anti–mouse Igs (Caltag). Irrelevant isotype-matched antibodies (Caltag) were used as controls. Sorting of pDCs and cDCs was performed using PE-conjugated mAbs against CD123 and CD13 (BD Biosciences), respectively, plus anti-PE–coupled beads (Miltenyi Biotec). DC maturation was induced after 24 hours of culture in RPMI 1640 (BioWhittaker) medium supplemented with 10% fetal calf serum (Invitrogen) and either 2.5 ng/mL recombinant human (rh) CD40L (R&D Systems) plus 60 ng/mL rhIL-3 (National Institute of Biochemical Standards and Controls) or 15 ng/mL rhTSLP (R&D Systems), and assessed by flow cytometry using FITC-conjugated anti-CD86 and PE-Cy5–conjugated anti-CD80 mAbs (BD Biosciences).
Isolation of thymocytes and peripheral T lymphocytes
CD4+CD8−CD25− SP thymocytes were sorted using anti-CD8 and anti-CD4 multisort beads (Miltenyi Biotec) and PE-conjugated anti-CD25 mAb (BD Biosciences) plus anti-PE beads. Large- (L) and small-sized (S) DP subsets were selected using Percoll density gradients (GE Healthcare; 1.068 and 1.08 densities, respectively).28 Further enrichment in CD69hi or CD69lo cells was performed by AutoMACs sorting using PE-conjugated anti-CD69 mAb (Caltag) and anti-PE beads. For isolation of CD4+CD25− T cells, peripheral blood (PB) mononuclear cells were labeled with PE-conjugated mAbs against CD8, CD15, CD19, and CD56 (Caltag), and against CD13, CD14, CD25, CD33, and CD123 (BD Biosciences), and then depleted of PE-labeled cells with anti-PE beads. CD4+ cells were then isolated by positive selection with anti-CD4 beads to reach more than 95% purity.
In vitro generation of Tregs
Autologous or allogeneic CD4+CD25− SP thymocytes, or allogeneic CD4+CD25− PB T cells (105 cells/well) were cocultured with either primary immature pDCs or CD40L plus IL3-cultured mature pDCs in round-bottomed 96-well culture plates at a final 2:1 T/DC ratio. T-cell proliferation was assessed by carboxyfluorescein succinimidyl ester (CFSE) labeling (Invitrogen). S-DP CD69lo, L-DP CD69hi, or L-DP CD69lo thymocytes (105cells/well) were cocultured with either mature pDCs or TSLP-activated cDCs, at a final 2:1 DP thymocyte/DC ratio. To preserve DP thymocyte viability, cocultures were set up in flat-bottomed 96-well culture plates onto a monolayer of OP9 stromal cells supplemented with 0.5 ng/mL rhIL-7 (National Institute of Biochemical Standards and Control), and 20 ng/mL rhIL-2 (NCI Biological Resources Branch) was added to support Treg generation.11,15 CD25 and Foxp3 expression was assessed by flow cytometry using APC-conjugated anti-CD25 (eBioscience) and PE-conjugated anti-Foxp3 (eBioscience) mAbs. For inducible costimulator (ICOS) expression, an FITC-conjugated anti-ICOS antibody29 was used. When indicated, cocultures were supplemented every other day with blocking mAbs against ICOS ligand (ICOSL, 2 μg/mL; eBioscience), CD86 (5 μg/mL), or CD80 (5 μg/mL) (BD Biosciences), or with anti-CD86 plus anti-CD80 mAbs (5 μg/mL each). Isotyped-matched control antibodies (5 μg/mL) were from Caltag.
Functional Treg assays
CD4+ PB T cells, or L-DP CD69hi, L-DP CD69lo, or S-DP CD69lo thymocytes cocultured with MpDC or McDC for 7 days were enriched in CD25hi or CD25lo/− cells by autoMACS sorting. Suppressive potential of isolated cells was assayed in triplicate by [3H]-thymidine incorporation after 5 days of coculture with CD4+CD25− PB T lymphocytes (1:1 ratio or as indicated) that were stimulated in flat-bottomed 96-well culture plates precoated with 10 μg/mL anti-CD3 mAb (UCHT1, ATCC-AN1009425) and supplemented with 2 μg/mL anti-CD28 mAb (BD Biosciences) plus IL-2 (20 ng/mL). To support nTreg cell survival,7 cultures were supplemented with 20 ng/mL rhIL-2. In some experiments, the suppression assay was performed in the presence of neutralizing anti-IL10 (200 ng/mL) plus anti-IL-10R (10 ng/mL) antibodies (R&D Systems), or transforming growth factor-β (TGF-β) R type I kinase (ALK5) inhibitor II (TGF-βRI inhibitor) (1μM; Calbiochem), added to the culture every other day. Irrelevant goat anti–rabbit Igs (200 ng/mL; Jackson ImmunoResearch Laboratories) and mouse IgG1 (10 ng/mL; Caltag) or dimethyl sulfoxide were used as controls. Cytokine production was measured in triplicate in supernatants collected at day 7 of culture, using a human IL-10 enzyme-linked immunosorbent assay (ELISA) kit (Immunotools) and a human TGF-β ELISArray kit (SA Biosciences).
Induction of CD40L and CD40L-dependent pDC activation
L-DP CD69hi thymocytes were cultured in 24-well culture plates (5 × 105/well) either untreated or precoated with anti-CD3 mAb, and supplemented with anti-CD28 mAbs plus 20 ng/mL rhIL-2. After 16 to 24 hours, CD40L expression was analyzed by flow cytometry using an APC-conjugated anti-CD40L mAb (BD Biosciences). Anti-CD3-stimulated or nonstimulated L-DP CD69hi thymocytes were then cocultured for 24 hours with primary thymic pDCs at a final 1:1 ratio in round-bottomed 96-well plates. Activation of pDCs was analyzed by 2-color flow cytometry using anti-CD123 and anti-CD86 mAbs.
Semiquantitative reverse-transcribed polymerase chain reaction
Total RNA was isolated using TRIzol (Invitrogen) from sorted S-DP CD69lo, L-DP CD69hi, and L-DP CD69lo thymocytes obtained from the very same thymus sample. cDNA was generated using oligo-d(T) primers and Expand Reverse Transcriptase (Roche Diagnostics). Serially diluted cDNA samples were amplified by polymerase chain reaction using specific CD40L primers, as described.30 Amplified products were subjected to agarose electrophoresis and visualized by ethidium bromide. β-Actin amplification was included as control of cDNA loading.
Immunohistochemistry
Frozen thymic sections were fixed on acetone, incubated with goat serum and avidin/biotin blocking kit (Vector Laboratories), and labeled using anti–human CD123 (BioLegend) and biotin-conjugated anti–human Foxp3 mAbs (eBioscience). Secondary reagents were Alexa 488–conjugated goat anti–mouse IgG1 and Alexa 455–conjugated streptavidin (Invitrogen). TECs were stained with antipan cytokeratin mAbs (Sigma-Aldrich) plus Alexa 488–conjugated donkey anti–mouse IgGs (Invitrogen). Nuclei were identified in all sections with TOPRO-3 (Invitrogen). Images were obtained in a confocal LSM510 (Carl Zeiss) microscope using 10× (numeric aperture [NA] 0.3), 25× (scan zoom of 2.0, NA 0.8), and 40× (NA 1.3) magnification and Carl Zeiss LSM510 4.2 software. Image analysis was performed using the Carl Zeiss LSM Image browser, Version 4.2 software.
Statistics
Statistical significance was determined with the 2-tailed Student t test, with the α level set at 0.05.
Results
pDCs and cDCs resident in the human thymus become activated in response to different stimuli
Thymic pDCs and cDCs can be identified in thymocyte suspensions depleted of monocytes and T-lineage cells by their reciprocal expression of CD123 versus CD13 (Figure 1A). As shown previously,21-23 primary thymocytes with high CD123 expression (0.05% ± 0.006% of total thymocytes, n = 40) lacked CD13 and CD11c myeloid markers but expressed the pDC marker BDCA2, whereas CD13+ cells (0.026% ± 0.003%) displayed a BDCA2lo/−CD11c+CD123lo phenotype characteristic of cDCs (Figure 1A-B). As expected of pDCs,21-23 CD123+CD13− cells expressed high CD45RA levels but very low CD45RO, whereas cDCs were homogeneously positive for CD45RO but heterogeneous for CD45RA (Figure 1B). Subsets of cDCs and pDCs thus identified displayed a typical immature DC phenotype, with no expression of the costimulatory molecule CD80 and weak or undetectable levels, respectively, of CD86 (Figure 1B). Besides these 2 DC subsets, we consistently observed a population of CD13− cells with low CD123 expression (0.062% ± 0.014%; Figure 1A), which lacked CD11c and expressed high CD45RA but weak CD45RO, thus resembling pDCs. However, CD123loCD13− cells displayed up-regulated levels of the activation markers CD80, CD86, and HLA-DR, but down-regulated BDCA2 expression, compared with CD123+CD13− pDCs (Figure 1B). Because down-regulation of both CD123 and BDCA2 is a common feature of human pDC activation,22,31 our results may suggest that the CD123loCD13− population actually includes activated pDCs.
pDCs and cDCs from human thymus mature in response to different stimuli. (A) CD123 versus CD13 flow cytometry analysis of thymocyte suspensions depleted of T cells and monocytes/macrophages shows the presence of CD123+CD13− (R1), CD123loCD13+ (R2), and CD123loCD13− (R3) cells (left histogram). Isotyped-matched control Abs were used to define background staining. Isolation of pDCs and cDCs was performed by magnetic sorting based on reciprocal CD123 and CD13 expression. Postsorting phenotype of one of 10 experiments is shown (right histogram). (B) Flow cytometry analysis of BDCA2, CD11c, CD86, CD80, HLADR, CD45RA, and CD45RO expression in the populations electronically gated as defined in panel A (shaded histograms). Background staining was defined with isotype-matched Abs (empty histograms). Results of 1 of 4 experiments are shown. (C) Cell recoveries of either pDCs cultured for 24 hours with TSLP or with CD40L plus IL-3 or cDCs cultured with TSLP. Absolute DC numbers were normalized to 105 input cells. Data (mean ± SD) correspond to 3 independent experiments. *P < .05. (D) pDCs do not mature in response to TSLP but mature in the presence of CD40L plus IL-3. Flow cytometry analysis of CD80 and CD86 expression (shaded histograms) of pDCs and cDCs cultured as described in panel C. (E) Bright-field microscopy of thymic pDCs cultured with CD40L plus IL-3 for 24 hours (top, original magnification ×40), showing an amplification of the lower right inset (bottom).
pDCs and cDCs from human thymus mature in response to different stimuli. (A) CD123 versus CD13 flow cytometry analysis of thymocyte suspensions depleted of T cells and monocytes/macrophages shows the presence of CD123+CD13− (R1), CD123loCD13+ (R2), and CD123loCD13− (R3) cells (left histogram). Isotyped-matched control Abs were used to define background staining. Isolation of pDCs and cDCs was performed by magnetic sorting based on reciprocal CD123 and CD13 expression. Postsorting phenotype of one of 10 experiments is shown (right histogram). (B) Flow cytometry analysis of BDCA2, CD11c, CD86, CD80, HLADR, CD45RA, and CD45RO expression in the populations electronically gated as defined in panel A (shaded histograms). Background staining was defined with isotype-matched Abs (empty histograms). Results of 1 of 4 experiments are shown. (C) Cell recoveries of either pDCs cultured for 24 hours with TSLP or with CD40L plus IL-3 or cDCs cultured with TSLP. Absolute DC numbers were normalized to 105 input cells. Data (mean ± SD) correspond to 3 independent experiments. *P < .05. (D) pDCs do not mature in response to TSLP but mature in the presence of CD40L plus IL-3. Flow cytometry analysis of CD80 and CD86 expression (shaded histograms) of pDCs and cDCs cultured as described in panel C. (E) Bright-field microscopy of thymic pDCs cultured with CD40L plus IL-3 for 24 hours (top, original magnification ×40), showing an amplification of the lower right inset (bottom).
To assess whether thymic immature pDCs, like their cDC counterparts,20 could become activated in response to TSLP, both cell subsets were independently isolated based on their reciprocal CD123+/CD13+ expression (96.56% ± 1.6% and 97.96% ± 0.9% purity, respectively; n = 4; Figure 1A), and their activation profile was then analyzed on culture with rhTSLP. As shown in Figure 1C, absolute pDC numbers decreased up to 4-fold (4.34 ± 1.45; n = 3) after 24 hours of culture, compared with TSLP-treated cDCs, and induction of pDC maturation was very inefficient, as indicated by the low numbers of cells that up-regulated CD80 and CD86 (Figure 1D). In contrast, no pDC decrease was found under conventional pDC activating conditions32 with CD40L plus IL-3 (Figure 1C), and up-regulation of CD80 and CD86 activation markers was induced to levels similar to those of TSLP-activated cDCs (57.6% ± 7.0% CD86+ and 64.8% ± 10.4% CD80+ vs 83.8% ± 3.1% CD86+ and 52.4% ± 13.1% CD80+, respectively; n = 3; Figure 1D). Notably, acquisition of such a pDC mature phenotype paralleled the formation of characteristic cell aggregates and the appearance of a distinctive mature DC morphology with long dendrites (Figure 1E). Therefore, pDCs resident in the human thymus, in contrast to cDCs, are unresponsive to TSLP but mature in response to CD40L plus IL-3.
Tolerogenic potential of human thymic pDCs
To investigate whether human thymic pDCs could serve a tolerogenic role, primary immature pDCs and mature pDCs derived from them on 24 hours of culture with CD40L plus IL-3 (hereafter referred to as IpDCs and MpDCs, respectively) were analyzed using the allogeneic assay previously used to characterize tolerogenic cDCs.20 IpDCs or MpDCs were cocultured with either CD4+CD25− naive T cells from allogeneic PB (CD4PB) or CD4+CD25− SP thymocytes (CD4SP) from allogeneic donors (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article), and both T-cell proliferation and production of CD4+CD25+Foxp3+ Tregs were analyzed at day 7. In addition, autologous CD4SP thymocytes were used to examine the tolerogenic function of thymic pDCs under more physiologic conditions. As shown in Figure 2A, no cell proliferation of either allogeneic or autologous cells was induced in response to IpDCs, as CFSE intensity was comparable with that of cultures devoid of pDCs; neither CD25 nor Foxp3 up-regulation was observed. In contrast, MpDCs induced the proliferation of a significant fraction of allogeneic CD4PB and CD4SP cells, whereas proliferation of autologous CD4SP thymocytes was hardly detectable (Figure 2B). In all culture conditions, cell proliferation correlated with CD25 up-regulation (47.6% ± 24%, 39% ± 24%, and 15.4% ± 8% of CD25+ cells in CD4PB, allogeneic CD4SP, and autologous CD4SP cultures, respectively; n = 3); so that CD4+ proliferating cells were mostly CD25+, whereas CD4+ cells lacking CD25 were essentially resting cells (Figure 2B). Therefore, mature, but not immature, thymic pDCs induced a conventional allogeneic CD4+ T-cell proliferation response. More importantly, MpDCs specifically induced the expression of the Treg marker Foxp312 in a variable proportion of CD4PB and CD4SP allogeneic cells, which displayed high CD25 (CD25hi) expression (Figure 2B-C), although CD25hiFoxp3+ cell generation was consistently more efficient from the latter. However, no generation of CD25hiFoxp3+ cells was observed from autologous CD4SP thymocytes (Figure 2B). Given that Foxp3 was undetectable on naive CD4+CD25− PB T cells and thymocytes (supplemental Figure 1) and was not induced on culture with IpDCs (Figure 2A), our data suggest that thymic MpDCs specifically promoted Foxp3 up-regulation and differentiation of Tregs. Formal proof that such CD4+CD25hi Foxp3+ T cells were Tregs was provided at the functional level in a conventional suppression assay. As shown in Figure 2D, sorted CD25hi, but not CD25lo/− cells, derived from CD4PB lymphocytes, significantly inhibited the proliferation of naive PB CD4+ T cells activated with anti-CD3 and anti-CD28 mAbs plus IL-2 (60.8 ± 9.7 inhibition in 3 experiments). Together, our data indicate that thymic pDCs activated with CD40L plus IL-3 display tolerogenic potential and can induce the generation of allogeneic Tregs.
Tolerogenic potential of human thymic pDCs. (A) Ex vivo-isolated immature thymic pDCs (IpDCs) or (B) pDCs activated in response to CD40L plus IL-3 (MpDCs) were cocultured with CFSE-labeled autologous (Auto) or allogeneic (Allo) CD4+ SP thymocytes (CD4SP) or allogeneic PB CD4+ T lymphocytes (Allo CD4PB). CD25 expression of total cells (left black histograms) and CFSE expression of either total or electronically gated CD25+ and CD25− cells (middle empty histograms) was analyzed by flow cytometry after 7 days. The CFSE profile of total cells cultured in the absence of DCs is shown in black. Numbers correspond to total percentages of CD25+ cells. Analysis of CD25 versus Foxp3 expression of total cultured cells is shown on the right. Numbers correspond to percentages of CD25+Foxp3+ cells. Background staining was defined with isotype-matched Abs. Results of 1 of 3 experiments are shown. (C) Relative numbers of CD25+Foxp3+ cells recovered from cocultures of either allo CD4SP thymocytes or allo CD4PB lymphocytes with MpDCs. Data are mean ± SEM for 3 independent experiments. *P < .05. (D) Suppressive function of CD25hi cells derived from CD4PB lymphocytes primed with MpDCs. Naive peripheral CD4+ T lymphocytes stimulated with anti-CD3 and anti-CD28 mAbs plus IL-2 (20 ng/mL) were cultured in the absence (control) or presence of magnetically sorted CD25hi or CD25lo/− cells (upper right histograms). Data are mean ± SEM of [3H]-thymidine incorporation at day 5 of triplicate cultures (cpm) of 1 of 3 independent experiments. **P < .01.
Tolerogenic potential of human thymic pDCs. (A) Ex vivo-isolated immature thymic pDCs (IpDCs) or (B) pDCs activated in response to CD40L plus IL-3 (MpDCs) were cocultured with CFSE-labeled autologous (Auto) or allogeneic (Allo) CD4+ SP thymocytes (CD4SP) or allogeneic PB CD4+ T lymphocytes (Allo CD4PB). CD25 expression of total cells (left black histograms) and CFSE expression of either total or electronically gated CD25+ and CD25− cells (middle empty histograms) was analyzed by flow cytometry after 7 days. The CFSE profile of total cells cultured in the absence of DCs is shown in black. Numbers correspond to total percentages of CD25+ cells. Analysis of CD25 versus Foxp3 expression of total cultured cells is shown on the right. Numbers correspond to percentages of CD25+Foxp3+ cells. Background staining was defined with isotype-matched Abs. Results of 1 of 3 experiments are shown. (C) Relative numbers of CD25+Foxp3+ cells recovered from cocultures of either allo CD4SP thymocytes or allo CD4PB lymphocytes with MpDCs. Data are mean ± SEM for 3 independent experiments. *P < .05. (D) Suppressive function of CD25hi cells derived from CD4PB lymphocytes primed with MpDCs. Naive peripheral CD4+ T lymphocytes stimulated with anti-CD3 and anti-CD28 mAbs plus IL-2 (20 ng/mL) were cultured in the absence (control) or presence of magnetically sorted CD25hi or CD25lo/− cells (upper right histograms). Data are mean ± SEM of [3H]-thymidine incorporation at day 5 of triplicate cultures (cpm) of 1 of 3 independent experiments. **P < .01.
Positively selected CD69hiTCR-αβhi DP thymocytes in human thymus develop efficiently into nTregs in response to activated autologous pDCs
Generation of nTregs under physiologic conditions must be driven by autologous DCs, but activated pDCs induced few, if any, nTregs from autologous CD4SP thymocytes (Figure 2B). A possible explanation for these negative results may rely on the current view that nTreg thymic progenitors express self-reactive TCRs with affinities falling within the range between positive selection of conventional CD4+ T cells and negative selection of high-affinity self-reactive T cells.17 In this scenario, nTreg progenitor frequencies would be expected to be extremely low at the CD4SP stage, but higher immediately upstream of negative selection, probably at the stage of CD4+CD8+ DP TCR-αβ+ thymocytes, as suggested in mice.33 To test this hypothesis, we analyzed the nTreg potential of either activated (positively selected) or nonactivated (nonselected) DP thymocytes, isolated by Percoll density gradients and cell sorting.28 Two distinct DP populations were Percoll-isolated, namely, large- and small-sized DP thymocytes (L-DP and S-DP, respectively, > 95% CD4+CD8+; supplemental Figure 2), which differed in TCR-αβ+ cell proportions and TCR-αβ expression levels. L-DP thymocytes included a minor subset TCR-αβ+ (27.3% ± 6.5%) selectively enriched in cells with high TCR-αβ expression (TCRhi; log 3 fluorescence intensity [FI]; 42.7% ± 8.4% of L-DP TCR-αβ+ thymocytes). In contrast, S-DP thymocytes were mostly TCR-αβ+ cells (65.6% ± 3.0%), with low to intermediate TCR-αβ expression (TCRlo/int; log 1-2 FI). Importantly, essentially all TCRhi L-DP thymocytes displayed high levels of the early activation marker CD69 (CD69hi, log 2-3 FI), a phenotype expected for positively selected DP thymocytes,34 whereas both L and S TCR-αβlo/int DP thymocytes were mostly nonactivated cells with low CD69 (CD69lo, log 1-2 FI; supplemental Figure 2A).
Based on these results, we used CD69 expression levels as a criterion to further isolate positively selected thymocytes from the L-DP subset. Accordingly, L-DP thymocytes were enriched in CD69hi selected cells (referred to as L-DP CD69hi, 62.1% ± 7.3% CD69hi within TCR-αβ+ cells; n = 4) by magnetic sorting, whereas S-DP thymocytes, which were mostly devoid of CD69hi cells (referred to as S-DP CD69lo; 6.4% ± 0.9% CD69hi within TCR-αβ+ cells; n = 4), remained nonfractionated (Figure 3A left). To compare the nTreg potential of positively selected versus unselected DP thymocytes, L-DP CD69hi and S-DP CD69lo cells were cocultured with CD40L plus IL-3-activated pDCs isolated from the same thymus, and their T-cell progeny was analyzed after 7 days by flow cytometry (Figure 3A right). Primary L-DP CD69hi thymocytes were basically devoid of CD25+Foxp3+ cells, although they included a small CD25−Foxp3+ subset (7.9% ± 2.3%) whose relevance as uncommitted nTreg precursors remains unclear35 (supplemental Figure 2B). However, after coculture with activated pDCs, they gave rise to a significant production of CD25+Foxp3+ cells both in absolute (supplemental Figure 3) and relative (Figure 3A-B) numbers, which was impaired in the absence of MpDCs, indicating that pDCs play an active role in inducing the generation of nTregs. In contrast, few CD25+Foxp3+ cells were derived from S-DP CD69lo thymocytes, which were unresponsive to MpDCs, as judged by their reduced survival and expansion compared with L-DP CD69hi cells (Figure 3A-B; supplemental Figure 3). Consistent with a true de novo generation of nTregs, acquisition of the CD25+Foxp3+ phenotype was accompanied by a sequential down-regulation of CD8 from L-DP-CD69hi thymocytes, and thereby to the generation of CD4+CD8− cells (Figure 3C). More importantly, CD8 down-regulation paralleled CD25 and FoxP3 up-regulation, so that the highest expression of these 2 markers was restricted to CD4+CD8− SP thymocytes. Because generation of CD25+Foxp3+CD4+ cells was impaired when L-DP thymocytes were depleted of CD69hi cells (L-DP CD69lo; supplemental Figure 4), our results further indicate that nTreg progenitors in the human thymus selectively reside within the subset of recently selected CD69hiTCR-αβhi DP self-reactive thymocytes.
MpDCs induce the generation of CD25+Foxp3+ natural Tregs from positively selected CD69hiTCR-αβhi DP autologous thymocytes. (A) CD69 versus TCR-αβ phenotype of primary large-sized DP thymocytes enriched in CD69hi cells by magnetic sorting (L-DP CD69hi) and unselected small-sized DP thymocytes mostly composing CD69lo cells (S-DP CD69lo) (left histograms, day 0). Numbers indicate percentages of gated cells. Flow cytometric analysis of Foxp3 versus CD25 expression on L-DP CD69hi and S-DP CD69lo thymocytes cultured onto OP9 stromal cells with rhIL-2 plus rhIL-7 for 7 days either in the presence or absence of autologous MpDCs is shown on the right (day 7). Numbers in quadrants indicate percentages of CD25+Foxp3+ cells. Results are representative of 1 of 3 independent experiments. (B) Relative numbers of CD25+Foxp3+ nTregs generated from L-DP CD69hi and S-DP CD69lo thymocytes cultured as in panel A. Data are mean ± SEM (n = 3). *P < .05. (C) Flow cytometric analysis of CD4 (left) or CD8 (right) versus CD25 expression on L-DP CD69hi thymocytes cultured as in panel A (top panels). Foxp3 expression on electronically gated CD8hiCD25− (R1), CD8intCD25+ (R2), and CD8−CD25+ (R3) populations is shown (bottom panels). Numbers indicate MFI values. Results are representative of 1 of 3 independent experiments.
MpDCs induce the generation of CD25+Foxp3+ natural Tregs from positively selected CD69hiTCR-αβhi DP autologous thymocytes. (A) CD69 versus TCR-αβ phenotype of primary large-sized DP thymocytes enriched in CD69hi cells by magnetic sorting (L-DP CD69hi) and unselected small-sized DP thymocytes mostly composing CD69lo cells (S-DP CD69lo) (left histograms, day 0). Numbers indicate percentages of gated cells. Flow cytometric analysis of Foxp3 versus CD25 expression on L-DP CD69hi and S-DP CD69lo thymocytes cultured onto OP9 stromal cells with rhIL-2 plus rhIL-7 for 7 days either in the presence or absence of autologous MpDCs is shown on the right (day 7). Numbers in quadrants indicate percentages of CD25+Foxp3+ cells. Results are representative of 1 of 3 independent experiments. (B) Relative numbers of CD25+Foxp3+ nTregs generated from L-DP CD69hi and S-DP CD69lo thymocytes cultured as in panel A. Data are mean ± SEM (n = 3). *P < .05. (C) Flow cytometric analysis of CD4 (left) or CD8 (right) versus CD25 expression on L-DP CD69hi thymocytes cultured as in panel A (top panels). Foxp3 expression on electronically gated CD8hiCD25− (R1), CD8intCD25+ (R2), and CD8−CD25+ (R3) populations is shown (bottom panels). Numbers indicate MFI values. Results are representative of 1 of 3 independent experiments.
Thymic pDCs and cDCs drive differentiation of autologous TCRhiCD69hi DP thymocytes into nTregs with reciprocal cytokine expression profiles
Next, we compared mature thymic cDCs and pDCs for their autologous tolerogenic potential. As shown in Figure 4A and B, both CD40L plus IL-3–activated MpDCs and TSLP-activated cDCs (referred to as McDCs) were capable of inducing CD25+Foxp3+ nTregs from autologous L-DP CD69hi thymocytes. However, titration analyses using sorted CD25hi cells derived from either MpDC- or McDC-primed L-DP CD69hi thymocytes (referred to as CD25hi-pDC or CD25hi-cDCs, respectively; Figure 4B) showed that the former were more efficient suppressors of CD4+ T-cell proliferation (Figure 4C). To further delineate the functional potential of the 2 nTreg subsets, we assessed their cytokine expression profiles and found that both nTregs produced IL-10, although MpDCs-primed nTregs were better producers. Conversely, McDCs-primed nTregs produced greater amounts of TGF-β (Figure 4D). Supporting a functional specialization, suppression activity of CD25hi-pDC nTregs was significantly inhibited by anti-IL-10 plus anti–IL-10R blocking antibodies, whereas TGF-βRI inhibitor significantly reduced suppression mediated by cDC-primed CD25hi nTregs (Figure 4E). Therefore, pDCs and cDCs resident in the human thymus can prime positively selected DP autologous thymocytes to differentiate into functionally distinct CD4+CD25+Foxp3+ nTregs.
Thymic mature pDCs and cDCs can prime autologous CD69hiTCR-αβhi DP thymocytes to differentiate into nTregs with distinct cytokine expression profiles. (A) Absolute (top panel) and relative numbers (bottom panel) of CD25+Foxp3+ cells derived from CD69hiTCR-αβhi DP thymocytes cocultured with either MpDCs or McDCs as described in Figure 3A. Data are mean ± SEM (n = 4). *P < .05. **P < .01. (B) Flow cytometry of CD25 and Foxp3 expression on cells generated from McDC- or MpDC-primed L-DP CD69hi thymocytes as described in panel A (top panels) and on the CD25hi-enriched population isolated from them by magnetic sorting (bottom panels). (C) Suppressive function of CD25hi cells isolated as in panel B was assessed by [3H]-thymidine incorporation as described in Figure 2D at the indicated CD25hi/T-cell ratios Data are mean ± SD (cpm) of triplicate cultures from a representative experiment. **P < .01. (D) IL-10 and TGF-β production by L-DP CD69hi thymocytes cultured either in the absence or presence of McDCs or MpDCs as in panel A was assessed by ELISA. Data are mean ± SD (pg/mL) of triplicates for a representative experiment of 3. *P < .05. **P < .01. (E). Suppressive function of CD25hi cells generated from L-DP CD69hi thymocytes on MpDC or McDC priming and isolated as in panel B was assessed by [3H]-thymidine incorporation at a 1:2 CD25hi/T-cell ratio either in the absence or presence of anti-IL-10 plus anti-IL-10R blocking Abs or TGF-βRI inhibitor. Isotyped-matched Abs and dimethyl sulfoxide carrier were used as controls. Data represent fold inhibition of PBCD4 T-cell proliferation normalized to cpm values obtained in cultures performed in the absence of blocking reagents. Data are mean ± SEM values of triplicate cultures from a representative experiment. **P < .01.
Thymic mature pDCs and cDCs can prime autologous CD69hiTCR-αβhi DP thymocytes to differentiate into nTregs with distinct cytokine expression profiles. (A) Absolute (top panel) and relative numbers (bottom panel) of CD25+Foxp3+ cells derived from CD69hiTCR-αβhi DP thymocytes cocultured with either MpDCs or McDCs as described in Figure 3A. Data are mean ± SEM (n = 4). *P < .05. **P < .01. (B) Flow cytometry of CD25 and Foxp3 expression on cells generated from McDC- or MpDC-primed L-DP CD69hi thymocytes as described in panel A (top panels) and on the CD25hi-enriched population isolated from them by magnetic sorting (bottom panels). (C) Suppressive function of CD25hi cells isolated as in panel B was assessed by [3H]-thymidine incorporation as described in Figure 2D at the indicated CD25hi/T-cell ratios Data are mean ± SD (cpm) of triplicate cultures from a representative experiment. **P < .01. (D) IL-10 and TGF-β production by L-DP CD69hi thymocytes cultured either in the absence or presence of McDCs or MpDCs as in panel A was assessed by ELISA. Data are mean ± SD (pg/mL) of triplicates for a representative experiment of 3. *P < .05. **P < .01. (E). Suppressive function of CD25hi cells generated from L-DP CD69hi thymocytes on MpDC or McDC priming and isolated as in panel B was assessed by [3H]-thymidine incorporation at a 1:2 CD25hi/T-cell ratio either in the absence or presence of anti-IL-10 plus anti-IL-10R blocking Abs or TGF-βRI inhibitor. Isotyped-matched Abs and dimethyl sulfoxide carrier were used as controls. Data represent fold inhibition of PBCD4 T-cell proliferation normalized to cpm values obtained in cultures performed in the absence of blocking reagents. Data are mean ± SEM values of triplicate cultures from a representative experiment. **P < .01.
Generation of human nTreg cells induced by pDCs and cDCs involves CD80/86 but not ICOSL coreceptors
Two subsets of Treg cells with different IL-10/TGF-β expression potentials have been identified in human thymus and periphery based on surface expression of ICOS molecule (ICOS+ and ICOS−).36 Thus, we next analyzed ICOS expression on nTregs induced by MpDCs or McDCs (Figure 5A). L-DP CD69hi precursors displayed low ICOS expression in vivo (log 1-2 FI), although somewhat higher than L-DP CD69lo thymocytes (70.6% ± 5.6% vs 48.9% ± 10.9% ICOS+ cells and 11.9 ± 1.3 vs 6.8 ± 1.4 mean FI [MFI], respectively; Figure 5B). However, ICOS up-regulation was induced (log 2-3 MFI) on CD25+CD4+ nTregs derived in the presence of either McDCs or MpDCs (Figure 5A), although a slightly higher expression was induced on the former (35.7 ± 6.4 vs 26.9 ± 3.3 MFI, respectively). Importantly, we also identified 2 independent subsets of ICOSlo and ICOS+ nTregs in the adult human thymus in vivo (supplemental Figure 5), which may be equivalent to the ICOS− and ICOS+ thymic subsets reported before.36 Whereas these data could suggest a functional role of ICOS and ICOSL in the generation of human nTregs, ICOSL expression on thymic DCs has not been reported as yet. We found that ICOSL was up-regulated on maturation on both pDCs and cDCs (77.5% ± 18.1% and 74.4% ± 13.4% respectively; Figure 5C). However, blocking mAbs to ICOSL weakly affected nTreg generation induced by MpDCs or McDCs, compared with isotype-matched controls (Figure 5D-E), and a similar effect was observed with anti-CD80 mAbs. In contrast, generation of nTregs was markedly impaired by anti-CD86, and simultaneous CD80/CD86 blockade resulted in a synergistic inhibition of up to 80% of nTreg production (Figure 5D-E), whereas cell recovery of CD25− cells was unaffected (supplemental Figure 6). Notably, both L-DP CD69hi and L-DP CD69lo thymocytes expressed the CD28 receptor of CD80/CD86 (98.9% ± 0.9% and 95.4% ± 3.9%, respectively), although at higher levels on the former (134.5 ± 4.5 vs 60.5 ± 19.9 MFI, respectively; Figure 5B). Collectively, these data support a functional role of the CD28-CD80/CD86, but not of the ICOS-ICOSL, signaling pathway in the generation of human nTregs.
Differentiation of nTregs from L-DP CD69hi thymocytes primed with MpDCs or McDCs involves CD80 and CD86 coreceptors. (A) Flow cytometric analysis of ICOS versus CD25 expression on nTregs derived from L-DP CD69hi thymocytes primed with McDCs or MpDCs as in Figure 4. (B) Flow cytometry analysis of ICOS and CD28 expression on L-DP CD69hi and L-DP CD69lo primary thymocytes. Numbers indicate percentages of positive cells. MFI values of CD28 expression are indicated. (C) ICOSL expression analyzed by flow cytometry on thymic pDCs and cDCs either ex vivo-isolated or after maturation in vitro. Numbers indicate percentages of positive cells. Results of one of 3 experiments are shown. (D-E) Effect of blocking anti-ICOSL, anti-CD80, anti-CD86, or both anti-CD80 and anti-CD86 mAbs in the generation of Tregs from MpDC- or McDC-primed L-DP CD69hi thymocytes. Generation of CD25+Foxp3+ nTregs was analyzed by flow cytometry at day 7. (D) Data are mean ± SEM of relative numbers of CD25+Foxp3+ cells normalized to numbers in control cultures with isotype-matched control antibodies (CAb) (n = 3). *P < .05. **P < .01. (E) Flow cytometry analysis of CD25+Foxp3+ cell generation of 1 representative experiment of 3 in panel D.
Differentiation of nTregs from L-DP CD69hi thymocytes primed with MpDCs or McDCs involves CD80 and CD86 coreceptors. (A) Flow cytometric analysis of ICOS versus CD25 expression on nTregs derived from L-DP CD69hi thymocytes primed with McDCs or MpDCs as in Figure 4. (B) Flow cytometry analysis of ICOS and CD28 expression on L-DP CD69hi and L-DP CD69lo primary thymocytes. Numbers indicate percentages of positive cells. MFI values of CD28 expression are indicated. (C) ICOSL expression analyzed by flow cytometry on thymic pDCs and cDCs either ex vivo-isolated or after maturation in vitro. Numbers indicate percentages of positive cells. Results of one of 3 experiments are shown. (D-E) Effect of blocking anti-ICOSL, anti-CD80, anti-CD86, or both anti-CD80 and anti-CD86 mAbs in the generation of Tregs from MpDC- or McDC-primed L-DP CD69hi thymocytes. Generation of CD25+Foxp3+ nTregs was analyzed by flow cytometry at day 7. (D) Data are mean ± SEM of relative numbers of CD25+Foxp3+ cells normalized to numbers in control cultures with isotype-matched control antibodies (CAb) (n = 3). *P < .05. **P < .01. (E) Flow cytometry analysis of CD25+Foxp3+ cell generation of 1 representative experiment of 3 in panel D.
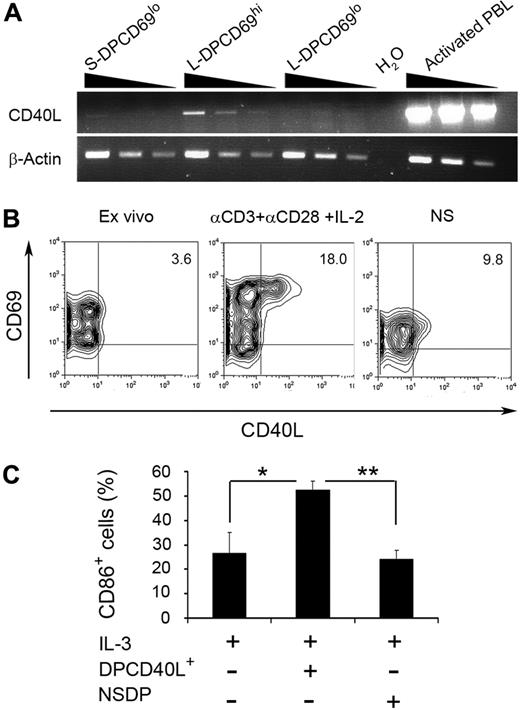
TCR-mediated up-regulation of CD40L on nTreg thymic progenitors induces the activation of autologous pDCs
Next, we sought to get some insights into the physiologic mechanisms responsible for the generation of tolerogenic pDCs and the production of nTregs in the human thymus in vivo. To this end, we searched for putative intrathymic sources of CD40L production and focused on positively selected L-DP CD69hi nTreg precursors. Although surface CD40L was hardly detectable on those cells under steady-state conditions, analysis of CD40L expression at the transcriptional level showed that CD40L is indeed selectively transcribed in vivo in the human thymus in L-DP CD69hi nTreg precursors (Figure 6A-B). Accordingly, a fast (18-24 hours) CD40L up-regulation was consistently induced (9.7% ± 4.2%) on cell activation with anti-CD3 and -CD28 mAbs plus IL-2 (Figure 6B); these data further support a selective expression of CD40L on positively selected DP thymocytes. More importantly, CD40L-expressing L-DP CD69hi thymocytes induced a significant maturation of thymic pDCs supplemented with IL-3, whereas a discrete maturation was observed in response to either nonstimulated L-DP CD69hi thymocytes or IL-3 alone (Figure 6C). Therefore, TCR engagement on L-DP CD69hi nTreg precursors may lead to the up-regulation of CD40L, which thereby interacts with CD40 on thymic pDCs consequently promoting their maturation.
TCR-mediated activation of L-DP CD69hi thymocyte-expressing CD40L transcripts leads to CD40L up-regulation and activation of thymic pDCs. (A) Reverse-transcribed polymerase chain reaction analysis of CD40L mRNA expression was performed using 2-fold serial dilutions of cDNA from primary S-DP CD69lo, L-DP CD69hi, and L-DP CD69lo thymocyte populations isolated from the same thymus sample (top panel). cDNA obtained from phorbol myristate acetate plus ionomycin-stimulated PB CD4+ T lymphocytes was used as a positive control. (B) Flow cytometry analysis of CD69 versus CD40L expression on primary L-DP CD69hi thymocytes before culture (left panel), after activation with anti-CD3 and anti-CD28 mAbs plus IL-2 for 24 hours (middle panel), or after culture in medium alone (right panel). NS indicates nonstimulated. Numbers in quadrants indicate percentages of positive cells. (C) Relative numbers of CD86-expressing pDCs after 24 hours of culture with IL-3 in the absence or presence of L-DP CD69hi thymocytes either stimulated (DPCD40L+) or nonstimulated (NS) as in panel A. Data are mean ± SEM (n = 3). *P < .05. **P < .01.
TCR-mediated activation of L-DP CD69hi thymocyte-expressing CD40L transcripts leads to CD40L up-regulation and activation of thymic pDCs. (A) Reverse-transcribed polymerase chain reaction analysis of CD40L mRNA expression was performed using 2-fold serial dilutions of cDNA from primary S-DP CD69lo, L-DP CD69hi, and L-DP CD69lo thymocyte populations isolated from the same thymus sample (top panel). cDNA obtained from phorbol myristate acetate plus ionomycin-stimulated PB CD4+ T lymphocytes was used as a positive control. (B) Flow cytometry analysis of CD69 versus CD40L expression on primary L-DP CD69hi thymocytes before culture (left panel), after activation with anti-CD3 and anti-CD28 mAbs plus IL-2 for 24 hours (middle panel), or after culture in medium alone (right panel). NS indicates nonstimulated. Numbers in quadrants indicate percentages of positive cells. (C) Relative numbers of CD86-expressing pDCs after 24 hours of culture with IL-3 in the absence or presence of L-DP CD69hi thymocytes either stimulated (DPCD40L+) or nonstimulated (NS) as in panel A. Data are mean ± SEM (n = 3). *P < .05. **P < .01.
Plamacytoid DCs and natural Tregs colocalize within the human thymic medulla
To analyze whether cross-talk between pDCs and nTreg cell precursors could take place in vivo, we assessed the distribution of both cell types within the human postnatal thymus. Immunohistochemistry and confocal microscopy of anti–pan-cytokeratin (Cytok) and Topro-3–labeled thymic sections allowed the identification of thymic cortex and medulla, as cytokeratin expression is lower in the former (Figure 7A top panels). Foxp3+ nTregs selectively localized to the thymic medulla (Figure 7A bottom panels), together with CD123+ pDCs (Figure 7B). Notably, interactions between pDCs and Foxp3+ cells could be found within the medullary area (Figure 7C top left inset). Quantification analysis of distinct sections showed that 20.9 plus or minus 4.0 of pDCs were in contact with Foxp3+ cells, whereas 7.7 plus or minus 1.7 of Foxp3+ cells interacted with pDCs (Figure 7D). These results suggest that medullary pDCs could interact with developing thymocytes and control their differentiation into nTregs in the steady-state human thymus.
Human Foxp3+ nTreg cells colocalize with CD123+ pDCs within the human thymic medulla. (A) Pan-cytokeratin (Cytok, green) immunostaining of human TECs, and nuclei staining with TOPRO-3 (blue) showing differential expression of Cytok in thymic medulla (M) and cortex (C). (Top panels) Original magnification 10×. Bar represents 100 μm. Double staining of Cytok (green) and Foxp3 (red) showing the localization of Foxp3+ nTregs in the thymic medulla (bottom panels). Original magnification 25×. Bar represents 50 μm. (B-C) Double immunostaining of CD123 (green) and Foxp3 (red) showing colocalization of pDCs and nTregs within the thymic medulla (B). Original magnification 10×. Bar represents 100 μm and highlighting close interactions among them (C arrows and upper left inset). Original magnification 40×. Bar represents 50 μm. (D) Quantification of Foxp3+ nTregs interacting with CD123+ pDCs, and vice versa, in the human thymus. Data are mean ± SEM (percent) of interacting cells within 1465 Foxp3+ and 518 CD123+ cells in 7 fields from 3 independent tissue sections.
Human Foxp3+ nTreg cells colocalize with CD123+ pDCs within the human thymic medulla. (A) Pan-cytokeratin (Cytok, green) immunostaining of human TECs, and nuclei staining with TOPRO-3 (blue) showing differential expression of Cytok in thymic medulla (M) and cortex (C). (Top panels) Original magnification 10×. Bar represents 100 μm. Double staining of Cytok (green) and Foxp3 (red) showing the localization of Foxp3+ nTregs in the thymic medulla (bottom panels). Original magnification 25×. Bar represents 50 μm. (B-C) Double immunostaining of CD123 (green) and Foxp3 (red) showing colocalization of pDCs and nTregs within the thymic medulla (B). Original magnification 10×. Bar represents 100 μm and highlighting close interactions among them (C arrows and upper left inset). Original magnification 40×. Bar represents 50 μm. (D) Quantification of Foxp3+ nTregs interacting with CD123+ pDCs, and vice versa, in the human thymus. Data are mean ± SEM (percent) of interacting cells within 1465 Foxp3+ and 518 CD123+ cells in 7 fields from 3 independent tissue sections.
Discussion
In this study, we developed an autologous experimental system that provided important information on the cellular and molecular mechanisms underlying generation of nTregs in the human thymus. We show that thymic pDCs are efficient tolerogenic DCs, able to induce nTreg generation, and provide evidence that nTreg precursors specifically reside within the subset of positively selected DP thymocytes expressing high CD69 and TCR-αβ levels.9,34 Therefore, human nTreg generation may be induced immediately downstream of positive selection in recently selected DP thymocytes. Importantly, pDC-induced nTreg development seems to involve the CD40-CD40L pathway, as nTreg progenitors up-regulated CD40L on TCR engagement and induced maturation of pDCs. Given that mature, but not immature, pDCs induced nTregs, we propose that CD40-CD40L-mediated cross-talk between pDCs and self-reactive nTreg precursors may provide critical feedback signals required for pDC maturation and nTreg differentiation in the steady-state human thymus.
Besides pDCs, cDCs also induced nTregs from CD69hiTCRhi DP, but not from CD4+CD25− SP, autologous thymocytes, although the latter have been shown to generate nTregs in an allogeneic setting.20 These data indicate that CD4+ SP, but not CD69hi DP, thymocytes may be devoid of nTreg potential in the steady-state thymus, as expected of mature thymocytes that have succeeded negative selection and display a nonautoreactive TCR repertoire.37 Accordingly, formal evidence has recently been provided that the efficiency of nTreg generation inversely correlated with progressive maturation in the murine thymus.38 Therefore, nTreg cell generation may happen in a narrow developmental window, immediately downstream of positive selection of those DP thymocytes that express anti-self-TCRs with moderate to high avidity below the threshold of negative selection. Because nTreg generation paralleled a selective expansion of CD25+ cells and Foxp3+ nTregs extensively proliferated in our cultures, expansion of minute numbers of preexisting Foxp3+ progenitors cannot be formally excluded. However, Foxp3 and CD25 were up-regulated in DP thymocytes, which simultaneously down-regulated CD8, suggesting that nTreg differentiation occurred de novo.
The nature of thymic APCs that induce nTregs is still controversial. Some studies showed that Treg development involved TCR-mediated high-affinity selection on cortical TECs,9,18 whereas others suggested the participation of a “saturable” thymic niche with a selective capacity much smaller than the niche supporting conventional positive selection.39,40 Thus, DCs located in the thymus medulla may represent the limiting niche providing unique signals for nTreg generation from self-reactive DP progenitors positively selected by cortical TECs. Supporting this view, mouse thymic DCs make a significant contribution to nTreg generation in vivo,19 although other cell types, such as TECs, may play such an instructive role in their absence.41 As a whole, available information concurs with the recent proposal that instructive generation of nTregs does not require unique features of a dedicated APC, but rather is dependent on quantitative features of external cues.38
Regarding the DC-derived signals required for nTreg priming, CD80 and CD86 coreceptors appear critical for the induction of both pDC- and cDC-primed nTregs, suggesting the participation of the CD28 pathway in humans, as shown in mice.13 Besides CD80/86, ICOSL is up-regulated on both pDCs and cDCs on maturation, although ICOSL expression is specific of pDCs in the periphery and selectively contributes to the generation of IL-10–producing Tregs.42 The identification of ICOS+ and ICOS− human Tregs with a reciprocal pattern of IL-10 and TGF-β production36 supports a specific role of the ICOS-ICOSL pathway in priming IL-10–producing Tregs. In our system, pDC-primed nTregs were also poised to IL-10 production and IL-10–dependent function, whereas cDC-induced nTregs were better TGF-β producers. However, thymic pDCs and cDCs appear to control the functional dichotomy of nTreg cells in an ICOS-ICOSL-independent manner. Thus, nTreg functional specialization could rely on the capacity of pDCs and cDCs to induce largely nonoverlapping Treg repertoires because of their distinct antigen-presenting abilities.43
Thymic pDCs and cDCs were found to become tolerogenic in response to distinct stimuli, suggesting that specific niches of mature DCs may be restricted to particular areas of the thymus. Indeed, cDCs are known to respond to TSLP locally produced by medullary TECs of Hassal corpuscles.20 However, IL-3 required for pDC survival is produced by all TECs,44 although CD40L expression seems restricted to positively selected thymocytes,45 which could then selectively induce pDC maturation in the medulla. Accordingly, CD40L transcription was confined in vivo to activated CD69hi DP human thymocytes, which, however, displayed undetectable CD40L surface expression. In this regard, weak but functional CD40L levels could be expressed in vivo on a restricted CD69hi DP subset, as judged by both the low frequency of CD40L+ cells derived from CD69hi DP thymocytes on in vitro polyclonal activation and their low levels of CD40L expression. Supporting a regulatory role for CD40L in pDC function, ligation of CD40 on thymic IpDCs by CD40L, expressed on activated CD69hi DP thymocytes on TCR engagement, induced pDC maturation and thereby nTreg cell production, at least in vitro. Similarly, ligation of CD40 is required for maturation of peripheral DCs.2 Therefore, as shown for peripheral Tregs,46,47 the CD40-CD40L pathway may play a relevant role in nTreg development in the human thymus, as proposed in mouse.48 Strengthening this possibility, defective CD40-CD40L signaling leads to autoimmunity in both humans and mice.46-48 In this scenario, we propose that recognition of self-peptide-major histocompatibility complexes by high-affinity TCRs expressed on positively selected DP thymocytes may be the mechanism that triggers CD40L expression on nTreg progenitors and leads to feedback maturation of thymic pDCs in vivo, thereby facilitating a functional cross-talk between pDCs and developing thymocytes, resulting in terminal nTreg differentiation. Still, further in vivo studies are required to establish the physiologic relevance of CD40-CD40L in such cross-talk. Our finding, that a substantial fraction of pDCs in the steady-state human thymus displays an activated phenotype, suggests that they may well be capable of inducing nTregs under physiologic conditions. Nevertheless, the existence of “inducer” stromal cells other than pDCs cannot be excluded.38 Histologic evidence is provided of pDC-nTreg colocalization within the human thymic medulla, which may also support a functional interaction between the 2 cell types in vivo. However, Foxp3 up-regulation during nTreg development takes more than 2 days. Therefore, limitations inherent to the one-point-in-time histologic technique require dynamic analyses to clarify whether CD123/Foxp3 colocalization represents long-lasting interactions between “inducer” pDCs and nTreg progenitors still in contact. We think that this is probable considering that stable DC–T-cell interactions can last for longer periods than initially thought, up to several days, and persistent Treg-DC contacts do actually precede the inhibition of autoimmune responses in nonobese diabetic mice.49
The functional role of peripheral pDCs in several physiopathologic situations related to tolerance induction is well established.49,50 Still, understanding pDC function in the pathogenesis of human diseases has just begun. Therefore, understanding how thymic DCs, either resident or recirculating through the blood,19 control generation of an autologous polyclonal repertoire from intrathymic progenitors could open new opportunities for the development of therapeutic strategies to prevent or reverse unwanted immunity.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank P. Portolés for providing the anti-ICOS mAb, J. Ochando, F. Sánchez-Madrid, P. Martín, A. Urzainki, M. García-Peydró, and M.J. García-León for helpful discussions, J. Alcain for technical support, and the Pediatric Cardiosurgery Units from Centro Especial Ramón y Cajal and Ciudad Sanitaria La Paz (Madrid, Spain) for thymus samples.
This work was supported by Plan Nacional (grants SAF2004-01122, PLE2007-0110, and BFU 2007-60990), Comunidad de Madrid (grant S-SAL0304-2006), Fundación MM, Instituto de Salud Carlos III (grant RECAVA RD06/0014/1012), and Fundación Ramón Areces (institutional grant). E.M.-G. was supported by MICINN (FPU program) and by CAM.
Authorship
Contribution: E.M.-G. performed most experiments; E.S.-F. and A.L.C. performed some experiments and provided reagents; and M.L.T. designed and directed the study and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: María L. Toribio, Centro de Biología Molecular Severo Ochoa, Consejo Superior de Investigaciones Científicas, Universidad Autónoma de Madrid, C/Nicolás Cabrera, 1 Cantoblanco, 28049 Madrid, Spain; e-mail: mtoribio@cbm.uam.es.


![Figure 2. Tolerogenic potential of human thymic pDCs. (A) Ex vivo-isolated immature thymic pDCs (IpDCs) or (B) pDCs activated in response to CD40L plus IL-3 (MpDCs) were cocultured with CFSE-labeled autologous (Auto) or allogeneic (Allo) CD4+ SP thymocytes (CD4SP) or allogeneic PB CD4+ T lymphocytes (Allo CD4PB). CD25 expression of total cells (left black histograms) and CFSE expression of either total or electronically gated CD25+ and CD25− cells (middle empty histograms) was analyzed by flow cytometry after 7 days. The CFSE profile of total cells cultured in the absence of DCs is shown in black. Numbers correspond to total percentages of CD25+ cells. Analysis of CD25 versus Foxp3 expression of total cultured cells is shown on the right. Numbers correspond to percentages of CD25+Foxp3+ cells. Background staining was defined with isotype-matched Abs. Results of 1 of 3 experiments are shown. (C) Relative numbers of CD25+Foxp3+ cells recovered from cocultures of either allo CD4SP thymocytes or allo CD4PB lymphocytes with MpDCs. Data are mean ± SEM for 3 independent experiments. *P < .05. (D) Suppressive function of CD25hi cells derived from CD4PB lymphocytes primed with MpDCs. Naive peripheral CD4+ T lymphocytes stimulated with anti-CD3 and anti-CD28 mAbs plus IL-2 (20 ng/mL) were cultured in the absence (control) or presence of magnetically sorted CD25hi or CD25lo/− cells (upper right histograms). Data are mean ± SEM of [3H]-thymidine incorporation at day 5 of triplicate cultures (cpm) of 1 of 3 independent experiments. **P < .01.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/115/26/10.1182_blood-2009-10-248260/4/m_zh89991053780002.jpeg?Expires=1767699605&Signature=yKPzMH0RQxbT~oEsBzl~LppS~3xmbeIYyHSw80ah5rbeL5sZikltPJB8xnWIFqJ4Z3Yk28zb-wzcHUKn3wKZNOUyJQpMsfWaD-35iqDUD9ZajYr06Q4MIOf3hhcc8x1n6o-3HIrFrNLjWwa~OMi4pIcfh2Hb1J6gSNOOIDn5kX6yghbq4JOjz0QvUm715sKJsg0q0bo44-Y1o4FNNywVS7Zwd6hS6~xZ-8QDf8QH3NGBpJibFKalffm-4ePOqqz5c0sLwXNOD0pKkR2fJsHlto824Jnu9n9UeZH7esi5NT3VBU-fFagwABJHunKyOAEzOTfcS4UMUvAKZ9vkieMc9g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Figure 4. Thymic mature pDCs and cDCs can prime autologous CD69hiTCR-αβhi DP thymocytes to differentiate into nTregs with distinct cytokine expression profiles. (A) Absolute (top panel) and relative numbers (bottom panel) of CD25+Foxp3+ cells derived from CD69hiTCR-αβhi DP thymocytes cocultured with either MpDCs or McDCs as described in Figure 3A. Data are mean ± SEM (n = 4). *P < .05. **P < .01. (B) Flow cytometry of CD25 and Foxp3 expression on cells generated from McDC- or MpDC-primed L-DP CD69hi thymocytes as described in panel A (top panels) and on the CD25hi-enriched population isolated from them by magnetic sorting (bottom panels). (C) Suppressive function of CD25hi cells isolated as in panel B was assessed by [3H]-thymidine incorporation as described in Figure 2D at the indicated CD25hi/T-cell ratios Data are mean ± SD (cpm) of triplicate cultures from a representative experiment. **P < .01. (D) IL-10 and TGF-β production by L-DP CD69hi thymocytes cultured either in the absence or presence of McDCs or MpDCs as in panel A was assessed by ELISA. Data are mean ± SD (pg/mL) of triplicates for a representative experiment of 3. *P < .05. **P < .01. (E). Suppressive function of CD25hi cells generated from L-DP CD69hi thymocytes on MpDC or McDC priming and isolated as in panel B was assessed by [3H]-thymidine incorporation at a 1:2 CD25hi/T-cell ratio either in the absence or presence of anti-IL-10 plus anti-IL-10R blocking Abs or TGF-βRI inhibitor. Isotyped-matched Abs and dimethyl sulfoxide carrier were used as controls. Data represent fold inhibition of PBCD4 T-cell proliferation normalized to cpm values obtained in cultures performed in the absence of blocking reagents. Data are mean ± SEM values of triplicate cultures from a representative experiment. **P < .01.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/115/26/10.1182_blood-2009-10-248260/4/m_zh89991053780004.jpeg?Expires=1767699605&Signature=w6CqETyn6s2Vbs5DV78r1Czm9YTO9q6IO9anH~bNZavMqfTdfxRCTkkHd8FpdHXMe1qWbUr0famkWzfQLEMSKipzBuemPOPzXhfnESf4jJat8EuPxCw5tAvqO27XBjzCEXqu8E9zfOk-Bv-pLYv8NxQoo3FDYPF5mEK4F3VaVfMZiDua-612fXH9m1Hn~5jEvA1piZTARF59obCMKQZh84dqwu~u-ZY0jx3Zb2Pa~AcCtd3Q5Jj5N9DXKWOLpLuupmC0dNfIU-KtLbQ1MwxyCKigrcU~am14SyR3Pu~SUfRCOBw5nm~mk0S91J~qrsaWn6HqagTHaLGARbavD7RPTA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal