Abstract
The development of the vertebrate vascular system is mediated by both genetic patterning of vessels and by angiogenic sprouting in response to hypoxia. Both of these processes depend on the detection of environmental guidance cues by endothelial cells. A specialized subtype of endothelial cell known as the tip cell is thought to be involved in the detection and response to these cues, but the molecular signaling pathways used by tip cells to mediate tissue vascularization remain largely uncharacterized. To identify genes critical to tip cell function, we have developed a method to isolate them using laser capture microdissection, permitting comparison of RNA extracted from endothelial tip cells with that of endothelial stalk cells using microarray analysis. Genes enriched in tip cells include ESM-1, angiopoietin-2, and SLP-76. CXCR4, a receptor for the chemokine stromal-cell derived factor-1, was also identified as a tip cell-enriched gene, and we provide evidence for a novel role for this receptor in mediating tip cell morphology and vascular patterning in the neonatal retina.
Introduction
During embryogenesis, the vertebrate circulatory system develops through the overlapping processes of vasculogenesis and angiogenesis. Vasculogenesis is the migration and differentiation of endothelial cells from hematopoietic precursors. Angiogenesis involves the sprouting of blood vessel networks and their maturation into an organized, functional circulatory system.1,2 Many aspects of angiogenesis are also recapitulated in the adult during normal conditions, such as pregnancy, and pathologic situations, such as inflammation and cancer.3 An understanding of the molecular mechanisms that govern this process is critical for the development of novel proangiogenic and antiangiogenic therapeutics.
A specialized subset of endothelial cells known as tip cells is thought to mediate vessel growth and patterning. Tip cells are found at the growing end of a nascent vascular sprout and are characterized by abundant and dynamic filopodia. These filopodia are thought to detect cues in the local environment and translate them into directed motility.4,5 Tip cells are responsive to angiogenic factors, such as vascular endothelial growth factor-A (VEGF-A),6 as well as classic axon guidance factors, such as netrin-1.7 The generation of new tip cells is limited by the activity of Delta-like 4 (Dll4),8-11 which is specifically up-regulated in tip cells.12 Signaling of Dll4 through Notch receptors on adjacent cells has been proposed to inhibit their development into tip cells.13 Despite these advances, the mechanisms that may promote or mediate the generation of new tip cells remain incompletely understood.
Recently, endothelial tip cells have become a topic of intense scrutiny,3 but many critical questions remain about their function and how they may differ from neighboring stalk cells. Expression of several genes, including VEGF receptor 2 (VEGFR2),6 Dll4, PDGF-B,6 and VEGFR3,14 has been shown to be higher in retinal tip cells compared with adjacent endothelial cells. Components of other signaling, morphogenetic, or guidance pathways that may be involved in tip cell function have yet to be characterized. Furthermore, as the vasculature matures into an organized and generally quiescent system of veins, arteries, and capillaries, tip cells are no longer required for sprouting angiogenesis, and their ultimate fate remains unknown.
To identify genes enriched in developing vessels and tip cells during angiogenesis, we developed methods to isolate elements of the vasculature from murine postnatal retina using laser capture microdissection. We analyzed gene expression in these elements using microarrays. Endothelial tip cells were collected separately, and their gene expression patterns were compared with those of endothelial stalk cells. Several novel tip cell genes were identified and their expression confirmed by in situ hybridization (ISH).
The chemokine receptor CXCR4 was identified as a tip cell-enriched gene. Together with its ligand stromal-cell derived factor-1 (SDF-1), this receptor has been previously implicated in vascular development; however, its precise role in this process has not yet been defined. Inhibition of CXCR4 signaling in neonatal mice results in defects in retinal tip cell morphology and attenuation of the intercellular connections between tip cells. This suggests a role for the SDF-1/CXCR4 signaling axis in tip cell behavior and vascular patterning.
Methods
Laser-capture microdissection
Retinas from CD-1 pups were collected 24 to 36 hours after birth (postnatal day 1-1.5), dissected in RNAse-free phosphate-buffered salt solution (PBS), placed vitreous-side down in cryostat molds, and frozen in Optimal Cutting Temperature medium (OCT). Retinas were sectioned en face, adhered to laser-capture microdissection (LCM)–membrane-mounted metal slides (no. 50102; Molecular Machines), and stored at −80°C until use. For LCM, slides were individually thawed for 30 seconds, fixed in cold methanol for 1 minute, and rinsed rapidly in PBS. Sections were stained with Alexa-594–labeled isolectin (no. I21413; Invitrogen) at 0.1 mg/mL in PBS plus 1 U/μL SUPERase-In (no. AM2694; Applied Biosystems) for 2 minutes. Slides were washed twice rapidly in PBS, dehydrated through 70% to 100% ethanol, and allowed to dry thoroughly (30 seconds to 1 minute). Cells were excised within 15 minutes of drying using a Molecular Machines CellCut Plus system (Molecular Machines and Industries) equipped with a Nikon Eclipse TE200E fluorescence microscope using a Nikon 40×/0.60 objective and MMI CellCut Plus software Version 3.4.7. Cells were adhered to capture tube lids (no. 50202; Molecular Machines). Tubes were placed on dry ice and kept at −80°C until RNA extraction.
RNA extraction
RNA was extracted using the Arcturus PicoPure kit (no. KIT0204; MDS Analytical Technologies) according to the manufacturer's instructions. RNA quality was assessed using an Agilent 2100 Bioanalyzer.
Microarray analysis
Starting RNA quantity was not assessed because of low yield but was estimated to be less than 10 ng. Message Amp II aRNA Amplification kit (no. 1751; Ambion) was used for 2 rounds of amplification (first round cDNA and aRNA and second round cDNA). First, in vitro transcription (to get aRNA) was done for 6 hours at 37°C. Cy5 dye was incorporated into the samples during second round in vitro transcription (IVT) according to protocol using the Low RNA Input Fluorescent Amplification kit (no. 5184-3523; Agilent). Second round aRNA was purified using RNeasy Mini Kit (no. 74104; QIAGEN). Probes were quantified on Nanodrop-1000 and quality was checked on Agilent 2100 Bioanalyzer (Eukaryote Total RNA Pico assay; Agilent Technologies). A total of 750 ng of each Cy5 probe was hybridized, along with 750 ng of the Cy3-labeled Universal Mouse Reference (no. 740100; Stratagene). Probes were hybridized to Agilent 44k Whole Mouse Genome arrays. Data were processed using Agilent's Feature Extraction software Version 8.5. The complete dataset from this analysis is available at the NCBI Gene Expression Omnibus using accession no. GSE19284.
Tip- and stalk-specific signals were identified by processing the data as described; all statistical methods were performed using Partek Version 6.4 (Partek Inc). Raw ratios generated from Agilent Feature Extraction software were log2 transformed. Statistically significant differences between tip and stalk cell data were identified using an unpaired t test with equal variance. A q value was then calculated to estimate the false discovery rate as described by Storey and Tibshirani.15 Data were included in the tip cell-specific or stalk cell-specific lists if they met the following criteria: P less than .05, q less than 0.1, fold change more than 2. Pathway analysis was performed using MetaCore from GeneGo Inc.
ISH of whole-mount retina
Eyes were fixed for 30 minutes in 4% (wt/vol) paraformaldehyde (PFA), dissected in PBS with 0.05% Tween-20 (PBST), and then fixed overnight in 4% PFA at 4°C. Retinas were dehydrated in methanol, bleached in 25% vol/vol hydrogen peroxide/methanol, and stored in methanol at −20°C. Before hybridization, retinas were rehydrated to PBS and then digested for 5 minutes in 20 μg/mL collagenase-1 (no. C1639; Sigma-Aldrich) plus proteinase K (20 μg/mL in PBST) followed by fixation in 4% PFA and 0.2% glutaraldehyde in PBS. After washing (PBST), retinas were preincubated in hybridization buffer (50% formamide/5 × saline sodium citrate/5 × Denhardt/250 μg/mL yeast tRNA/500 μg/mL herring sperm DNA/2.5 mM ethylenediaminetetraacetic acid/0.1% Tween-20/0.25% 3(3-cholamidopropyl) dimethylammonio-1-propane sulfonate) at 55°C and then incubated with RNA probes in hybridization buffer at 55°C overnight. Probe labeling with digoxigenin-uridine triphosphate (no. 11175025910; Roche Diagnostic) and visualization of RNA hybrids with alkaline phosphatase (AP)–conjugated antidigoxigenin antibodies (no. 11093274910; Roche Diagnostic) were carried out according to the manufacturer's instructions using nitroblue tetrazolium/5-bromo-4-chloro-3-indolyl-phosphate as a color reagent (Sigma-Aldrich). Antibody labeling was performed after the AP reaction using anti-glial fibrillary acidic protein (GFAP, 1:100; clone G-A-5; Sigma-Aldrich; and Alexa-594–labeled isolectin. Retinas were mounted on slides using Fluoromount-G (Electron Microscopy Sciences). Slides were analyzed at room temperature using a Zeiss Axioplan 2 microscope. AP product stain was imaged with a color camera (Zeiss Axiocam HRc), and fluorescent signal was imaged with a black-and-white camera (Zeiss Axiocam MRm). The following objectives (Carl Zeiss) were used as indicated in the figure legends: 2.5×/0.075 Plan-Neofluar; 5×/0.12 A-Plan; 10×/0.03 Plan-Neofluar; 20×/0.5 Plan-Neofluar; 40×/0.75 Plan-Neofluar. Axiovision 4.6.3 software was used for image acquisition and Adobe Photoshop 8.0 for image processing where indicated.
Immunohistochemistry on retinal whole-mount preparations
Retinas were dissected and fixed as described and then incubated in PBS containing 10% fetal bovine serum and 0.05% Tween-20. Incubations with antibodies (in PBS containing 10% fetal bovine serum and 0.05% Tween-20) were carried out overnight at 4°C (primary) and for 3 hours at room temperature (secondary). Antibodies used were anti-glial fibrillary acidic protein, anti–ZO-1 (1:00; no. 617300; Invitrogen), anti–collagen IV (Meridian T40263R), Alexa Fluor 488 or 594 secondary antibodies (Invitrogen) and Alexa Fluor 488 or 594 isolectin (Invitrogen). Images were acquired and analyzed as described for ISH of whole-mount retina or at 37°C using Leica DMI6000CS (Leica Microsystems) upright laser scanning confocal microscope with a 40 × 1.25 NA HCX PL APO CS objective and LAS AF image acquisition software.
In vitro capillary formation assay
Human umbilical vein endothelial cells (HUVECs; no. C2517A; Lonza Walkersville) were maintained in EGM-2 media and cultured for capillary formation on Cytodex beads as previously described16 in 4-well Nuclon Δ tissue culture multidishes (Nunc 176740). Capillary outgrowth was maintained by addition of EGM-2 media conditioned by a layer of confluent fibroblasts (D551; ATCC) for 7 days or by coculture with a layer of D551 human fibroblasts as described. Recombinant human SDF-1a (StemCell Technologies) and/or AMD3100 (Sigma-Aldrich) were added to the media for outgrowth assays. Vessels were stained by the addition of anti-CD31 antibody (1:100; no. 555444; BD Biosciences PharMingen) and Alexa Fluor 488-labeled (Invitrogen) secondary antibody directly to the culture medium overnight in a tissue culture incubator. Cultures were washed with fresh medium followed by PBS before imaging. A Zeiss Axiovert 200 microscope equipped with an Axiocam MRm camera, Axiovision 4.6.3 software. A 2.5×/0.075 Zeiss Plan-Neofluar and a 40×/0.75 Zeiss LD A-Plan objective were used for image acquisition. Sprout lengths and numbers were quantified using ImageJ 1.5.0_13 (National Institutes of Health).
In vivo AMD3100 or antibody administration
All studies were conducted in accordance with the Guide for the Care and Use of Laboratory Animals.17 The Genentech Institutional Animal Care and Use Committee approved all animal protocols. CD-1 neonates from timed pregnancies were administered AMD3100 in PBS or PBS alone by intraperitoneal injection starting on the day of birth. For function blocking antibody experiments, 50 mg/kg anti-CXCR4 antibody (MAB21651; R&D Systems) or control antibody (MAB 0061; R&D Systems) was used. After 24 to 48 hours, dams and pups were killed and the retinas analyzed by immunofluorescence staining and microscopy.
Image analysis and quantification
For Sholl analysis and vascular area measurements, images of the entire vascular area of isolectin-stained whole-mount retinas were converted to binary images using Metamorph. Area was measured in a region bounded by the distal edge of the vasculature. Binary images were imported into ImageJ, and Sholl analysis was done using a plugin available at: www.biology.ucsd.edu/labs/ghosh/software/index.html. For vascular protrusion measurements, images of the vascular area were traced in ImageJ using the NeuronJ plugin (www.imagescience.org/meijering/software/neuronj) according to criteria detailed in supplemental Figure 2 (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Results
Isolation of endothelial tip cells
In mice, vascularization of the retina begins at birth. A network of endothelial cells, known as the primary vascular plexus, emerges from the optic disc and grows outward, reaching the distal edge of the retina approximately 1 week later.18 Preceding and underlying the primary vascular plexus is a network of astrocytes that is critical for the normal outgrowth of this vasculature. Astrocytes secrete proangiogenic factors, including VEGF-A, and provide a permissive substrate for endothelial cell migration.19
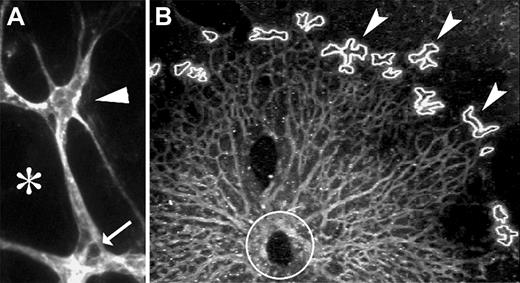
The primary vascular plexus emerges in a stereotypical and well-characterized process and is initially confined to a single layer of the neural retina. For these reasons, the early postnatal mouse retina was selected as a source of actively developing vasculature and tip cells. We developed an approach to efficiently isolate retinal vasculature using laser capture microdissection. Retinas were flattened and sectioned en face; we could routinely isolate the entire vasculature in a single 10-μm section. Sections were fixed and stained with fluorescently labeled isolectin using a rapid-fix technique (“Laser-capture microdissection”). Tip cells, identified by their location at the most distal region of the vascular plexus and by the presence of filopodia, were excised by laser capture microdissection (Figure 1). Stalk cells were also identified by position and morphology for harvest, and tissue surrounding the vasculature was harvested for comparison to the vasculature. We expect that this protocol will produce samples substantially enriched for tip or stalk cell RNA, although contaminating RNA from nonvascular retina may be included as well.
Retinas were sectioned en face to isolate the vascular layer and stained with fluorescent-labeled isolectin. Tip cells (arrowhead) were identified by their position at the distal edge of the vasculature and by the presence of filopodia in P1 or P2 retinas. (A) Stalk cells (arrows) and adjacent nonvascular retina tissue (*) were also harvested. (B) Burn marks indicate where tip cells have been removed by LCM (arrowheads) from P2 retina. Circle represents the optic nerve head region.
Retinas were sectioned en face to isolate the vascular layer and stained with fluorescent-labeled isolectin. Tip cells (arrowhead) were identified by their position at the distal edge of the vasculature and by the presence of filopodia in P1 or P2 retinas. (A) Stalk cells (arrows) and adjacent nonvascular retina tissue (*) were also harvested. (B) Burn marks indicate where tip cells have been removed by LCM (arrowheads) from P2 retina. Circle represents the optic nerve head region.
Between 50 and 100 tip cells were typically harvested per retina. To prevent RNA degradation, the fixation, staining, and laser capture of the cells were executed in less than 30 minutes. Tip cells from several retinas were pooled, and the RNA was isolated. Quality of the mRNA was assessed before use in further experiments by observing the 28S and 18S ribosomal peaks. RNA samples from tips, stalks, and adjacent tissue underwent 2 amplification cycles each and hybridized to Agilent Whole Mouse Genome Oligo Microarray (“Microarray analysis”). Gene expression in tip cells was compared with stalk cell gene expression to identify genes enriched in either subpopulation of endothelial cells.
Gene expression analysis
Tip- and stalk-specific probe sets were identified by comparing microarray data from tip cell samples to stalk cell samples (“Microarray analysis”). From this analysis, 194 probe sets were found to have a higher signal in tip cells compared with stalk cells, whereas 234 probe sets were found to have a higher signal in stalk cells compared with tip cells. The probe sets with higher signals in tip cells mapped to genes enriched for pathways involved in axon guidance signaling (P = .002; “Microarray analysis”), including Bmp7, CXCR4, Sema3F, and Sema3A. Probe sets with higher signals in stalk cells corresponded to genes enriched for components of the Notch signaling pathway, including Jagged 1 and Jagged 2 (P = .08). Tip cell-enriched genes are listed in supplemental Table 1.
Several gene transcripts have previously been reported to be specifically enriched in retinal tip or stalk cells. Our data were in agreement with several previously published reports but were inconsistent with a few others. Microarray data pertaining to these transcripts are summarized in Table 1. Consistent with prior data, mRNA for the secreted protein apelin20 also revealed a significantly higher signal in tip cells in 2 of 3 probes in our dataset. Likewise, the probe for Delta-like 412 reported significantly higher levels in tip cells. Conversely, the Notch receptor Jagged-1 has been reported to be selectively expressed in retinal stalk cells.21 In our dataset, 2 probes for Jagged-1 show higher expression in stalk cells compared with tips; one of these was highly significant. Contrasting with the prior literature, platelet-derived growth factor-B mRNA was previously reported to be enriched in tip cells,6 but our probe sets failed to reflect this difference. Similarly, VEGFR2 and VEGFR3 were both reportedly enriched in tip cells,6,14 but this enrichment was not reflected in the microarray data. These differences may reflect some contamination of the tip cell samples with RNA from stalk cells, as the laser capture protocol is expected to produce samples enriched for, but not exclusively composed of, tip or stalk RNA. Alternatively, the differences could reflect technical limitations of the microarrays used in this analysis. Together, the results indicate that the experimental approaches for tissue isolation and expression analysis described in this report can successfully identify tip and stalk cell-enriched genes, although some may be missed.
Expression of genes with previously documented tip or stalk cell expression
| Transcript . | Previously reported expression pattern . | Probe . | Fold-expression tips/stalks . | P . |
|---|---|---|---|---|
| Apelin | Retinal tip cells20 | A_51_P209327 | 1.8 | .006 |
| A_52_P62284 | 1.8 | .001 | ||
| A_51_P133345 | 0.8 | .136 | ||
| Delta-like four | Retinal tip cells, retinal arteries12 | A_51_P384402 | 1.4 | .023 |
| PDGF-β | Retinal tip cells6 | A_51_P175262 | 0.94 | .494 |
| A_52_P618427 | 1.1 | .328 | ||
| VEGFR2 | Retinal tip cells6 | A_51_P316553 | 0.69 | .027 |
| A_52_P478193 | 0.71 | .2 | ||
| VEGFR3 | Retinal tip cells15 | A_51_P179531 | 0.84 | .01 |
| Jagged-1 | Retinal stalk cells21 | A_52_P634090 | 0.4 | 1.92 × 10−5 |
| A_51_P280906 | 0.77 | .209 |
| Transcript . | Previously reported expression pattern . | Probe . | Fold-expression tips/stalks . | P . |
|---|---|---|---|---|
| Apelin | Retinal tip cells20 | A_51_P209327 | 1.8 | .006 |
| A_52_P62284 | 1.8 | .001 | ||
| A_51_P133345 | 0.8 | .136 | ||
| Delta-like four | Retinal tip cells, retinal arteries12 | A_51_P384402 | 1.4 | .023 |
| PDGF-β | Retinal tip cells6 | A_51_P175262 | 0.94 | .494 |
| A_52_P618427 | 1.1 | .328 | ||
| VEGFR2 | Retinal tip cells6 | A_51_P316553 | 0.69 | .027 |
| A_52_P478193 | 0.71 | .2 | ||
| VEGFR3 | Retinal tip cells15 | A_51_P179531 | 0.84 | .01 |
| Jagged-1 | Retinal stalk cells21 | A_52_P634090 | 0.4 | 1.92 × 10−5 |
| A_51_P280906 | 0.77 | .209 |
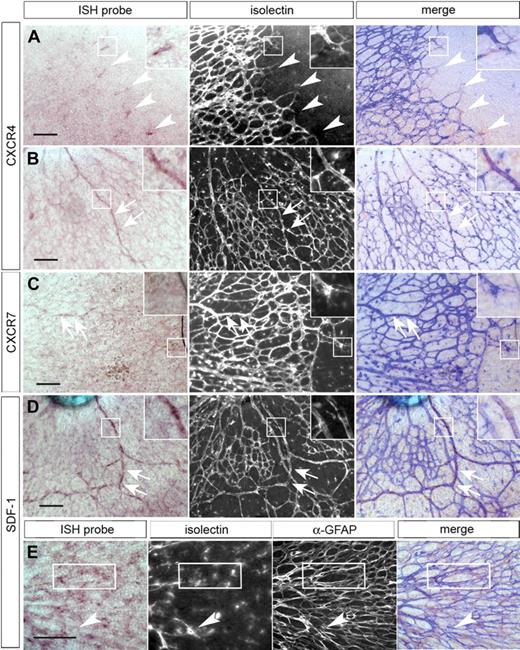
We selected several putative novel retinal tip cell genes whose transcripts were indicated to be enriched by the microarray data. To confirm their localization, we performed ISH on whole-mount postnatal retina. Several genes were confirmed to be enriched in tip cells, including CXCR4 (Figure 2), angiopoietin-2, SLP76/Lcp2, and Esm-1 (supplemental Figure 3; supplemental Table 1). Interestingly, several others that were enriched in the tip cell sample were actually expressed in a discrete region of the astrocyte layer directly adjacent to the tip cells, including VEGF-A,6 adrenomedullin, and cyclin D2 (supplemental Figure 3; and data not shown). These results indicate that our data provide a useful starting point for identifying genes enriched in tip cells or in cells adjacent to tip cells, but their precise localization then needs to be confirmed by further analysis.
Components of the SDF-1 signaling system are expressed in the retinal vasculature. ISH was performed on whole-mount postnatal day 1 or 2 retinas, and fluorescently labeled isolectin was used to counterstain the entire vasculature. ISH of whole-mount neonatal retina shows CXCR4 mRNA enriched in tip cells (A, arrowheads) and in the developing arteries (B, arrows). CXCR7 is expressed at low levels in the developing arteries (C, arrows) and absent from tip cells (C, inset box). SDF-1 mRNA is detected in the larger vessels of the developing retina (D, arrows) and in the retinal astrocyte layer (merged image shows ISH and anti-GFAP stain; E). Boxed area shows representative area of overlap with anti-GFAP staining of the astrocytes. Black scale bars represent 100 μm. Images were acquired as described in “ISH of whole-mount retina” using a 20× (A-D) or a 40× (E) objective. For ISH and merged images, RGB levels were adjusted using Photoshop; for merged images, fluorescent images were inverted and hue, levels, and transparency were adjusted to make both light and fluorescent images discernible.
Components of the SDF-1 signaling system are expressed in the retinal vasculature. ISH was performed on whole-mount postnatal day 1 or 2 retinas, and fluorescently labeled isolectin was used to counterstain the entire vasculature. ISH of whole-mount neonatal retina shows CXCR4 mRNA enriched in tip cells (A, arrowheads) and in the developing arteries (B, arrows). CXCR7 is expressed at low levels in the developing arteries (C, arrows) and absent from tip cells (C, inset box). SDF-1 mRNA is detected in the larger vessels of the developing retina (D, arrows) and in the retinal astrocyte layer (merged image shows ISH and anti-GFAP stain; E). Boxed area shows representative area of overlap with anti-GFAP staining of the astrocytes. Black scale bars represent 100 μm. Images were acquired as described in “ISH of whole-mount retina” using a 20× (A-D) or a 40× (E) objective. For ISH and merged images, RGB levels were adjusted using Photoshop; for merged images, fluorescent images were inverted and hue, levels, and transparency were adjusted to make both light and fluorescent images discernible.
The chemokine receptor CXCR4 is enriched in tip cells
Among the transcripts enriched in the tip cell sample were those for CXCR4 and CXCR7/Cmkor1, receptors for the chemokine SDF-1. CXCR4 is a multifunctional G-protein–coupled receptor involved in developmental and pathologic angiogenesis. Mice lacking SDF-1 or CXCR4 die prenatally or perinatally with multiple developmental abnormalities,22 including defects in the vasculature of the gut.23 The vascular phenotype is cell-autonomous and recapitulated in a vascular-specific knockout of CXCR4.24 CXCR4 is also expressed by tumor cells and tumor-associated vasculature.25 Similarly, SDF-1 is frequently associated with tumor progression and metastasis.26 ISH confirmed specific tip cell expression of CXCR4 (Figure 2A) in the developing retina. CXCR4 mRNA was also detected in larger vessels near the optic nerve head (Figure 2B). However, in contrast to the microarray data, CXCR7/Cmkor1 showed no expression in tip cells (Figure 2C) and only a very low, almost undetectable level of expression in developing arteries.
SDF-1 was strongly expressed in differentiating arteries proximal to the optic nerve head and in the astrocytes underlying the vasculature (Figure 2D-E). Interestingly, VEGF6 expression has also been observed in astrocytes adjacent to the retinal vascular front. This is consistent with a critical role for the astrocyte layer in mediating vascular growth and patterning in the retina. In the developing arteries, apparent coexpression of SDF-1 and CXCR4 suggests an autocrine and/or paracrine signaling mechanism, a mode of activity previously observed for this system.27
SDF-1 promotes capillary formation in vitro
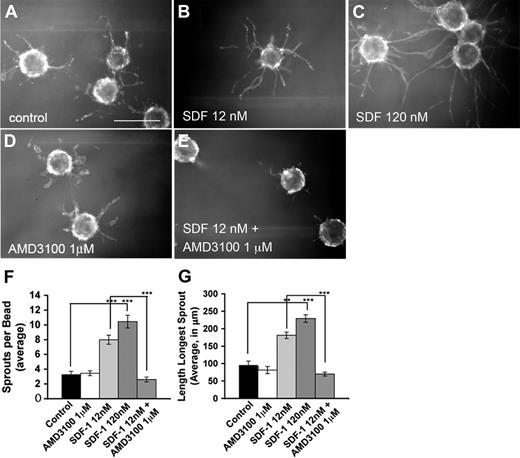
SDF-1 promotes migration in several types of endothelial cells, including retinal endothelial cells.28-30 SDF-1 also promotes endothelial tube formation.31-33 We examined the effects of SDF-1 on capillary formation and outgrowth in vitro. HUVECs were coated onto polystyrene beads and embedded in a fibrin matrix, which was then overlaid with primary human fibroblasts. In this assay, addition of SDF-1 results in a significant dose-dependent increase in sprout length and number (data not shown). To eliminate the possibility that these effects were secondary to an effect of SDF-1 on the supporting fibroblasts, HUVEC-coated beads were cultured without fibroblasts but supplemented with fibroblast-conditioned medium. Under these conditions, SDF-1 had a similar effect on sprout length and number (Figure 3), indicating that it acts directly on HUVECs.
SDF-1 stimulates capillary formation in vitro. (A-E) HUVECs were coated on polystyrene beads and embedded in a fibrin matrix; 12nM (B) or 120nM (C) SDF-1 added to the media stimulates both the length of the longest sprout per bead and sprout number per bead in a dose-dependent manner. A total of 1μM AMD3100 (D) alone has no effect on capillary outgrowth but reduces SDF-1–stimulated outgrowth to control levels (E). Quantification is shown in panels F and G. Original magnification × 40. Scale bar represents 100 μm. For quantification, 2.5× images were taken of the center of a 4-well plate. Sprout lengths and numbers were quantified from 10 beads from the upper left-hand quadrant of this image. Beads were not counted if their sprouts did not lie entirely within the image. If fewer than 10 beads were found in the upper left quadrant, beads were counted from the upper right quadrant and so on in a clockwise manner until 10 were quantified. For each experiment, 2 wells for each condition were quantified. Experiments were repeated 3 separate times, and representative data are shown. Error bars represent SEM. Statistical analysis was performed using the unpaired Student t test assuming equal variance for pairwise comparison of treatment groups: **P < .05, ***P < .005.
SDF-1 stimulates capillary formation in vitro. (A-E) HUVECs were coated on polystyrene beads and embedded in a fibrin matrix; 12nM (B) or 120nM (C) SDF-1 added to the media stimulates both the length of the longest sprout per bead and sprout number per bead in a dose-dependent manner. A total of 1μM AMD3100 (D) alone has no effect on capillary outgrowth but reduces SDF-1–stimulated outgrowth to control levels (E). Quantification is shown in panels F and G. Original magnification × 40. Scale bar represents 100 μm. For quantification, 2.5× images were taken of the center of a 4-well plate. Sprout lengths and numbers were quantified from 10 beads from the upper left-hand quadrant of this image. Beads were not counted if their sprouts did not lie entirely within the image. If fewer than 10 beads were found in the upper left quadrant, beads were counted from the upper right quadrant and so on in a clockwise manner until 10 were quantified. For each experiment, 2 wells for each condition were quantified. Experiments were repeated 3 separate times, and representative data are shown. Error bars represent SEM. Statistical analysis was performed using the unpaired Student t test assuming equal variance for pairwise comparison of treatment groups: **P < .05, ***P < .005.
Inhibition of CXCR4 in vivo alters tip cell morphology
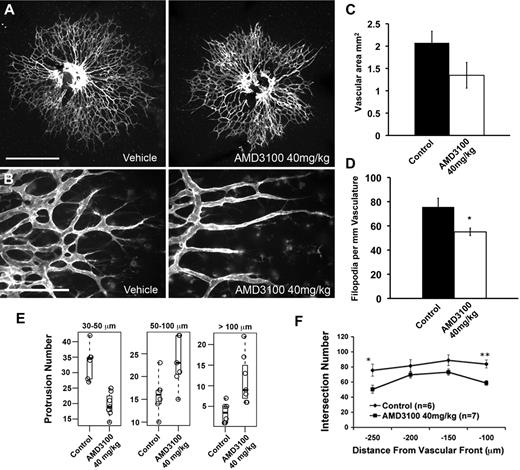
To assess the role of the SDF-1/CXCR4 signaling system in the retina, we used the bicyclam compound AMD3100, a specific and potent inhibitor of the SDF-1/CXCR4 interaction.34 In vitro, AMD3100 inhibited SDF-1–dependent capillary outgrowth but had little effect on sprout length or number in the absence of SDF-1 (Figure 3). AMD3100 (40 mg/kg) or vehicle (PBS) was administered by intraperitoneal injection to mice on postnatal day 1. After 24 hours, the retinal vasculature of mice treated with AMD3100 consistently showed defects in the morphology and patterning in the distal region. The vascular plexus in this region appeared less dense, and the tip cells appeared to lack the intercellular contacts typically formed between them, resulting in a “spiky” appearance. To analyze the effects of CXCR4 inhibition over a longer time period of postnatal retinal development, AMD3100 was administered every 12 hours starting at postnatal day 0 and retinas were examined 48 hours after the first dose (Figure 4). This treatment resulted in a dramatic alteration in tip cell morphology and patterning, including loss of lateral tip cell interconnections. To verify this effect using an independent method to block CXCR4 signaling, neonates were injected with anti-CXCR4 antibody or isotype-matched control antibody. Retinas from neonates treated with anti-CXCR4 but not from those treated with control antibody showed similar defects in tip cell morphology, although these effects were slightly less pronounced (supplemental Figure 1).
Inhibition of CXCR4 results in morphologic defects in the developing retinal vasculature. Treatment with AMD3100 results in spiky appearance to the distal vasculature and a reduction in the lateral intercellular connections normally seen between tip cells. Newborn mice were treated with 40 mg/kg AMD3100 or PBS control (A-B) twice daily for 2 days. Retinas were harvested 48 hours after first injections and stained with fluorescently labeled isolectin and imaged using 5× (A; scale bar represents 500 μm) or 40× (B; scale bar represents 100 μm) objectives. For each of the measurements in panels C to F, a litter was divided into treatment groups, and 1 eye from each neonate was quantified. Typical results for 1 litter are shown. Average vascularized retinal area is moderately but not significantly reduced after AMD3100 treatment. One-way analysis of variance indicates P = .18 for this dataset (C). Filopodia numbers are significantly reduced in AMD3100-treated retinas; 40× images of the vascular periphery were acquired in z-stacks encompassing the full vascular layer. A maximum projection image of each stack was generated, and filopodia were counted manually using ImageJ software and normalized to the length of the distal vascular front (D). Numbers of unbranched vascular protrusions between 50 and 100 mm and greater than 100 mm in length are increased in AMD3100-treated retinas. Total counts of protrusions were identified for each retina in each of 3 length bins (30-50 μm, 50-100 μm, and > 100 μm). The Student t test was used to test whether the means of the distributions for the vehicle or AMD-treated counts were different for a particular length bin. The resulting P values were corrected for multiple testing using the Bonferroni method (E). Sholl analysis indicates significantly reduced vascular densities at 100 and 250 μm from the vascular front; these regions correlate with areas of CXCR4 expression in the retinal vasculature (F). Error bars represent SEM. Statistical analysis was performed using the unpaired Student t test assuming equal variance for pairwise comparison of treatment groups unless otherwise indicated: *P < .05, **P < .005.
Inhibition of CXCR4 results in morphologic defects in the developing retinal vasculature. Treatment with AMD3100 results in spiky appearance to the distal vasculature and a reduction in the lateral intercellular connections normally seen between tip cells. Newborn mice were treated with 40 mg/kg AMD3100 or PBS control (A-B) twice daily for 2 days. Retinas were harvested 48 hours after first injections and stained with fluorescently labeled isolectin and imaged using 5× (A; scale bar represents 500 μm) or 40× (B; scale bar represents 100 μm) objectives. For each of the measurements in panels C to F, a litter was divided into treatment groups, and 1 eye from each neonate was quantified. Typical results for 1 litter are shown. Average vascularized retinal area is moderately but not significantly reduced after AMD3100 treatment. One-way analysis of variance indicates P = .18 for this dataset (C). Filopodia numbers are significantly reduced in AMD3100-treated retinas; 40× images of the vascular periphery were acquired in z-stacks encompassing the full vascular layer. A maximum projection image of each stack was generated, and filopodia were counted manually using ImageJ software and normalized to the length of the distal vascular front (D). Numbers of unbranched vascular protrusions between 50 and 100 mm and greater than 100 mm in length are increased in AMD3100-treated retinas. Total counts of protrusions were identified for each retina in each of 3 length bins (30-50 μm, 50-100 μm, and > 100 μm). The Student t test was used to test whether the means of the distributions for the vehicle or AMD-treated counts were different for a particular length bin. The resulting P values were corrected for multiple testing using the Bonferroni method (E). Sholl analysis indicates significantly reduced vascular densities at 100 and 250 μm from the vascular front; these regions correlate with areas of CXCR4 expression in the retinal vasculature (F). Error bars represent SEM. Statistical analysis was performed using the unpaired Student t test assuming equal variance for pairwise comparison of treatment groups unless otherwise indicated: *P < .05, **P < .005.
To analyze this phenotype, we first measured the areas of vascularized retina in vehicle and AMD3100-treated neonates. There was a moderate reduction in vascularized area in AMD3100-treated retinas; however, this reduction was typically not statistically significant (Figure 4C). Because AMD3100-treated neonates often show slightly less weight gain than their littermates over the course of treatment, this reduction may be a result of a slight developmental delay. To assess the overall network density, a modified Sholl analysis was used. Sholl analysis35 is a morphometric analysis used to quantify dendritic arborization of neurons, and it has also been adapted to measure vascular density.36,37 A series of concentric circles spaced 50 μm apart and centered about the optic nerve head was overlaid onto an image of the vasculature. The number of intersections of the vasculature with circles 100, 150, 200, and 250 μm from the distal vascular front (supplemental Figure 2) was quantified. Treatment with AMD3100 resulted in a reduction in vascular density at all points measured and a significant reduction at 100 and 250 μm from the distal edge (Figure 4F). The region 100 μm from the distal edge is largely populated by tip cells, and the significant reduction in vascular density in this region is consistent with a role for CXCR4 in mediating tip cell morphology and branching. The region at 250 μm, close to the optic nerve head, represents a region where the primitive vascular plexus is beginning to remodel into arteries and veins. As these nascent arteries express CXCR4, the loss of vascular density is also consistent with a role for CXCR4 in vessel patterning in this region.
We quantified vascular protrusion length by measuring the lengths of vascular protrusions from the distal edge of the network. Protrusions in this case were defined as isolectin-positive structures composed of 1 or more cells that protrude from the vascular network without contacting adjacent vasculature (diagrammed in supplemental Figure 2). Retinas from AMD3100-treated neonates showed a significant increase in the percentage of vascular protrusions between 50 to 100 μm and greater than 100 μm in length and a decrease in shorter protrusions. Because the overall diameter of the retinal vasculature is unchanged or even decreased in AMD3100 treated neonates, it is unlikely that this increase in vascular protrusion length represents an increase in tip cell growth or migration. More probably, it reflects a loss in tip cell branching or lateral intercellular connections.
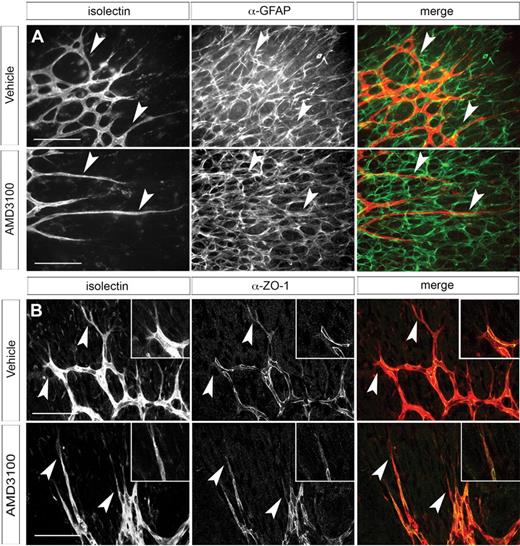
Intercellular connections between endothelial cells are mediated in part by tight junction protein ZO-1.38 Both tip and stalk cells express ZO-1. In tip cells, ZO-1 is localized to the cytoplasmic compartment; in contrast, ZO-1 in stalk cells is enriched at the cell membrane where the tight junctions characteristic of mature blood vessels are forming. ZO-1 immunostaining indicated that this distinct localization pattern was unaffected by CXCR4 inhibition (Figure 5B), suggesting that formation of tight junctions between endothelial cells proceeds normally.
Analysis of retinal astrocyte and vascular intercellular adhesion morphology in AMD3100-treated retinas. Whole-mount retinas were dissected from neonates treated with AMD3100 (40 mg/kg twice daily as in Figure 4) or vehicle control. Retinas were stained with anti-GFAP antibody (A) and fluorescently labeled isolectin and imaged as described in “Immunohistochemistry on retinal whole-mount preparations” using fluorescent Zeiss Axioplan 2 microscope with 40× objective (A) or Leica DMI6000CS confocal microscope with 40× objective (B). Although the vascular morphology is clearly aberrant, the underlying astrocytes have a normal morphology and vessels still appear to track over them. Arrowheads indicate tip cells (all panels). (B) Anti–ZO-1 immunostaining shows cytoplasmic localization in tip cells and recruitment to sites of intercellular contact in stalk cells. Scale bars represent 100 μm.
Analysis of retinal astrocyte and vascular intercellular adhesion morphology in AMD3100-treated retinas. Whole-mount retinas were dissected from neonates treated with AMD3100 (40 mg/kg twice daily as in Figure 4) or vehicle control. Retinas were stained with anti-GFAP antibody (A) and fluorescently labeled isolectin and imaged as described in “Immunohistochemistry on retinal whole-mount preparations” using fluorescent Zeiss Axioplan 2 microscope with 40× objective (A) or Leica DMI6000CS confocal microscope with 40× objective (B). Although the vascular morphology is clearly aberrant, the underlying astrocytes have a normal morphology and vessels still appear to track over them. Arrowheads indicate tip cells (all panels). (B) Anti–ZO-1 immunostaining shows cytoplasmic localization in tip cells and recruitment to sites of intercellular contact in stalk cells. Scale bars represent 100 μm.
We examined the morphology of the astrocyte layer in AMD3100-treated animals to ascertain whether the vascular phenotype was secondary to a defect in astrocyte patterning. The astrocyte layer in these retinas appears indistinguishable from controls (Figure 5A). Furthermore, tip cells still appear to track with the astrocyte meshwork even when their morphology is abnormal, suggesting that the interaction between these 2 cell types is functionally intact. Retinal endothelial cells secrete collagen IV, a component of the basement membrane, during migration.39 Collagen IV can be seen encapsulating the newly forming vessels in both control and AMD3100-treated retinas. No empty collagen “sleeves” or other evidence of retracting vessels is apparent (supplemental Figure 4).
Tip cells are characterized by their numerous filopodia. These filopodia extend laterally toward other tip cells in the vascular plexus as the first step in establishing intertip cell adhesions. To assess the effects of blocking CXCR4 signaling on filopodia formation, we counted tip cell filopodia in vehicle- and AMD3100-treated retinas (Figure 4D) and normalized to the length of the perimeter of the cells at the edge of the vascular plexus. Filopodia numbers were significantly impaired in AMD3100-treated tip cells, consistent with previous work implicating the SDF-1/CXCR4 signaling pathway in filopodia formation.40,41
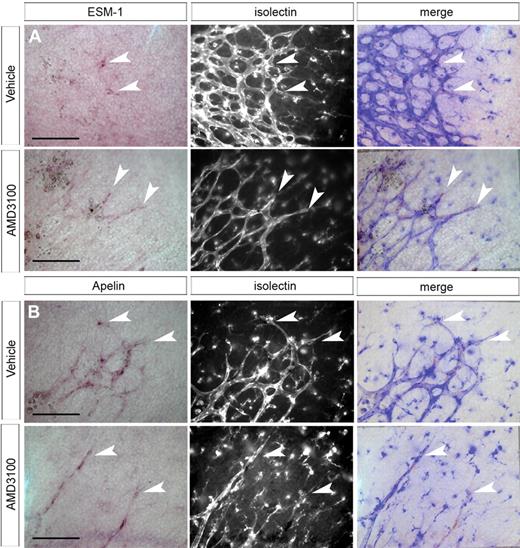
Because of their “spiky” or elongated appearance and reduced filopodia, we examined the possibility that loss of CXCR4 activity causes premature differentiation of tip cells into stalk cells. We examined expression of other tip cell marker genes in AMD3100- and control-treated retinas. The secreted protein apelin is specifically up-regulated in retinal tip cells.20 We observed strong expression of apelin mRNA in tip cells of both vehicle- and AMD3100-treated retinas (Figure 6B). An additional marker, endocan/ESM-1, was identified by our microarray analysis and confirmed to be highly specific to tip cells. Like apelin, expression of this gene in tip cells was unaffected by AMD3100 treatment (Figure 6A). Similarly, expression of Dll4 was detected in tip cells in both AMD3100- and control-treated retinas (supplemental Figure 5).
Tip cell identity is unaffected after inhibition of CXCR4 signaling. ISH of P1 to P1.5 whole-mount retina indicates that expression of ESM-1 and apelin are limited to tip cells in the developing retinal vasculature. ESM-1 mRNA is still detectable in tip cells after AMD3100 treatment (A). Similarly, apelin is expressed in tip cells at the vascular front in both control and AMD3100-treated retinas (B). Arrowheads indicate tip cells; scale bar represents 100 μm; imaging was performed as described in “ISH of whole-mount retina.”
Tip cell identity is unaffected after inhibition of CXCR4 signaling. ISH of P1 to P1.5 whole-mount retina indicates that expression of ESM-1 and apelin are limited to tip cells in the developing retinal vasculature. ESM-1 mRNA is still detectable in tip cells after AMD3100 treatment (A). Similarly, apelin is expressed in tip cells at the vascular front in both control and AMD3100-treated retinas (B). Arrowheads indicate tip cells; scale bar represents 100 μm; imaging was performed as described in “ISH of whole-mount retina.”
Discussion
Endothelial tip cells have been proposed to have a specialized function in the guidance and patterning of nascent blood vessels. To further characterize these cells, we harvested tip cells from postnatal mouse retina using laser capture microdissection and compared their gene expression profile with that of stalk cells harvested from the same tissue. Several novel tip cell-expressed genes were identified and confirmed to be up-regulated in tip cells by ISH.
The chemokine receptor CXCR4 was identified as a tip cell-enriched gene, and this expression was confirmed by ISH. The SDF-1/CXCR4 signaling system has long been known to be involved in both developmental and adult angiogenesis23,42 and to promote endothelial cell migration and proliferation. Our results suggest that this system may also be involved in vascular patterning. Neonatal mice treated with the CXCR4 antagonist showed dose-dependent defects in retinal vascular patterning and tip cell morphology, including a reduction in filopodia. This phenotype was most dramatic at 40 mg/kg of AMD3100. Doses of 5 to 10 mg/kg are routinely administered to adult mice for acute mobilization of hematopoietic progenitor cells.28,43,44 Because AMD3100 has a short half-life in vivo (3.6 hours in healthy humans45 ), an increased dose was used to antagonize CXCR4 activity long enough to effect a change in the developing retinal vasculature. Although AMD3100 has been reported to be highly specific to CXCR4, a recent report suggests that it may also bind the other known SDF-1 receptor, CXCR7.46 However, ISH suggests there is little, if any, expression of CXCR7 in the early postnatal retinal vasculature. Furthermore, the phenotype seen in AMD3100-treated retinas was verified using a function-blocking antibody specific to CXCR4. Although involvement of CXCR7 cannot be definitively excluded, it is unlikely to contribute to the phenotype seen in AMD3100-treated retinas.
Retinas from neonates treated with AMD3100 show a reduction in the density of the distal region of the retinal vasculature, where tip cells are abundant, and proximal to the optic nerve head, where the presumptive arteries are beginning to differentiate. This is consistent with our observation that endothelial cells in both of these regions express CXCR4. The vascular plexus between these 2 regions shows a moderate decrease in density. This decrease may be a residual effect resulting from growth and guidance defects of the tip cells in pioneering the network in its earlier stages. However, the partial recovery of a normal branching pattern suggests that factors other than CXCR4 contribute to patterning in this region. Further analysis of AMD3100-treated retinas indicated an overall increase in the percentage of vascular protrusions more than 100 μm in length. Because the overall vascular area is not increased, this phenotype probably does not result from increased outgrowth of tip cells but rather a reduction of intercellular connections of tip cells within the vascular network.
Tip cells form several types of intercellular connections: basal (with the underlying astrocytes), lateral (with adjacent tip cells), and radial (with trailing stalk cells). Previous work has implicated neuropilin-1 in the formation of lateral connections between tip cells in the murine hindbrain.47 Although neuropilin-1 is involved in retinal angiogenesis, blocking either its VEGF-binding domain or its semaphorin-binding domain does not affect tip cell morphology or intercellular connections.36 However, both the present study and data from the neuropilin-1 knockout illustrate the importance of lateral tip cell connections in driving vascular plexus formation.
Filopodia formation is a critical first step in the guidance and motility of tip cells5 and the subsequent formation of intercellular connections. Inhibition of CXCR4 results in a significant decrease in filopodia numbers, and this probably contributes to the gross morphologic changes in vessel patterning seen in these retinas. This phenotype is reminiscent of the gut vasculature defects seen in CXCR4– and SDF-1–deficient embryos. These defects are characterized by a lack of interconnecting vessels between the superior mesenteric artery and the primary capillary plexus surrounding the primitive gut. Furthermore, CXCR4−/− or SDF-1−/− gut vasculature is deficient in filopodia extensions and processes linking adjacent endothelial cells. These defects are endothelial-cell autonomous as they are also evident in the vascular-specific CXCR4 knockout.
Endothelial tip cells compose a distinct subpopulation of endothelial cells thought to have an important role in vascular guidance and patterning. In this study, we isolated tip cells from developing retinal vasculature and analyzed their gene expression pattern compared with that in endothelial stalk cells from the same tissue. The chemokine receptor CXCR4 was identified as a tip cell-enriched gene. CXCR4 was found to have a previously unidentified role in vascular growth and patterning in the neonatal retina, mediating intercellular connections between tip cells. Intercellular interactions between tip cells and their physiologic function in angiogenesis are poorly understood. Characterizing these interactions could provide new avenues of investigation for antiangiogenic therapeutics, particularly those that might specifically target nascent or actively sprouting vasculature.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Grazyna Fedorowicz, Zora Modrusan, Brendan Tarlow, Jeff Eastham-Anderson, and Houston Gilbert for excellent technical assistance and Anne Eichmann for helpful comments on the paper.
Authorship
Contribution: G.A.S. designed, performed, and analyzed research and wrote the paper; J.S.K. analyzed research and wrote the paper; and M.T.-L. designed and analyzed research and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Marc Tessier-Lavigne, Genentech Inc, 1 DNA Way, South San Francisco, CA 94080; e-mail: tessier-lavigne.marc@gene.com.