Abstract
Rituximab, a monoclonal antibody that targets CD20 on B cells, is now central to the treatment of a variety of malignant and autoimmune disorders. Despite this success, a substantial proportion of B-cell lymphomas are unresponsive or develop resistance, hence more potent anti-CD20 monoclonal antibodies (mAbs) are continuously being sought. Here we demonstrate that type II (tositumomab-like) anti-CD20 mAbs are 5 times more potent than type I (rituximab-like) reagents in depleting human CD20 Tg B cells, despite both operating exclusively via activatory Fcγ receptor–expressing macrophages. Much of this disparity in performance is attributable to type I mAb-mediated internalization of CD20 by B cells, leading to reduced macrophage recruitment and the degradation of CD20/mAb complexes, shortening mAb half-life. Importantly, human B cells from healthy donors and most cases of chronic lymphatic leukemia and mantle cell lymphoma, showed rapid CD20 internalization that paralleled that seen in the Tg mouse B cells, whereas most follicular lymphoma and diffuse large B-cell lymphoma cells were far more resistant to CD20 loss. We postulate that differences in CD20 modulation may play a central role in determining the relative efficacy of rituximab in treating these diseases and strengthen the case for focusing on type II anti-CD20 mAb in the clinic.
Introduction
The antibody rituximab is widely used in the treatment of B-cell diseases, and many second- and third-generation anti-CD20 monoclonal antibodies (mAbs) are currently undergoing clinical development.1 Persistent lack of clarity regarding the precise mode of action for anti-CD20 mAb is reflected in the variety of approaches being taken to improve the potency of these reagents.2 Although it is widely accepted that Fc/Fc γ receptor (FcγR) interactions are vital,3,4 the importance of the 2 other potential effector pathways, complement-dependent cytotoxicity (CDC) and programmed cell death, is still disputed.5 We have previously described 2 classes of anti-CD20 mAb, based on differences in their engagement of the antigen: type I (rituximab-like) and type II (tositumomab-like). Type I mAbs are able to redistribute CD20 into lipid rafts and evoke complement activation, whereas type II induce homotypic adhesion and nonapoptotic cell death.6-8 Interestingly, type I and II reagents cannot be distinguished by the region of CD20 they engage because almost all anti-CD20 mAbs share small, closely overlapping epitopes that focus on an A×P motif identified previously.9,10 It seems more likely that it is the orientation of binding and the degree of CD20 cross-linking that determine their type and nature.
The efficacy of rituximab in lymphoma therapy varies according to tumor burden11,12 and subtype. Although follicular lymphomas (FLs) are relatively sensitive, B-chronic lymphatic leukemia (B-CLL) and small lymphocytic lymphoma (SLL) are significantly less so,13 such that dose escalation with approximately 6 times more mAbs is required for significant clinical activity.14,15 Although the second-generation type I anti-CD20 mAb ofatumumab appears promising,16-18 large (2 g) quantities of mAbs are being administered in these trials. The lower level of CD20 expression on CLL is put forward as a possible explanation,19 although intuitively this might be expected to consume less mAbs. Similarly, it has been suggested that soluble CD20 might consume rituximab in CLL,20 but this result has not been confirmed and seems unlikely given the hydrophobic nature of CD20 which, with a predicted tetraspan structure, is doubtful to persist as a soluble, antigenically intact molecule. Some reports have also suggested that modulation of CD20 occurs on CLL and non-Hodgkin lymphoma (NHL) lines, but these data have been largely unconfirmed with little indication in the literature to suggest that this is an important mechanism that impedes efficacy.21,22 We now show that modulation is potentially a key factor in determining the potency of CD20 mAbs against certain B-cell targets and propose that type II reagents that avoid internalization are probably more effective drugs for deleting autoimmune and malignant B cells in various disorders.
Methods
Animals and cells
Mice were bred and maintained in local facilities. hCD20 Tg mice have been described previously.1 Other genetically altered strains used were γchain−/−, C1q−/−, C3−/− (Jax Mice), and Vav-Bcl-2 Tg. hCD20 Tg × γ chain−/− and Vav-Bcl-2 × hCD20 Tg mice were obtained by crossbreeding, with genotypes confirmed by polymerase chain reaction (PCR) and/or flow cytometry. Animal experiments were cleared through local ethical committee and were performed under Home Office license PPL30/2451. Human cell lines were obtained from ECACC (Daudi, Raji, Ramos, SU-DHL-4), a kind gift from Dr J. Teeling (Granta 519), or a kind gift from Dr L. Nolan (DOHH-2, HBL-1, RL) and were maintained in antibiotic-free media. Mouse splenic B cells and normal human peripheral B cells were purified by negative selection using MACS B-cell isolation kits (Miltenyi Biotec).
Antibodies and reagents
All mAbs have previously been described,1 except for FGM6, which is a novel type II anti-CD20 mAb constructed from patented published sequences with additional mutations. mAbs were produced in either the 293F Freestyle system (Invitrogen) or CHO-K1. IgG was purified on Protein A with purity assessed by electrophoresis (Beckman EP system; Beckman) and lack of aggregation by high-performance liquid chromatography. F(ab′)2 fragments were produced as described previously.23 Antibodies used for Western blotting were anti-CD20 (7D1; AbDSerotec), anti-actin (Sigma-Aldrich), and anti–Alexa 488 (Invitrogen). Clodronate (Sigma-Aldrich) or phosphate-buffered saline containing liposomes were produced as detailed by van Rooijen and Sanders.24 The actin inhibitor Latrunculin B was from Calbiochem and azide, etoposide, and dexamethasone from Sigma-Aldrich.
Flow cytometry
Fluorescently conjugated mAbs were from BD Biosciences, AbDSerotec, or made in-house. Flow cytometry was as described previously25 with samples assessed on a FACScan, FACSCalibur, or FACSCanto II with data analyzed with CellQuest Pro or FACSDiva (all BD Biosciences). To assay anti-CD20 mAb (mouse IgG2 subclass) concentrations, sera were incubated with human SU-DHL-4 cells (hCD20+), and cell-bound anti-CD20 detected using fluorescein isothiocyanate (FITC)-labeled goat anti–mouse Fc (Jackson ImmunoResearch Laboratories) with reference to a standard curve. To determine surface expression of CD20 after mAb treatment in vivo or in vitro, available antigen was saturated with excess anti-CD20 mAb (10 μg/mL), cells washed, and then surface anti-CD20 detected using R-phycoerythrin (RPE)–labeled goat anti–mouse Fc (Jackson ImmunoResearch Laboratories); B cells were then identified with allophycocyanin (APC)-labeled anti–mouse CD19. Immunofluorescent staining to detect Bcl-2 levels in hCD20 Tg B cells was performed with FITC-labeled anti–Bcl-2 (BD Biosciences) according to the manufacturer's instructions.
B-cell depletion experiments
hCD20 Tg mice received a single intravenous dose of mAbs and B cells remaining in the blood or organs assessed by flow cytometry or immunohistochemistry.1 Residual B cells were plotted as percentage of B cells in mice treated with irrelevant isotype-matched mAb (WR17) or phosphate-buffered saline. In adoptive transfer experiments, splenocytes from hCD20 Tg and wild-type (WT) mice were labeled with 5μM and 0.5μM carboxyfluorescein succinimidyl ester (CFSE; Invitrogen), respectively, mixed (1:1), and injected intravenously into recipients (1-3 × 106/mouse), followed 24 hours later by mAbs intravenously. Sixteen hours later, splenocytes were analyzed to determine target to nontarget CFSE-labeled B cells ratio. To assess depletion of hCD20 Tg × Vav-Bcl-2 B cells, hCD20 × Vav-Bcl-2 and hCD20 Tg splenocytes were labeled with 5μM and 0.5μM CFSE, respectively, and WT splenocytes with 20μM PKH26 (Sigma-Aldrich), mixed (1:1:1), injected, and treated with mAbs intravenously 24 hours later. Sixteen hours later the ratio of both target B-cell populations was compared with the PKH26-labeled nontarget cells.
Clinical samples
Ethical approval for the use of clinical samples was obtained by the Southampton University Hospitals National Health Service Trust from the Southampton and South West Hampshire Research Ethics Committee. Informed consent was provided in accordance with the Declaration of Helsinki. Samples were released from the Human Tissue Authority licensed University of Southampton, Cancer Science Division Tissue Bank. Samples were assessed as single-cell suspensions that had been isolated, Ficoll-purified, and cryopreserved for subsequent analysis.
Western blotting
Western blotting was performed as described previously.26 Briefly, 2.5 to 5 × 106 hCD20 Tg B cells were treated for 6 hours in vitro with Alexa 488 mAb (10 μg/mL), washed, and lysed in onyx buffer. Samples were then separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis, and proteins were transferred immediately onto polyvinylidene difluoride membrane. Membranes were blocked with 5% nonfat dried milk, incubated with the appropriately diluted primary antibodies, washed, and then incubated with horseradish peroxidase–conjugated anti–rabbit or anti–mouse IgG (Sigma-Aldrich), and visualized by enhanced chemiluminescence (ECL, GE Healthcare) and exposure to light-sensitive film (Hyperfilm ECL, GE Healthcare).
Internalization assay and Alexa 488 labeling
mAbs were labeled with Alexa 488 according to the manufacturer's instructions (Invitrogen). Samples were incubated with Alexa 488–labeled mAb (5 μg/mL), harvested after 2, 6, or 24 hours, washed, resuspended, and incubated at 4°C for 30 minutes in the presence or absence of anti–Alexa 488 quenching antibody (Invitrogen). Samples were then assessed by flow cytometry. With human peripheral blood, B cells were identified by staining with RPE-labeled anti-CD19.
PCR for CD20 mRNA
Purified B cells were treated as indicated and total RNA isolated, before conversion to cDNA (Invitrogen). PCR was performed using appropriate dilutions of cDNA and primers specific for human CD20 or mouse GAPDH as a control.
Phagocytosis assay
To generate bone marrow-derived macrophages, adherent cells were isolated from the femurs of C57Bl/6 mice and cultured in vitro using L929-conditioned media for 5 to 8 days. On the day of the assay, macrophages were harvested using trypsin/ethylenediaminetetraacetic acid (Invitrogen), resuspended in RPMI, plated into a 96-well tissue culture plate (5 × 104/well), and incubated for 2 to 4 hours at 37°C. Target B cells were prepared from spleens of hCD20 Tg mice and incubated for 16 hours in the presence or absence of anti-CD20 mAb (10 μg/mL) to allow any modulation to take place, and then labeled with 5μM CFSE. The cells that had not been treated with mAb were then labeled with anti-CD20 mAb for 30 minutes at room temperature to obtain the maximum level of surface binding. The B cells were then added to the macrophages at a ratio of 5:1, the plates incubated for 30 minutes at 37°C, and then APC-anti-F4/80 antibody (AbDSerotec) added to distinguish the macrophages. Cells were harvested and analyzed by flow cytometry. The percentage of cells that stained double positive for CFSE and APC-anti-F480, which includes macrophages with both surface-bound and internalized B cells, was determined as a measure of phagocytic potential. For qualitative evidence of phagocytosis, macrophages were seeded into chamber slides (ibidi) with CFSE-labeled targets. After removal of unbound targets, slides were then imaged as described in “Confocal microscopy” below.
Confocal microscopy
For in vitro modulation, cells were incubated with Alexa 488–conjugated anti-CD20 mAb as required, and 50nM Lysotracker Red DND-99 or 5 μg/mL Alexa 647–conjugated transferrin (both Invitrogen) used for lysosome and endosome analysis, respectively. Cells were transferred onto slides and images captured immediately. For in vivo modulation, mice were treated with Alexa 488–conjugated anti-CD20 (100 μg intravenously) for 24 hours, and then spleens were removed and frozen and sections (10 μm) acetone-fixed and counterstained with TOPRO-3 (Invitrogen). For analysis of splenic B-cell depletion, frozen sections were stained with rabbit anti–mouse CD3 (Abcam) and rat anti–mouse B220, and then Alexa 488–conjugated goat anti–rabbit and Alexa 546–conjugated goat anti–rat (both Invitrogen).
For confocal microscopy, samples were mounted in Vectashield (Vector Laboratories) and images collected sequentially on a Leica TCS SP5 (Leica Microsystems) with lens and magnification as detailed as in figure legends. Images were acquired using Leica software (LAS-AF v2) and processed using Adobe Photoshop CS2 Version 9.0.2.
Statistical analysis
To compare differences between the experimental groups, a 2-tailed t test was performed using GraphPad Prism software.
Results
FcγR-dependent depletion in human CD20 transgenic (hCD20 Tg) mice
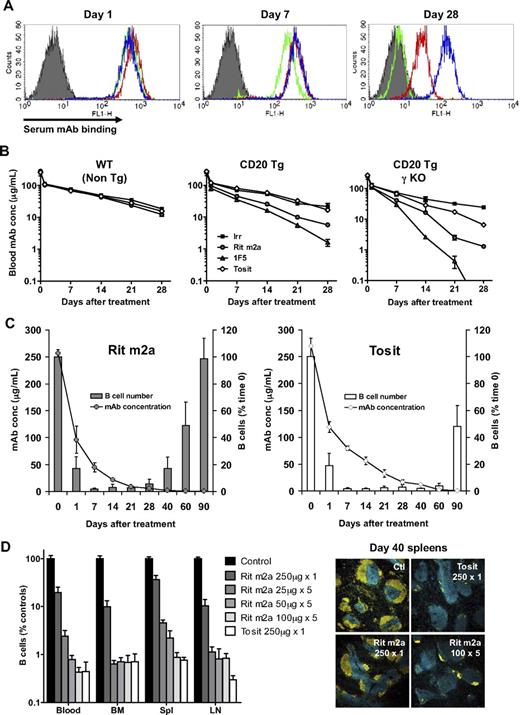
Type II (tositumomab-like) anti-CD20 mAbs demonstrate significantly prolonged clearance of circulating B cells in congenic hCD20 Tg models compared with their type I (rituximab-like) counterparts1,27 (Figure 1A). As a result of this enhanced potency, a single dose of 250 μg of type II mAb typically cleared circulating B cells for 60 days, compared with 20 to 30 days for type I reagents. Using an in vivo transfer model, we also demonstrated enhanced splenic B-cell depletion by type II mAb (Figure 1B). This depletion was dose dependent (Figure 1C); and comparing IgG2a isotype-matched versions of rituximab (Rit m2a) and tositumomab, the latter was approximately 5 times more potent. Type I mAbs have been variously proposed to engage complement28,29 and apoptotic cell death mechanisms.30,31 To examine these different effector mechanisms, a series of transfer experiments was performed either with recipient mice lacking key effector molecules or with transferred cells from modified donors (Figure 1D). In C1q−/− and C3−/− mice, the degree of B-cell depletion was equal to that in WT mice, suggesting no requirement for complement in this model.1 When apoptosis-resistant (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article)32 double-transgenic hCD20 × Vav Bcl-2 target cells were transferred, they were no less susceptible to depletion than normal hCD20 Tg targets with either type I or II mAb, indicating that induction of apoptosis is not a key effector mechanism in this model. In contrast, when engagement of mAb through FcγR was abrogated, either by transfer into γ-chain−/− mice or using F(ab′)2 fragments, B-cell depletion was completely curtailed (Figure 1D; supplemental Figure 2), supporting a vital role for FcγR-dependent effector mechanisms.3,33,34 Importantly, lack of activity with type II mAbs in both FcγR-blunted models also indicates the lack of a role for the Fc-independent nonapoptotic lysosome-mediated cell death we have previously described.6,8,35 Although seemingly at odds, these results can be reconciled by considering the different nature of the models used. Fc-independent cell death can be demonstrated in a range of malignant B cells, with both cell lines and primary samples and, we would postulate, could be an important mechanism in this setting. However, we and others36 have been unable to demonstrate potent directly induced cell death in normal B cells in the absence of a mitogenic signal whether in human cells or hCD20 Tg mouse cells ex vivo or in vivo, suggesting that this form of mAb-induced cell death may depend on cell cycling or prior triggering of the B-cell receptor. In this sense, the current observations regarding the depletion of normal B cells may have more relevance to the ever increasing use of anti-CD20 mAbs in autoimmune conditions.
Type II anti-CD20 mAbs depleted B cells more effectively than type I mAbs via a complement and apoptosis-independent mechanism that requires Fc/FcR engagement. (A) Systemic depletion: hCD20 Tg mice (BALB/c background) received 250 μg anti-CD20 mAb or isotype matched control intravenously on day 0 and the number of circulating B cells assessed. Points indicate means; n ≥ 4 mice for each time point from 2 to 4 independent experiments. (B) Adoptive transfer: hCD20 Tg target (T) or WT nontarget (NT) BALB/c splenocytes labeled with high or low CFSE, respectively, were injected intravenously into BALB/c mice. Twenty-four hours later, mice received mAbs (1 μg, intravenously); and 16 hours later spleens analyzed to determine the T/NT ratio. Left panel: bars represent mean ± SD; n = 3 to 5 mice for each treatment group. *Both type II mAbs significantly differ from both type I mAb: Rit m2a versus FGM6 (P < .02); Rit m2a versus Tosit P < .002; 1F5 versus FGM6 and 1F5 versus Tosit (P < .005). Right panel: Typical dot-plot data. (C) Dose-response to Rit m2a and tositumomab (Tosit) in a similar adoptive transfer: bars represent mean and range; n = 2 mice at each concentration. (D) Contribution of effector mechanisms: Ctl, transfer of T and NT into WT recipients as in panel B; CDC, NT, and T cells transferred into complement-deficient (C1q−/− and C3−/−) mice; programmed cell death, hCD20 Tg and hCD20 × Vav-Bcl-2 double Tg (both T), and WT (NT) labeled with high CFSE, low CFSE, and PKH26, respectively, transferred into WT mice and the level of both T compared with the NT; Fc/FcγR, T, and NT cells transferred into γ−/− or clodronate-treated (Clod) WT mice; also shown is the activity of F(ab′)2 fragments in WT recipients. Bars represent mean ± SD; n = 3 mice for each treatment group. Each condition is representative of at least 2 independent experiments. (E) Poor type I depletion is not the result of shaving: Transfer of T and NT cells into WT and CD64−/− mice as in panel B with Ctl, Rit m2a, or Tosit mAb. Bars represent mean and range; n = 2 mice for each treatment group; representative of at least 2 independent experiments.
Type II anti-CD20 mAbs depleted B cells more effectively than type I mAbs via a complement and apoptosis-independent mechanism that requires Fc/FcR engagement. (A) Systemic depletion: hCD20 Tg mice (BALB/c background) received 250 μg anti-CD20 mAb or isotype matched control intravenously on day 0 and the number of circulating B cells assessed. Points indicate means; n ≥ 4 mice for each time point from 2 to 4 independent experiments. (B) Adoptive transfer: hCD20 Tg target (T) or WT nontarget (NT) BALB/c splenocytes labeled with high or low CFSE, respectively, were injected intravenously into BALB/c mice. Twenty-four hours later, mice received mAbs (1 μg, intravenously); and 16 hours later spleens analyzed to determine the T/NT ratio. Left panel: bars represent mean ± SD; n = 3 to 5 mice for each treatment group. *Both type II mAbs significantly differ from both type I mAb: Rit m2a versus FGM6 (P < .02); Rit m2a versus Tosit P < .002; 1F5 versus FGM6 and 1F5 versus Tosit (P < .005). Right panel: Typical dot-plot data. (C) Dose-response to Rit m2a and tositumomab (Tosit) in a similar adoptive transfer: bars represent mean and range; n = 2 mice at each concentration. (D) Contribution of effector mechanisms: Ctl, transfer of T and NT into WT recipients as in panel B; CDC, NT, and T cells transferred into complement-deficient (C1q−/− and C3−/−) mice; programmed cell death, hCD20 Tg and hCD20 × Vav-Bcl-2 double Tg (both T), and WT (NT) labeled with high CFSE, low CFSE, and PKH26, respectively, transferred into WT mice and the level of both T compared with the NT; Fc/FcγR, T, and NT cells transferred into γ−/− or clodronate-treated (Clod) WT mice; also shown is the activity of F(ab′)2 fragments in WT recipients. Bars represent mean ± SD; n = 3 mice for each treatment group. Each condition is representative of at least 2 independent experiments. (E) Poor type I depletion is not the result of shaving: Transfer of T and NT cells into WT and CD64−/− mice as in panel B with Ctl, Rit m2a, or Tosit mAb. Bars represent mean and range; n = 2 mice for each treatment group; representative of at least 2 independent experiments.
Having demonstrated the importance of FcγR mechanisms in this model, we wanted to further elucidate the effector cells involved and used clodronate-containing liposomes to deplete the macrophage population24 in recipient mice. In line with previously published work,37,38 clodronate-treated mice were unable to eliminate B cells, implicating macrophages as the most probable effectors (Figure 1D; supplemental Figure 2). Finally, to determine whether the difference observed in potency between type I and II mAbs could be explained by the so-called “shaving” phenomenon described by Beum et al,39 where antibody/CD20 complexes are removed from the cell surface through engagement of CD64, we performed B-cell depletion in (1) CD64−/− × hCD20 Tg mice (not shown) and (2) by transfer of hCD20 Tg B cells into WT or CD64−/− mice (Figure 1E) and found no significant effect on the potency of depletion by type I or II mAbs. This would suggest that shaving does not play an important role in the in vivo difference between these mAbs. These data demonstrate, for the first time, that both type I and II mAbs use the same macrophage- and FcγR-dependent effector mechanism in vivo; therefore, differences in the mechanism of deletion cannot explain the superior potency of type II mAbs.
Rituximab rapidly modulates CD20 from the cell surface
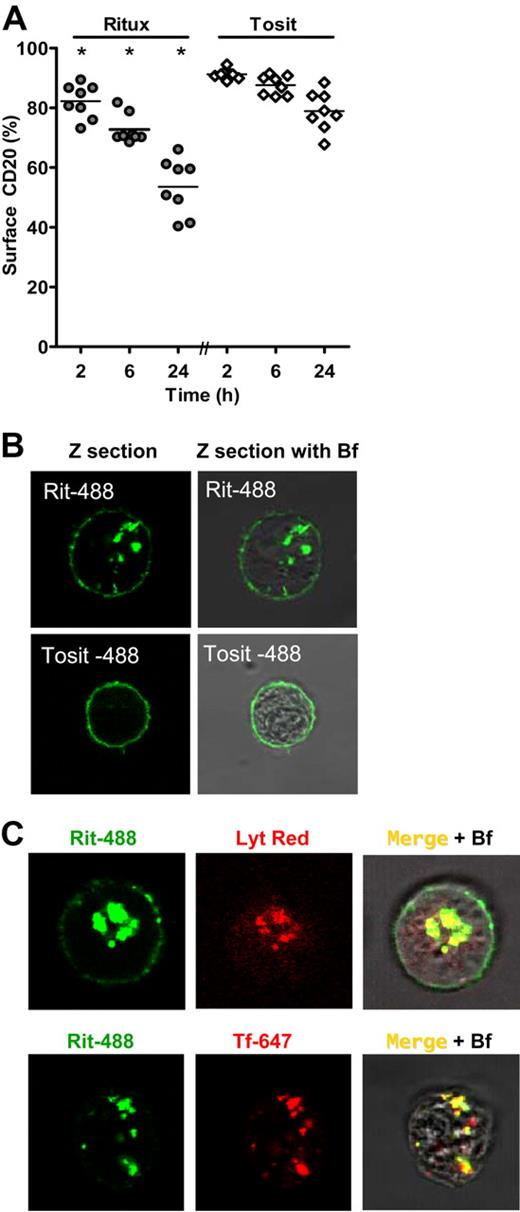
One indication of a critical difference between the activities of the 2 types of anti-CD20 mAbs came when CFSE-labeled hCD20 Tg B cells were transferred into γ-chain−/− mice. Here, the absence of activatory FcγR prevents deletion of target cells, permitting their study after mAb administration (Figure 1D). In these experiments, CD20 levels were determined by incubating cells with an excess of each anti-CD20 mAb and the same anti–mouse Fc specific RPE-conjugated secondary antibody, permitting a direct comparison of mAb-binding levels with a common secondary reagent. In this system, treatment with type I mAb for 16 hours evoked a reduction of approximately 80% to 90% in the level of CD20 present on the surface of B cells, whereas type II mAbs left CD20 largely unchanged (Figure 2A). This was confirmed in hCD20 Tg × γ−/− mice (Figure 2B) where all normal B-cell subsets express hCD20, precluding anomalies within the adoptive transfer model being responsible for the differences. Type I-mediated modulation of CD20 was dose dependent in vivo and required the continued presence of mAbs (supplemental Figure 3; and data not shown). Rapid modulation of CD20 also occurred on purified splenic B cells in vitro with type I but not type II mAbs (Figure 2C). These data, taken with the fact that modulation occurs in γ-chain−/− (activatory FcR-negative) mice and with purified B cells, illustrate that this process does not require FcR engagement by effector cells and therefore appears an intrinsic B-cell phenomenon separate from the CD20 shaving reaction.40 In light of these findings, we reassessed the potential of anti-CD20 mAbs to mediate phagocytosis of B cells after overnight treatment with mAbs and found that the drop in CD20 surface expression after Rit m2a treatment resulted in a 50% loss in the ability of macrophages to capture Ab-coated B cells (Figure 2D), whereas B cells treated with tositumomab remained equally sensitive to macrophage attack. These results clearly demonstrate that CD20 modulation by type I mAb impairs their ability to engage critical FcR-dependent effector cells, possibly explaining the reduced efficacy of type I mAb in B-cell depletion (Figure 1A).1 Although others have previously demonstrated modulation of CD20 from the cell surface as a direct result of mAb ligation,21,22 we think that this is the first study to demonstrate a potential therapeutic consequence of such B-cell intrinsic modulation.
Type I mAb treatment results in modulation of CD20 from the cell surface both in vivo and in vitro. (A) hCD20 Tg B cells transferred into nondepleting γ−/− mice as in Figure 1C were assessed 16 hours after mAb treatment for CD20 surface expression by detecting bound mAb with RPE-labeled anti–mouse Fc. Red indicates Rit m2a; blue, tositumomab; solid, background. Top histogram: CD20 expression on B cells from untreated mice labeled with Rit m2a and tositumomab ex vivo; bottom histogram, CD20 expression after treatment with Rit m2a or tositumomab in vivo. Bar chart: CD20 expression after treatment with type I and type II mAb. Bars represent mean ± SD; n ≥ 3mice, one of at least 3 experiments. *Rit m2a and 1F5 significantly differ from FGM6 and tositumomab (P < .001). (B) Surface CD20 expression on peripheral B cells in hCD20 Tg γ-chain−/− mice after mAb treatment (250 μg); red, Rit m2a; blue, tositumomab; solid, background; black, untreated. (C) Loss of surface CD20 in vitro. Isolated splenic hCD20 Tg B cells were incubated with Rit m2a or tositumomab (10 μg/mL) before detecting surface CD20. Bars represent mean and range for 2 experiments each performed in triplicate. (D) Phagocytic potential is reduced by treatment of CD20 Tg B cells with Rit m2a. Cells were treated for 16 hours with mAbs, labeled with CFSE, incubated with bone marrow-derived macrophages, and then APC-F4/80 labeled before flow cytometry to detect double-positive macrophages (left hand panel) or confocal microscopy (right hand panel) as described in “Methods” using an HCX PL APO lambda blue 63×/1.4 oil immersion lens with 1.7× optical zoom. Sixteen-hour incubation with Rit m2a but not tositumomab caused loss of CD20 and resulted in a reduction in the number of double-positive macrophages. *P < .001; ns indicates not significant. Bars represent mean ± SEM of triplicate samples, one of 3 similar experiments. Right hand panel shows typical image of macrophages capturing and engulfing CFSE-labeled B cells coated with tositumomab.
Type I mAb treatment results in modulation of CD20 from the cell surface both in vivo and in vitro. (A) hCD20 Tg B cells transferred into nondepleting γ−/− mice as in Figure 1C were assessed 16 hours after mAb treatment for CD20 surface expression by detecting bound mAb with RPE-labeled anti–mouse Fc. Red indicates Rit m2a; blue, tositumomab; solid, background. Top histogram: CD20 expression on B cells from untreated mice labeled with Rit m2a and tositumomab ex vivo; bottom histogram, CD20 expression after treatment with Rit m2a or tositumomab in vivo. Bar chart: CD20 expression after treatment with type I and type II mAb. Bars represent mean ± SD; n ≥ 3mice, one of at least 3 experiments. *Rit m2a and 1F5 significantly differ from FGM6 and tositumomab (P < .001). (B) Surface CD20 expression on peripheral B cells in hCD20 Tg γ-chain−/− mice after mAb treatment (250 μg); red, Rit m2a; blue, tositumomab; solid, background; black, untreated. (C) Loss of surface CD20 in vitro. Isolated splenic hCD20 Tg B cells were incubated with Rit m2a or tositumomab (10 μg/mL) before detecting surface CD20. Bars represent mean and range for 2 experiments each performed in triplicate. (D) Phagocytic potential is reduced by treatment of CD20 Tg B cells with Rit m2a. Cells were treated for 16 hours with mAbs, labeled with CFSE, incubated with bone marrow-derived macrophages, and then APC-F4/80 labeled before flow cytometry to detect double-positive macrophages (left hand panel) or confocal microscopy (right hand panel) as described in “Methods” using an HCX PL APO lambda blue 63×/1.4 oil immersion lens with 1.7× optical zoom. Sixteen-hour incubation with Rit m2a but not tositumomab caused loss of CD20 and resulted in a reduction in the number of double-positive macrophages. *P < .001; ns indicates not significant. Bars represent mean ± SEM of triplicate samples, one of 3 similar experiments. Right hand panel shows typical image of macrophages capturing and engulfing CFSE-labeled B cells coated with tositumomab.
Rituximab is consumed in vivo
In addition to reducing the efficiency of effector cell engagement, extensive modulation probably perturbs mAb half-life as a result of consumption and degradation by CD20+ B cells. However, we had previously reported that both type I and II mAb half-lives in depleting mice were largely equivalent based on assays using radiolabeled mAbs.1 Given our new findings, we reassessed antibody half-life using a new highly sensitive flow cytometry-based assay which, importantly, measures intact, bioactive mAb (capable of binding CD20+ cells) in the serum as opposed to measuring radioactivity recovered from whole blood, which could include degraded, inactive mAbs or mAbs internalized by cells giving falsely high readings for type I mAbs. In contrast to our previous results, using this system and consistent with the suggestion that modulation will affect mAb consumption, we found that in WT mice the half-life of all the mAbs was the same at 14 days, but in hCD20 Tg mice, the type I and II mAbs persisted with half-lives of 6 to 7 days and 14 days, respectively (Figure 3A-B center panel). Thus, these results correspond with the extent and duration of B-cell depletion seen in Figure 1 and demonstrate that the faster decay of type I mAbs shown in hCD20 Tg mice is a function of binding to and consumption by CD20+ B cells. Moreover, in hCD20 Tg × γ-chain−/− mice (Figure 3B right panel), where the anti-CD20 mAbs bind to B cells that are not depleted, a similar or slightly accelerated rate of type I mAb decay was observed, supporting the hypothesis that they are consumed by B cells in an active, antigen-specific process that does not require effector cell interactions and excludes a role for shaving by activatory FcγR-expressing effectors. Further to the observed correlation of mAb half-life and extent of depletion, peripheral B-cell repopulation correlated with the decline in serum mAb levels (Figure 3C). When mice treated with either type I or II mAb received a repeat dose of the same mAb just as their B cells returned to the circulation (days 40 and 90, respectively), they showed secondary depletion essentially the same as that after primary treatment (supplemental Figure 4). This suggests that B cells in type I mAb-treated mice are not inherently refractory but simply that the duration of depletion reflects the kinetics of mAb consumption. In this regard, we found that the reduced efficacy of type I mAbs can, at least in part, be overcome by maintenance administration. Using a regimen of 25, 50, or 100 μg/week for 5 weeks, Rit m2a was maintained in the serum at much higher levels (∼ 5, 12, and 40 μg/mL, respectively, at day 40, compared with 2 μg/mL with a single administration; supplemental Figure 5), leading to longer-lasting depletion of B cells compared with a single dose. However, maintenance dosing, although improving the performance of Rit m2a, still could not equal the depleting efficacy of a single dose of the type II mAb tositumomab at equivalent doses (Figure 3D).
Serum mAb levels correlate with B-cell depletion kinetics. (A-B) WT, hCD20 Tg, and CD20 Tg γ-chain−/− mice were treated with mAbs (250 μg), and the serum mAb concentration measured after 1, 7, and 28 days by incubating sera with SU-DHL-4 cells and then detecting cell-bound mAbs with FITC-labeled anti–mouse Fc, and comparing the level with a standard curve. (A) Representative histograms; solid histogram, background staining; blue, type II mAb tositumomab; red and green, type I mAb Rit m2a and 1F5, respectively. (B) The concentration of mAbs in the serum; n = 3 mice per group. Bars represent mean ± SD. (C) Correlation of serum mAb levels and B-cell repopulation in the periphery. hCD20 Tg mice were treated with a single dose of mAb (250 μg) and the mAb level in the serum and B-cell numbers assessed. Bars represent mean ± SD; n = 3 mice per group. (D) Repeated low-level dosing (25, 50, or 100 μg weekly for 5 weeks) with Rit m2a potentiates B-cell depletion compared with a single large dose (250 μg) of mAb to a level equivalent to that seen with tositumomab. The level of B cells in the blood and secondary lymphoid organs was assessed on day 40 by flow cytometry. T cells (blue) and B cells (yellow) in day 40 spleen sections were also visualized by confocal microscopy as described in “Methods” using an HCX PL FLUOTAR 10×/0.3 lens and 1× optical zoom. Serum mAb levels were also assessed (supplemental Figure 5) with repeated doses shown to enhance mAb serum levels.
Serum mAb levels correlate with B-cell depletion kinetics. (A-B) WT, hCD20 Tg, and CD20 Tg γ-chain−/− mice were treated with mAbs (250 μg), and the serum mAb concentration measured after 1, 7, and 28 days by incubating sera with SU-DHL-4 cells and then detecting cell-bound mAbs with FITC-labeled anti–mouse Fc, and comparing the level with a standard curve. (A) Representative histograms; solid histogram, background staining; blue, type II mAb tositumomab; red and green, type I mAb Rit m2a and 1F5, respectively. (B) The concentration of mAbs in the serum; n = 3 mice per group. Bars represent mean ± SD. (C) Correlation of serum mAb levels and B-cell repopulation in the periphery. hCD20 Tg mice were treated with a single dose of mAb (250 μg) and the mAb level in the serum and B-cell numbers assessed. Bars represent mean ± SD; n = 3 mice per group. (D) Repeated low-level dosing (25, 50, or 100 μg weekly for 5 weeks) with Rit m2a potentiates B-cell depletion compared with a single large dose (250 μg) of mAb to a level equivalent to that seen with tositumomab. The level of B cells in the blood and secondary lymphoid organs was assessed on day 40 by flow cytometry. T cells (blue) and B cells (yellow) in day 40 spleen sections were also visualized by confocal microscopy as described in “Methods” using an HCX PL FLUOTAR 10×/0.3 lens and 1× optical zoom. Serum mAb levels were also assessed (supplemental Figure 5) with repeated doses shown to enhance mAb serum levels.
Modulated rituximab is internalized, not shaved
Although using secondary antibodies to detect surface-bound mAb is able to provide a measure of the surface expression level of CD20, it does not permit the study of the fate of the anti-CD20 mAbs. To investigate this more fully, we used a method adapted from the surface fluorescence-quenching method of Austin et al,41 after comparing these techniques to ensure that they gave similar results (supplemental Figure 6). This fluorescence quenching technique permitted us to determine simultaneously the extent of modulation and whether the modulated mAbs were internalizing. First, we compared whether fluorescently labeled Rit m2a (Rit-488) or tositumomab (Tosit-488) internalized with CD20 as it modulated from the cell surface. We found that, when administered to cells in vitro (Figure 4A) or in vivo (Figure 4B), Rit-488 but not Tosit-488 was rapidly internalized as detected by its inaccessibility to surface quenching. Further, splenic B cells from Rit-488–treated mice accumulated appreciable levels of intracellular fluorescence, in marked contrast to results with Tosit-488 (Figure 4B-C left panel). This accumulation resulted in B cells becoming approximately 4 times more strongly stained with Rit-488 than Tosit-488 (Figure 4B top panel). Moreover, the Rit-488 was localized predominately inside the cells, with little remaining on the surface, whereas the Tosit-488 remained almost exclusively on the cell surface (Figure 4C bottom panel). Internalization correlated with a localized punctate staining pattern, which was also observed in spleen sections after in vivo administration of Rit-488 but not Tosit-488 (Figure 4C; supplemental Movie 1). Importantly, type I mAb-mediated internalization occurs in WT FcγR-expressing hCD20 Tg mice (data not shown), indicating that it occurs in vivo during B-cell depletion. This internalization is an active process requiring permissive temperature, energy production, and actin cytoskeletal rearrangements (Figure 4D). Furthermore, treatment with Rit m2a, but not tositumomab, leads to a reduction in the total levels of CD20 protein and a concomitant degradation of internalized mAb as detected by the loss of IgG–Alexa 488 conjugate (Figure 4E). CD20 mRNA levels remain relatively constant throughout, suggesting that the cells are unable, at least in the short term, to up-regulate CD20 in response to these events (Figure 4F). Taken together, these data provide an explanation for the consumption of rituximab in vivo, the kinetics of its decay in the serum, and the concordant recovery of B-cell numbers.
Type I mAbs modulate CD20 from the cell surface through internalization, leading to mAb and CD20 degradation. (A) hCD20 Tg B cells were incubated in vitro with Rit m2a–Alexa 488 (Rit-488) or Tosit–Alexa 488 (Tosit-488) mAbs (5 μg/mL) for 1, 2, or 6 hours, washed, and then incubated in the presence or absence of anti–Alexa 488 quenching Ab. The fluorescence remaining after quenching indicates the proportion of internalized mAbs (histogram top panel). Bars represent mean with range for duplicate determinations, one of 3 similar experiments. (B) hCD20 Tg × γ−/− mice were administered Rit-488 or Tosit-488 (100 μg) intravenously, and the amount of fluorescence associated with splenic B cells was assessed 24 hours later (top panel), Rit-488-treated B cells accumulated approximately 4 times the fluorescence compared with that of Tosit-488-treated mice (n = 3 mice). Despite the Rit m2a-treated B cells having a higher level of B cell–associated fluorescence, the Tosit-treated B cells (blue) expressed more CD20 on their surface than Rit-488–treated cells (red; bottom panel), histograms from one of 3 similar experiments. (C) Left panel: In vivo treatment: 10 μm spleen sections from mice treated, as in panel B, were analyzed by confocal microscopy. Assessed at the same gain intensity, far greater mAb accumulation is evident after Rit m2a (top panels), with its more punctate staining apparent when Rit m2a and Tosit were compared at optimized gain intensity (bottom panels). Right panel, in vitro treatment: hCD20 Tg B cells treated with Rit-488 (5 μg/mL) in vitro and assessed by confocal microscopy with a maximal projection, Z section through the center of the cell, and overlaid versus the bright field (Bf). In both panels an HXC PL APO CS 100×/1.4 oil immersion lens, 2× optical zoom was used. (D) Internalization of Rit-488 or Tosit-488 on hCD20 Tg B cells under normal conditions (NT), at 4°C, in the presence of azide (Azd, 15mM) or latrunculin B (Lat B, 50μM). Bars represent mean with range for duplicate determinations, one of 3 similar experiments. (E) hCD20 Tg B cells treated with Rit-488 or Tosit-488 for 6 hours and assessed by Western blot for the expression of intact Alexa 488–IgG, CD20, and actin (as a loading control). (F) mRNA levels of CD20 and GAPDH (as a control) were assessed in the same cells.
Type I mAbs modulate CD20 from the cell surface through internalization, leading to mAb and CD20 degradation. (A) hCD20 Tg B cells were incubated in vitro with Rit m2a–Alexa 488 (Rit-488) or Tosit–Alexa 488 (Tosit-488) mAbs (5 μg/mL) for 1, 2, or 6 hours, washed, and then incubated in the presence or absence of anti–Alexa 488 quenching Ab. The fluorescence remaining after quenching indicates the proportion of internalized mAbs (histogram top panel). Bars represent mean with range for duplicate determinations, one of 3 similar experiments. (B) hCD20 Tg × γ−/− mice were administered Rit-488 or Tosit-488 (100 μg) intravenously, and the amount of fluorescence associated with splenic B cells was assessed 24 hours later (top panel), Rit-488-treated B cells accumulated approximately 4 times the fluorescence compared with that of Tosit-488-treated mice (n = 3 mice). Despite the Rit m2a-treated B cells having a higher level of B cell–associated fluorescence, the Tosit-treated B cells (blue) expressed more CD20 on their surface than Rit-488–treated cells (red; bottom panel), histograms from one of 3 similar experiments. (C) Left panel: In vivo treatment: 10 μm spleen sections from mice treated, as in panel B, were analyzed by confocal microscopy. Assessed at the same gain intensity, far greater mAb accumulation is evident after Rit m2a (top panels), with its more punctate staining apparent when Rit m2a and Tosit were compared at optimized gain intensity (bottom panels). Right panel, in vitro treatment: hCD20 Tg B cells treated with Rit-488 (5 μg/mL) in vitro and assessed by confocal microscopy with a maximal projection, Z section through the center of the cell, and overlaid versus the bright field (Bf). In both panels an HXC PL APO CS 100×/1.4 oil immersion lens, 2× optical zoom was used. (D) Internalization of Rit-488 or Tosit-488 on hCD20 Tg B cells under normal conditions (NT), at 4°C, in the presence of azide (Azd, 15mM) or latrunculin B (Lat B, 50μM). Bars represent mean with range for duplicate determinations, one of 3 similar experiments. (E) hCD20 Tg B cells treated with Rit-488 or Tosit-488 for 6 hours and assessed by Western blot for the expression of intact Alexa 488–IgG, CD20, and actin (as a loading control). (F) mRNA levels of CD20 and GAPDH (as a control) were assessed in the same cells.
CD20 mAbs rapidly internalize and are targeted to the lysosome in normal human B cells and CLL
Having established the rapid and extensive internalization of type I mAb with hCD20 Tg B cells, we sought to clarify whether this was an artifact of this Tg model. Although there have been previous reports demonstrating CD20 internalization with mAb ligation,21,22 the majority of studies have shown that CD20 does not modulate.42,43 Indeed, the first clinical use of anti-CD20 mAb, 1F5, by Press et al,44 showed that mAbs did not undergo antigenic modulation from patient tumor cells, and this was highlighted as a benefit of such a reagent. Furthermore, we have reported that CD20 did not substantially modulate from Raji BL cells or a primary CLL sample in a short-term 2-hour culture.45 To clarify these apparent discrepancies, we determined to study modulation on a variety of B-cell targets. Michel and Mattes have previously demonstrated the internalization of CD20 on certain human B-cell lines after mAb engagement but found it to be slow with little internalization until 18 hours.22 We found very similar results in our experiments where internalization of CD20 occurred in a panel of 8 human B-cell lines in vitro after rituximab-488 (Ritux-488) but not Tosit-488 treatment (Figure 5A), again with internal punctate staining (Figure 5B), albeit considerably less rapidly than in the hCD20 Tg mouse B cells. Using these cell lines, we were able to confirm22 the trafficking of type I CD20 mAbs to early endosomes and lysosomes as detected by fluorescent transferrin and Lysotracker, respectively (Figure 5C), illustrating the route of CD20/type I mAb complex internalization and degradation. In sharp contrast, modulation and internalization of CD20/type I mAb occurred much more rapidly in normal human peripheral blood B cells (Figure 6A), being comparable with that seen in the hCD20 Tg mouse model (Figure 3A). We next examined a panel of primary tumors and revealed marked heterogeneity in the rate of Ritux-488 CD20 internalization. As shown in Figure 6B, CLL cells demonstrated the most rapid kinetics and internalization levels followed by MCL, then FL, and finally diffuse large B-cell lymphoma (DLBCL) cells, which displayed very little internalization out to 24 hours of culture (Figure 6B; and data not shown). For CLL, there was considerable variation in the rate of modulation, with internalization rates straddling that seen in normal B cells. In contrast, FL and DLBCL were generally much slower to internalize than CLL (P < .05 at 6 hours), with the exception of 2 outlier FL samples, which displayed atypical rapid internalization. This heterogeneity, especially if studying small patient cohorts with mixed tumor types, might help explain some of the confusion as to the tendency of CD20 to be lost from tumor cells. The data also offer the intriguing possibility that modulation of CD20 by type I mAb might be a useful predictor of therapeutic outcome. For example, it could help explain why SLL and CLL, which as a rule respond relatively poorly to rituximab, also consume mAbs in larger amounts than equivalent cases of FL.46,47 We are currently exploring the mechanisms underlying these differences. Furthermore, the outlier cases of FL, which modulate rapidly, provide an ideal opportunity to investigate phenotypic or genotypic differences that might control CD20 modulation. However, irrespective of the molecular explanation, the modulation and consumption of type I of anti-CD20 mAbs have clear implications for the use of rituximab or nonmodulating type II reagents in malignant and autoimmune settings.
Type I mAbs internalize in human cell lines and traffic to the lysosome. (A) A selection of NHL cell lines (Raji, Daudi, SU-DHL-4, DOHH2, Ramos, Granta-519, RL, and HBL-1) was treated with rituximab–Alexa 488 (Ritux-488) or Tosit-488 (5 μg/mL) for 2, 6, or 24 hours and then assessed for internalization as before. *At each time point, the level of surface-accessible CD20 with Ritux-488 was significantly lower than with Tosit-488: P < .001 at each time point. (B) Appearance of Raji B cells treated with Ritux-488 or Tosit-488 (5 μg/mL) for 24 hours in vitro and assessed by confocal microscopy using an HCX PL APO CS 100×/1.4 lens with 1× optical zoom (Rit-488) and 1.5× optical zoom (Tosit-488) with a Z section through the center of the cell and overlaid versus the bright field (Bf). (C) Colocalization of Ritux-488 with transferrin-647 or Lysostracker on Raji cells.
Type I mAbs internalize in human cell lines and traffic to the lysosome. (A) A selection of NHL cell lines (Raji, Daudi, SU-DHL-4, DOHH2, Ramos, Granta-519, RL, and HBL-1) was treated with rituximab–Alexa 488 (Ritux-488) or Tosit-488 (5 μg/mL) for 2, 6, or 24 hours and then assessed for internalization as before. *At each time point, the level of surface-accessible CD20 with Ritux-488 was significantly lower than with Tosit-488: P < .001 at each time point. (B) Appearance of Raji B cells treated with Ritux-488 or Tosit-488 (5 μg/mL) for 24 hours in vitro and assessed by confocal microscopy using an HCX PL APO CS 100×/1.4 lens with 1× optical zoom (Rit-488) and 1.5× optical zoom (Tosit-488) with a Z section through the center of the cell and overlaid versus the bright field (Bf). (C) Colocalization of Ritux-488 with transferrin-647 or Lysostracker on Raji cells.
Type I mAbs internalize in primary normal and malignant human B cells. (A) Normal human peripheral blood B cells were isolated by negative selection, treated with Ritux-488 or Tosit-488 (5 μg/mL) for 2, 6, or 24 hours, and then assessed for internalization as before. Results from 8 different donors are shown. *At each time point, the level of surface-accessible CD20 with Ritux-488 was significantly lower than with Tosit-488 (P < .001). (B) CLL, MCL, FL, or DLBCL samples were treated with Ritux-488 or Tosit-488 (5 μg/mL) for 2 or 6 hours and then assessed for internalization as before. The amount of surface-accessible CD20 after Ritux-488 was significantly lower than after Tosit-488 at 6 hours for CLL, MCL, and FL (P < .001, P = .001, and P < .001, respectively). The table indicates the significance of the difference in the rate of internalization of Ritux-488 between CLL and other disease subtypes. Each data point represents a sample from a different patient: CLL, n = 26; MCL, n = 5; FL, n = 9; and DLBCL, n = 5.
Type I mAbs internalize in primary normal and malignant human B cells. (A) Normal human peripheral blood B cells were isolated by negative selection, treated with Ritux-488 or Tosit-488 (5 μg/mL) for 2, 6, or 24 hours, and then assessed for internalization as before. Results from 8 different donors are shown. *At each time point, the level of surface-accessible CD20 with Ritux-488 was significantly lower than with Tosit-488 (P < .001). (B) CLL, MCL, FL, or DLBCL samples were treated with Ritux-488 or Tosit-488 (5 μg/mL) for 2 or 6 hours and then assessed for internalization as before. The amount of surface-accessible CD20 after Ritux-488 was significantly lower than after Tosit-488 at 6 hours for CLL, MCL, and FL (P < .001, P = .001, and P < .001, respectively). The table indicates the significance of the difference in the rate of internalization of Ritux-488 between CLL and other disease subtypes. Each data point represents a sample from a different patient: CLL, n = 26; MCL, n = 5; FL, n = 9; and DLBCL, n = 5.
Discussion
Anti-CD20 immunotherapy is rapidly evolving, with multiple new mAbs designed to harness different effector pathways now in clinical trials.2 The diversity of the mAbs being tested reflects the uncertainty regarding the critical mechanism of action for these reagents and which effector pathways should be harnessed for optimal efficacy.
This study demonstrates, for the first time, that the same Fc:FcγR-dependent mechanism is critical for the in vivo activity of both type I and type II reagents. Despite this common mode of action, it is clear that type II mAbs produce more effective B-cell depletion. The approximately 5-fold enhanced efficacy of tositumomab shown here was independent of complement activity or direct induction of apoptosis. Although we cannot entirely rule out the possibility that type II mAbs achieve this efficacy in part through the induction of a nonapoptotic cell death pathway, such as described for NHL cell lines,6,8,35 we have so far been unable to demonstrate this on isolated hCD20 Tg B cells (data not shown). Furthermore, cell death through this route does not require additional cross-linking or Fc presence6,8,35 ; and given the lack of depletion in γ-chain−/− mice and with F(ab′)2 fragments, it seems doubtful that direct induction of nonapoptotic cell death plays a major role in this model. However, presumably in NHL, both mechanisms will potentially operate to further improve the efficacy of type II versus type I anti-CD20 mAbs.
The enhanced ability of type II reagents to deplete B cells appears to be related to the fact that they elicit little modulation of CD20 from the cell surface. In contrast, the binding of type I mAb induces a profound modulation of CD20, resulting in 2 concomitant effects: a reduction in effector cell-mediated clearance of B cells and enhanced mAb consumption.
The literature on CD20 modulation is conflicting, with some reports suggesting that it occurs21,22,48 and others reporting that it does not.42,43,49 These discrepancies may reflect the cell types and different mAbs used. In many cases, heavily passaged B-cell lines and xenograft models failed to show significant internalization unless incubated with type I mAbs over a long period (eg, 18-24 hours). In contrast, here, human B cells taken from the blood of healthy volunteers underwent internalization with similar kinetics to hCD20 Tg B cells, within just 2 hours. The reasons underlying these differences in kinetics are currently under investigation.
Variable resistance to depletion in vivo may also reflect how long the B cells are coated with type I anti-CD20 mAbs and allowed to modulate before they encounter FcγR-expressing effectors. As such, the length of time before encountering tissue resident effector cells or release into the circulation to gain access to the reticulo-endothelial system could explain the differential sensitivity of B cells in the periphery (most sensitive), bone marrow, and spleen (least sensitive).
It is also possible that internalization may explain the differing sensitivity of B-cell malignancies to rituxumab. We have shown, in a cohort of 26 CLL samples treated ex vivo, that samples display a range of internalization rates, with some markedly enhanced compared with normal B cells. This may predict defective cellular clearance and consumption of large quantities of mAbs, as is observed clinically. Furthermore, this wide heterogeneity in modulation rate probably explains why we previously concluded that rituximab did not modulate rapidly from the cell surface based on a far smaller cohort of CLL samples and BL cell lines.45 Interestingly, whereas we see some CLL cases that modulate rapidly, for example, up to 80% loss of CD20 at 6 hours, we do not see the almost complete loss reported by Jilani et al21 after only 1 to 2 hours, which possibly reflects differences in methodology. Interestingly, MCL samples also modulated rituximab quite rapidly, in keeping with the relatively poor response of these lymphomas to rituximab treatment. Conversely, FL and DLBCL samples in general demonstrate significantly slower internalization, corresponding to their greater sensitivity to rituximab and consistent with the literature in the field that CD20 is not lost from the cell surface during rituximab treatment. The molecular basis underlying the more rapid internalization seen in 20% of the FL samples is particularly intriguing and currently under study. Intriguingly, polymorphisms in FcγRIIIa have been found to correlate with clinical responses to rituximab with FL50,51 but not CLL.52 It is tempting to speculate that these associations are related to the rate of modulation observed in each disease, with the rapid modulation observed in CLL potentially negating the enhanced potency of the high affinity FcγRIIIa allele.
Importantly, the deficiencies of rituximab resulting from mAb consumption could be largely overcome by maintenance treatment, suggesting that the reduced level of anti-CD20 mAb bound to “modulated” targets is still sufficient (albeit not optimal) to trigger deletion by effectors. However, this compensation was less apparent in the tissues where maintenance Rit m2a, although improved, was no more effective than a single dose of tositumomab, even when serum mAb levels were higher. Furthermore, our experiments demonstrate that FcR-dependent CD20 shaving does not play an important role in limiting the efficacy of Rit m2a in the current model because depletion efficacy was dose-dependent and low repeated doses of Rit m2a, as suggested by Beum et al,39 did not improve performance. It is important to point out, however, that shaving may only occur when the reticulo-endothelial phagocytic systems, such as those of the liver, become fully saturated, which may not occur when depleting B cells as opposed to large numbers of tumor cells.
The data presented here suggest that type II mAbs may be the most effective in depleting large numbers of B cells as in CLL. Type II mAbs are essentially nonmodulatory, are not rapidly consumed, and maintain their ability to engage FcR-expressing effectors, avoiding the need for multiple dosing regimens and reducing the overall amount of mAbs required. An alternative strategy, given the current clinical penetrance of type I mAbs, would be to enhance their efficacy by blocking CD20 internalization after treatment, for example, by interfering with the membrane fluidity,53 energy production, or the actin machinery of the target cells. Indeed, it is possible that cytotoxic agents act in this way by directly or indirectly affecting such pathways, perhaps helping to explain the efficacy of rituximab in combination with chemotherapy and radiotherapy.
Recent clinical trials have assessed the efficacy of another type I mAb (ofatumumab)16-18 with improved affinity and CDC activity in CLL.9,29 Interestingly, our preliminary analysis (data not shown) indicates that this mAb, in keeping with its type I nature, will also suffer from rapid modulation, although its higher affinity and lower off-rate may provide greater activity at lower mAb serum levels.
In conclusion, these results suggest that type II anti-CD20 mAbs have considerable therapeutic potential in the treatment of B-cell diseases. Until very recently, tositumomab was the only type II mAb to have been investigated clinically, and this almost exclusively as a radioimmunoconjugate. The second type II reagent, GA101, is currently undergoing clinical trials, with promising effects seen in both CLL and rituximab-treated FL.54 Importantly, our data suggest an overlooked mechanism for resistance to anti-CD20 mAb. The relevance of this to other target antigens and mAbs is unknown, but this study highlights the importance of fine specificity in regulating mAb effector functions7 and suggests that internalization and consumption may be important criteria for selecting therapeutic mAbs. Knowledge of the central pathways involved in modulation should allow the design of rational combination therapies to augment the effect of existing mAb treatments.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank members of the CD20 research team, past and present, for their contributions, together with Prof Tim Illidge, Dr J. Teeling, and Dr Luke Nolan for the supply of anti-CD20 mAb (tositumomab and rituximab) and cell lines; Prof Andrew George for insightful discussions; Prof Mark Shlomchik for the provision of hCD20 Tg mice; Prof Marina Botto and Aras Kadioglu for C1q−/− mice; Dr Sjef Verbeek and Prof Jan van de Winkel for FcγR−/− mice; Dr George Hacker and Prof Jerry Adams for Vav-Bcl-2 Tg mice; and Dr Kathleen N. Potter, Dr C. Ian Mockridge, Isla Wheatley, and the Department of Health/Cancer Research United Kingdom Experimental Cancer Medicine Centre for provision and assistance with clinical material.
This work was supported by Leukaemia Research (grants 07048 and 07010), a Medical Research Council fellowship (S.H.L.), Cancer Research UK (grants C328/A2738 and C328/A2737), and Tenovus, Cardiff.
Authorship
Contribution: S.A.B. designed the research, performed experiments, analyzed results, produced figures, and wrote the manuscript; R.R.F. performed experiments, produced figures, and edited the manuscript; H.T.C.C. produced critical reagents; J.S.V. provided critical mice and unique reagents; T.C.J., R.M.V., S.S.W., S.V.D., H.J., K.L.C., J.P.K., S.H.L., and D.A.J. performed experiments; P.W.M.J. analyzed results and edited the manuscript; M.J.G. designed the research, analyzed results, and edited the manuscript; and M.S.C. designed the research, analyzed results, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Mark S. Cragg, Tenovus Laboratory, Cancer Sciences Division, Southampton University School of Medicine, General Hospital, Southampton SO16 6YD, United Kingdom; e-mail: msc@soton.ac.uk.
References
Author notes
S.A.B. and R.R.F. contributed equally to this study and should be considered first authors.
M.J.G. and M.S.C. contributed equally to this study and are the senior authors.