Abstract
Neurolymphomatosis (NL) is a rare clinical entity. The International Primary CNS Lymphoma Collaborative Group retrospectively analyzed 50 patients assembled from 12 centers in 5 countries over a 16-year period. NL was related to non-Hodgkin lymphoma in 90% and to acute leukemia in 10%. It occurred as the initial manifestation of malignancy in 26% of cases. The affected neural structures included peripheral nerves (60%), spinal nerve roots (48%), cranial nerves (46%), and plexus (40%) with multiple site involvement in 58%. Imaging studies often suggested the diagnosis with 77% positive magnetic resonance imaging, and 84% (16 of 19) positive computed tomography-positron emission tomography studies. Cerebrospinal fluid cytology was positive in 40%, and nerve biopsy confirmed the diagnosis in 23 of 26 (88%). Treatment in 47 patients included systemic chemotherapy (70%), intra-cerebrospinal fluid chemotherapy (49%), and radiotherapy (34%). Response to treatment was observed in 46%. The median overall survival was 10 months, with 12- and 36-month survival proportions of 46% and 24%, respectively. NL is a challenging diagnosis, but contemporary imaging techniques frequently detect the relevant neural invasion. An aggressive multimodality therapy can prevent neurologic deterioration and is associated with a prolonged survival in a subset of patients.
Introduction
The term neurolymphomatosis (NL) encompasses nerve infiltration by neurotropic neoplastic cells in the setting of an unknown or a known hematologic malignancy. It is a rare neurologic manifestation of non-Hodgkin lymphoma (NHL) and leukemia with a poorly defined incidence. The most comprehensive review identified 72 cases of NL caused by NHL that were reported during a 28-year period.1 The majority of patients with NL described in the literature had NHL; and in that setting, NL appears to represent a unique subtype of extranodal disease. However, nerve-infiltrating disease may occur rarely in leukemia2-4 ; therefore, the present study assessed this clinical entity in patients with either NHL or leukemia.
The typical manifestations of NL are of a neuropathy that may affect peripheral nerves, nerve roots, plexus, or cranial nerves. The most common presentations include painful peripheral neuropathy or radiculopathy, cranial neuropathy, painless polyneuropathy, and peripheral mononeuropathy or a mononeuropathy multiplex. Successful therapy is contingent on the recognition of this unique neurologic complication, yet the diagnosis is difficult and often elusive. Because NL is rare, there is limited information available on mode of presentation, clinical course, yield of diagnostic procedures, and response to therapy. As well, the survival after diagnosis and treatment is poorly characterized as it has never been reported in a systematic study.
The International Primary Central Nervous System Lymphoma Collaborative Group (IPCG) is a multidisciplinary group established in 2002 under the sponsorship of the International Extranodal Lymphoma Study Group. Given the rarity of NL, this neurologic complication of lymphoma and leukemia was identified as an area for which collaborative work could further our understanding of the disease. We report a retrospective analysis of 50 cases of NL that were assembled by 9 IPCG investigators from 12 centers in 5 countries. Because malignant infiltration of peripheral nerves occurs in both NHL and leukemia, the current study investigated both conditions under the common term of NL.
Methods
A retrospective chart review was conducted by the IPCG investigators to collect information on HIV-seronegative adult patients whose final diagnosis was compatible with the definition of clinical neuropathy characterized by infiltration of malignant lymphocytes. As this study is retrospective, inclusion of patients was based on final diagnosis of NL that often required the full perspective of the course of neurologic manifestations and the related diagnostic workup. Eligible were patients with neoplasms categorized as either lymphoma or leukemia. Other hematologic malignancies were excluded. Eligibility criteria included patients whose clinical manifestation was consistent with either a primary NL, defined as NL that is the first manifestation of the hematologic malignancy, or patients with secondary NL, in which NL is a site of relapse or progression of a previously diagnosed lymphoma or leukemia. In principle, NL was defined as neuropathy that is characterized by infiltration of malignant cells. Yet, manifestation of either cranial neuropathy or cauda equina involvement in the presence of positive cytology was not considered as NL unless evidence existed for intradural as well as extradural infiltration of the affected nerves or, alternatively, additional data indicated that malignant infiltration of either peripheral nerves and/or neural plexi has also developed. Malignant infiltration of nerve structures that occurred in the setup of a bulky disease that entrapped and infiltrated the neural elements was excluded. In primary NL, infiltration of the affected neural structure had to be proved by a biopsy or at autopsy. In secondary NL, the diagnosis required exclusion of other causes of neuropathy, presence of positive imaging findings that detected specific neural involvement, and evidence for disease progression. If diagnosis remained in doubt, a biopsy of the affected structure was required or otherwise autopsy findings indicated the final diagnosis.
A data collection form was sent to investigators, and each one received ethics committee approval from all participating institutions for the release of case information that was rendered anonymous. Requested information included patient demographics, details of clinical history and presentation, prognostic parameters such as Eastern Cooperative Oncology Group Performance Status (ECOG-PS), serum lactate dehydrogenase level, disease stage, extranodal site(s) involved at the diagnosis of lymphoma, International Prognostic Index (IPI) score, cell type or karyotype, and total white blood cell count at diagnosis. Information was requested on neurologic status (muscle weakness, sensory deficit, and autonomic abnormalities) as well as on the neurologic function5 at the diagnosis of NL. The neurologic function was assessed according to the following scale: 0 indicates no neurologic symptoms (fully active at home/work without assistance); 1, minor neurologic symptoms (fully active at home/work without assistance); 2, moderate neurologic symptoms (fully active at home/work but requires assistance); 3, moderate neurologic symptoms (less than fully active at home/work and requires assistance); and 4, severe neurologic symptoms (totally inactive requiring complete assistance at home or in institution, unable to do work). Data were collected on diagnostic measures conducted for evaluation of the neuropathy, including imaging studies, cerebrospinal fluid (CSF) evaluation, and biopsy and DNA analysis of body fluids or tissues. In addition, the form requested information on types of treatment given for NL, response to treatment that was evaluated by posttreatment ECOG-PS, neurologic function score, neurologic status, and posttreatment imaging studies. Data on disease progression, site(s) of involvement, and survival were reported.
Descriptive summaries included proportions for categorical variables and medians, minimums, and maximums for numeric variables. Overall survival was calculated from the date of diagnosis of NL to the date of death. Surviving patients were censored at the date of last follow-up. Survival curves were estimated using the Kaplan-Meier product-limit methods,6 and comparisons between primary and secondary NL were examined by the log-rank test.
Results
Information on a total of 50 patients was assembled. Patients with NL were diagnosed from January 1993 to November 2008. During this 16-year period, only 15 (30%) patients were diagnosed during the first 8 years (until December 2000) and 35 patients were diagnosed afterward with 50% of all cases identified after January 2004. Of the 11 cases diagnosed up to 1998, in 4 the final diagnosis was established only at autopsy, whereas in all the rest diagnosis was arrived antemortem. The increased rate of detection of NL during the last 5 years raised the issue of whether the presumably earlier or facilitated diagnosis is associated with changes in clinical features and treatment outcome compared with those described previously. Therefore, we present the main features of our series in parallel to those enumerated in the English literature (Tables 1–2). The literature search was conducted for reports of adult patients with high-grade lymphoma or acute leukemia presenting with cranial, spinal, or peripheral nerve infiltration by malignant cells of these hematologic malignancies.
Patient characteristics and clinical features of neurolymphomatosis
| Characteristic . | Group A: literature review with MGH case series (1972-2000), N = 72 . | Group B: literature review, case reports (2001-2008), N = 44 . | Group C: current IPCG case series (1993-2008), N = 50 . |
|---|---|---|---|
| Sex | |||
| Male | 39 (54) | 26 (59) | 30 (60) |
| Female | 33 (46) | 16 (36) | 20 (40) |
| Not reported | 2 (4.5) | ||
| Median age, y (range) | 63 (18-84) | 56 (16-71) | 55.5 (18-80) |
| NL as an extranodal site of systemic lymphoma | 29 (40) | 27 (61) | 33 (66) |
| NL as the presentation of malignancy | NA | 13 (29.5) | 13 (26) |
| Malignant cell type | |||
| B cells | 59 (82) | 29 (66) | 41 (82) |
| T cells | 4 (5) | 11 (25) | 5 (10) |
| NK cells | 1 (2) | ||
| Not classified | 9 (13) | 3 (6.8) | 4 (8) |
| Parenchymal brain involvement | 19 (26) | NA | 11 (22), PCNSL |
| Affected neural structures* | |||
| Peripheral nerves | 46 (64) | 9 (20) | 30 (60) |
| Spinal nerves | 47 (65) | 14 (32) | 24 (48) |
| Neural plexus | 23 (32) | 15 (34) | 20 (40) |
| Cranial nerves | 37 (51) | 15 (34) | 23 (46) |
| Painful neuropathy | 34 (47) | 25 (57) | 38 (76) |
| Type of neuropathy | |||
| Pure motor | NA | 7 (16) | 11 (22) |
| Pure sensory | NA | 8 (18) | 1 (2) |
| Sensorimotor | NA | 23 (52) | 36 (72) |
| Not reported | 6 (14) | 2 (4) |
| Characteristic . | Group A: literature review with MGH case series (1972-2000), N = 72 . | Group B: literature review, case reports (2001-2008), N = 44 . | Group C: current IPCG case series (1993-2008), N = 50 . |
|---|---|---|---|
| Sex | |||
| Male | 39 (54) | 26 (59) | 30 (60) |
| Female | 33 (46) | 16 (36) | 20 (40) |
| Not reported | 2 (4.5) | ||
| Median age, y (range) | 63 (18-84) | 56 (16-71) | 55.5 (18-80) |
| NL as an extranodal site of systemic lymphoma | 29 (40) | 27 (61) | 33 (66) |
| NL as the presentation of malignancy | NA | 13 (29.5) | 13 (26) |
| Malignant cell type | |||
| B cells | 59 (82) | 29 (66) | 41 (82) |
| T cells | 4 (5) | 11 (25) | 5 (10) |
| NK cells | 1 (2) | ||
| Not classified | 9 (13) | 3 (6.8) | 4 (8) |
| Parenchymal brain involvement | 19 (26) | NA | 11 (22), PCNSL |
| Affected neural structures* | |||
| Peripheral nerves | 46 (64) | 9 (20) | 30 (60) |
| Spinal nerves | 47 (65) | 14 (32) | 24 (48) |
| Neural plexus | 23 (32) | 15 (34) | 20 (40) |
| Cranial nerves | 37 (51) | 15 (34) | 23 (46) |
| Painful neuropathy | 34 (47) | 25 (57) | 38 (76) |
| Type of neuropathy | |||
| Pure motor | NA | 7 (16) | 11 (22) |
| Pure sensory | NA | 8 (18) | 1 (2) |
| Sensorimotor | NA | 23 (52) | 36 (72) |
| Not reported | 6 (14) | 2 (4) |
Values are no. (%), except for age (y). Group A consists of a retrospective case series of 25 patients diagnosed in Massachusetts General Hospital (MGH) and literature review of additional 47 cases that were published together.1 Group B is based on literature review.4,7-41 Group C is the current case series.
NA indicates not available; and PCNSL, primary central nervous system lymphoma.
Involvement of multiple sites was common.
Diagnostic modalities and response to treatment of neurolymphomatosis
| Diagnostic/treatment modality . | Group A: literature review with MGH case series (1972-2000), N = 72 . | Group B: literature review, case reports (2001-2008), N = 44 . | Group C: current IPCG case series (1993-2008), N = 50 . |
|---|---|---|---|
| Imaging | |||
| CT | NA | 3/11 (27) | 7/11 (64) |
| MRI | 28/40 (70) | 28/35 (80) | 36/47 (77) |
| FDG-PET | NA | 19/21 (90) | 16/19 (84) |
| CSF cytology | 21/52 (40) | 10/24 (42) | 18/45 (40) |
| CSF PCR gene rearrangement | NA | 2/2 (100) | 3/11 (27) |
| Biopsy of affected nerve | 24/30 (80) | 19/21 (90) | 23/26 (88) |
| Diagnosis established only by autopsy | 33 (46) | 2 (5) | 4 (8) |
| No. of patients treated for NL* | 43 (60) | 34 (77) | 47 (94) |
| IV HD-MTX | 5/43 (12) | 8/34 (23.5) | 23/47 (49) |
| Intra-CSF chemotherapy | 15/43 (35) | 14/34 (41) | 23/47 (49) |
| Radiotherapy | 10/43 (23) | 17/34 (50) | 16/47 (34) |
| Response rate† | 31/43 (72) | 20/34 (58) | 16/35 (46) |
| Diagnostic/treatment modality . | Group A: literature review with MGH case series (1972-2000), N = 72 . | Group B: literature review, case reports (2001-2008), N = 44 . | Group C: current IPCG case series (1993-2008), N = 50 . |
|---|---|---|---|
| Imaging | |||
| CT | NA | 3/11 (27) | 7/11 (64) |
| MRI | 28/40 (70) | 28/35 (80) | 36/47 (77) |
| FDG-PET | NA | 19/21 (90) | 16/19 (84) |
| CSF cytology | 21/52 (40) | 10/24 (42) | 18/45 (40) |
| CSF PCR gene rearrangement | NA | 2/2 (100) | 3/11 (27) |
| Biopsy of affected nerve | 24/30 (80) | 19/21 (90) | 23/26 (88) |
| Diagnosis established only by autopsy | 33 (46) | 2 (5) | 4 (8) |
| No. of patients treated for NL* | 43 (60) | 34 (77) | 47 (94) |
| IV HD-MTX | 5/43 (12) | 8/34 (23.5) | 23/47 (49) |
| Intra-CSF chemotherapy | 15/43 (35) | 14/34 (41) | 23/47 (49) |
| Radiotherapy | 10/43 (23) | 17/34 (50) | 16/47 (34) |
| Response rate† | 31/43 (72) | 20/34 (58) | 16/35 (46) |
Values are no. positive/no. of tests (%). Group A consists of a retrospective case series of 25 patients diagnosed in Massachusetts General Hospital (MGH) and literature review of additional 47 cases that were published together.1 Group B is based on literature review.4,7-41 Group C is the current case series.
NA indicates not available; PCR, polymerase chain reaction; and IV HD-MTX, intravenous high-dose methotrexate.
Some patients were treated by chemotherapy other than high-dose methotrexate.
Includes complete and partial response by clinical improvement or by posttreatment imaging.
Clinical features and diagnostic modalities
Patient characteristics are summarized in Table 1. The median age of the current IPCG series was 55.5 years (range, 18-80 years) and 30 (60%) were males. The underlying malignancy was NHL in 45 (90%) patients and 5 had acute lymphoblastic leukemia. Of the 45 patients with NHL, 11 (24%) had initial diagnosis of primary central nervous system lymphoma (PCNSL). The predominant malignant cell type was B-cell; and of the 5 patients with T-cell disease, 3 had acute leukemia. The most common subtype of lymphoma was diffuse large B-cell (34 of 45; 75.5%). Four patients had follicular lymphoma (9%), 2 peripheral T-cell lymphoma (4%), and one mantle cell lymphoma (2%) and, in 4 cases (9%) information on the subtype of the disease was not available.
NL occurred as the first manifestation of malignancy (primary NL) in 14 (28%) cases (including 11 patients with systemic NHL, 2 with PCNSL, and 1 with leukemia) and as a relapse or progression of a previously treated disease in the remaining 36 (23 patients with systemic NHL, 9 with PCNSL, and 4 with leukemia). The 2 patients with primary NL that initially were diagnosed as PCNSL presented with cranial neuropathy as the initial manifestation of their disease. Cranial nerve infiltration by malignant lymphocytes was proved by biopsy. Both were then defined as PCNSL, but at disease progression they continued to manifest features compatible with NL that included extension of neural involvement beyond the dura matter. One patient also developed parenchymal brain involvement during disease progression.
Secondary NL was diagnosed within a median interval of 10 months (range, 4-120 months) after initial diagnosis of the hematologic malignancy. The median ECOG-PS at the diagnosis of NL was 2. The IPI was reported in 32 of 34 (94%) patients with systemic NHL, and 26 (81%) of them had intermediate to high IPI (presence of 2 or more prognostic factors) at diagnosis.
NL affected more than one anatomic structure in 29 (58%) patients (Table 1; Figure 1A-C). Peripheral nerves were the most frequently involved site, whereas spinal, cranial nerve involvement, and neural plexus infiltration occurred at a similar rate. The manifestation of painful neuropathy was recorded in 38 (76%) patients, with sensorimotor neuropathy being the most common type (36 cases). Notwithstanding the infiltrative nature of NL, pure motor neuropathy was described in 20% of the patients and pure sensory neuropathy was noted in a single patient.
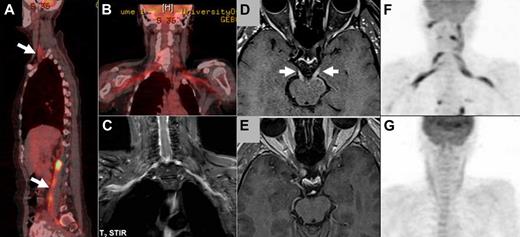
Imaging studies in NL. (A-B) FDG-PET imaging of a patient with neurolymphomatosis (NL). (A) Multiple sites of involvement, including the brachial and lumbosacral plexi (arrows). (B-C) Bilateral involvement of the brachial plexus in the same patient clearly detected by both FDG-PET (B) and by MRI (C) T2 short T1 inversion recovery imaging. (D-E) Enhanced MRI imaging (T1-weighted with gadolinium) of a patient with NL that affected multiple cranial nerves. (D) Bilateral abnormal enhancement of the oculomotor nerves that corresponded to the clinical presentation of bilateral ophthalmoplegia. (E) Complete resolution of abnormal enhancement after 2 cycles of treatment with intravenous high-dose methotrexate and intra-CSF treatment with cytarabine. These imaging findings matched the marked neurologic improvement observed under treatment. (F-G) FDG-PET imaging of a patient with NL who presented with severe painful sensorimotor neuropathy and bilateral brachial plexus involvement. (F) FDG-PET findings at diagnosis of NL compatible with bilateral brachial plexus involvement by lymphoma. (G) Complete resolution of abnormal tracer uptake after 2 courses of treatment with systemic high doses of methotrexate and cytarabine. The treatments lead to clear neurologic improvement and good control of the painful neuropathy.
Imaging studies in NL. (A-B) FDG-PET imaging of a patient with neurolymphomatosis (NL). (A) Multiple sites of involvement, including the brachial and lumbosacral plexi (arrows). (B-C) Bilateral involvement of the brachial plexus in the same patient clearly detected by both FDG-PET (B) and by MRI (C) T2 short T1 inversion recovery imaging. (D-E) Enhanced MRI imaging (T1-weighted with gadolinium) of a patient with NL that affected multiple cranial nerves. (D) Bilateral abnormal enhancement of the oculomotor nerves that corresponded to the clinical presentation of bilateral ophthalmoplegia. (E) Complete resolution of abnormal enhancement after 2 cycles of treatment with intravenous high-dose methotrexate and intra-CSF treatment with cytarabine. These imaging findings matched the marked neurologic improvement observed under treatment. (F-G) FDG-PET imaging of a patient with NL who presented with severe painful sensorimotor neuropathy and bilateral brachial plexus involvement. (F) FDG-PET findings at diagnosis of NL compatible with bilateral brachial plexus involvement by lymphoma. (G) Complete resolution of abnormal tracer uptake after 2 courses of treatment with systemic high doses of methotrexate and cytarabine. The treatments lead to clear neurologic improvement and good control of the painful neuropathy.
The diagnostic modalities included CSF analysis (in 45 patients), imaging studies that were reported for all patients, and nerve biopsy that was obtained in 26 (52%) of the patients (Table 2). CSF analysis was remarkable for elevated protein in 61%, low glucose level in 11%, and elevated cell count (> 5 cells/mm3) in 44%. Malignant cells were detected in the CSF in 18 (40%) of the studies, and suspicious cytology was reported in another 6 (13%). Of the 20 patients with elevated cell count, 12 had positive cytology and 4 had suspicious cytology. Thus, 8 patients had positive or suspicious cytology in the face of a normal CSF cell count. Of patients with abnormal CSF cell count, in 6 (30%) cases no cranial or spinal root involvement was evident. All the rest manifested a combined affliction of cranial and/or spinal roots together with plexus and/or peripheral nerves involvement. The CSF evaluation also included polymerase chain reaction–based gene rearrangement analysis of either the immunoglobulin heavy chain or of the T-cell receptor in 12 cases (24%) and was positive in 4 (33%).
All patients were evaluated by imaging and 23 (46%) were assessed by more than one modality. Magnetic resonance imaging (MRI) was done in 47 (94%) cases whereas 18F-fluoro-2-deoxy-D-glucose (FDG) positron emission tomography–computed tomography (PET-CT) was performed in 19 (38%) patients, the majority (16; 84%) attained in patients diagnosed after 2004. The diagnostic yield of MRI and FDG-PET was high (Table 2), with abnormal findings found in 77% and 84%, respectively. MRI findings were detailed in 41 of 47 (87%) patients and included abnormal enhancement of the affected neural structure in 31 (76%) cases. The affected nerves were most commonly characterized as thickened (22 of 41; 53%), in 7 (17%) the involvement was diffuse, and in 12 (30%) it was nodular. Despite the high yield of imaging evaluation, the findings were often not definitive and consequently nerve biopsy was performed in 26 (52%) patients. The biopsy demonstrated NL in 23 (88%) patients. Biopsy was performed from cranial nerves (2 cases), L2 spinal nerve root (1), brachial plexus (1), and peripheral nerves (15), including sciatic, peroneal, sural, femoral, and median nerves. Of the 5 sural nerve biopsies, 3 were negative, whereas all other biopsies demonstrated neural infiltration by malignant cells. For 7 patients, the site of biopsy was not specified.
Therapeutic management and outcome
Treatment was administered to 47 (90%) patients with NL (Table 2). Treatment varied, with systemic chemotherapy given to 33 (70%), intra-CSF chemotherapy to 23 (49%), and radiotherapy in 16 (34%). Both intravenous and intra-CSF treatment was administered to 19 (40%) patients, 4 (8.5%) received only intra-CSF therapy, and 10 patients were managed by radiotherapy alone.
Systemic chemotherapy included high-dose methotrexate in 23 patients (given as monotherapy to 10 patients), high-dose cytarabine in 18 patients (given as monotherapy to 5 patients), and other combination chemotherapy in 5 cases (including 4 rituximab–cytoxan, hydroxyrubicin, oncovin, prednisone combination). Intra-CSF chemotherapy was administered by lumbar puncture or through an intraventricular Ommaya device. This treatment was given to 13 patients who manifested cranial and/or spinal root involvement and to 11 patients who had high CSF cell count. Of the 23 patients who received intra-CSF chemotherapy, 11 (48%) were treated with more than one agent. Intra-CSF chemotherapy included cytarabine given to 17 patients, methotrexate in 14, and 3 patients were treated with rituximab.
Radiotherapy was delivered to symptomatic sites of NL; however, 3 patients received a craniospinal field. Because of the retrospective nature of this case series, data related to treatment toxicity were inadequately reported.
The response to treatment was assessed by comparing the pretreatment and posttreatment scores of ECOG-PS, neurologic function score, report of neurologic status, and objective response as detected by imaging. Of the 47 treated patients, pretreatment and posttreatment evaluations were available as follows: ECOG-PS and neurologic function in 35 patients, detailed neurologic status in 28 cases, and imaging in 25 patients. Response to treatment based on 35 pretreatment and posttreatment evaluations was noted in 16 (46%) patients who presented with a complete or partial resolution of their symptoms and signs. An additional 9 (26%) patients stabilized on treatment, and the remaining 10 patients progressed despite treatment. The median ECOG-PS changed from 2 (pretreatment) to 1.5 (posttreatment) and median neurologic function score from 2 to 1. These changes were not statistically significant (paired t test). Posttreatment imaging demonstrated complete resolution of the previously documented abnormalities related to NL in 14 (56%) of 25 patients (Figure 1D-G), partial response in another 3 (12%) cases, 1 patient showed no change, and 7 (28%) worsened. Objective response as demonstrated by imaging corresponded to clinical and neurologic improvement in all 17 patients.
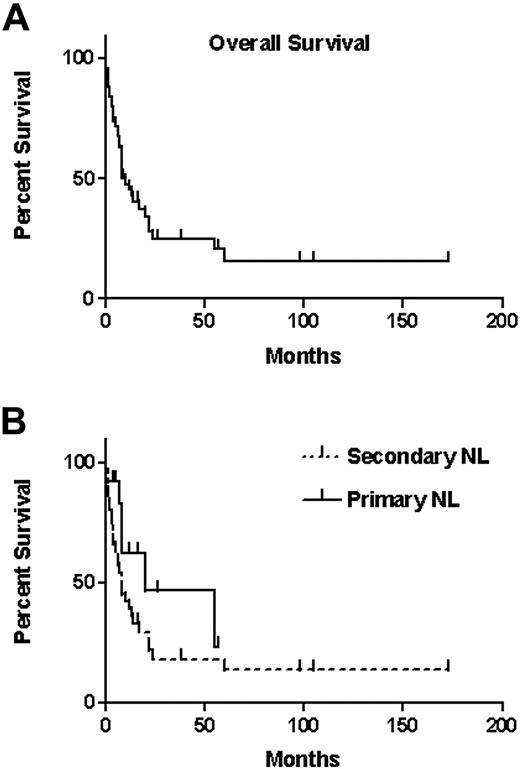
The overall median survival calculated from the diagnosis of NL was 10 months (Figure 2A) with 12-month and 36-month survival proportions of 46% and 24%, respectively. When survival of patients with primary NL was compared with survival of secondary NL, no statistically significant difference was observed (P = .129), although the median survival of the 13 patients with primary NL was 20 months and that of secondary NL was 8 months (Figure 2B).
Survival of patients with NL. (A) Overall survival of the 50 patients from the date of diagnosis of NL (median, 10 months). Vertical lines indicate censored observations. (B) Survival of patients with either primary NL (median, 20 months; 13 patients) or secondary NL (median, 8 months; 37 patients). Vertical lines indicate censored observations.
Survival of patients with NL. (A) Overall survival of the 50 patients from the date of diagnosis of NL (median, 10 months). Vertical lines indicate censored observations. (B) Survival of patients with either primary NL (median, 20 months; 13 patients) or secondary NL (median, 8 months; 37 patients). Vertical lines indicate censored observations.
Discussion
Diagnosis of NL requires integration of clinical presentation (symptoms/signs), imaging findings, and pathologic data obtained from neural, extraneural tissue, and the CSF. A high index of suspicion and familiarity with the clinical manifestations of NL are necessary. Because it is a rare manifestation of hematologic malignancies, diagnosis is often delayed and its incidence remains unknown.
The current series is the largest detailed series ever collected. It describes the presentation, treatment, and outcome of 50 NL cases that were diagnosed over a 16-year period (group C, Tables 1–2). A previous literature review covered a 28-year period and identified 47 cases, which were reported together with 25 cases identified retrospectively by the authors1 (group A, Tables 1–2). After this publication, we found an additional 44 cases whose report was published during an 8-year period (group B, Tables 1–2).
The majority (> 50%) of patients in the present report were diagnosed in the last 5 years. This might be a product of an innate bias linked with the retrospective nature of our series as more recent cases are probably easier to identify and their records may be more accessible for review. Alternatively, it may be related to an increased awareness of this rare manifestation of lymphoma and leukemia or simply reflect a facilitated diagnosis associated with the use of contemporary imaging techniques. Lastly, it may signify a trend in the biologic behavior of hematologic malignancies related to a selection of specific neurotropic clones associated with either more aggressive treatment or longer survival. To clarify whether any of the factors described might have had an impact on our findings, we have tried to compare the current series with previous publications (groups A and B). Group B corresponds to the period of diagnosis of the greater fraction of our patients (group C).
Diagnostic modalities
Clinically, NL mimics non-neoplastic and paraneoplastic neuropathies. Clinical findings that suggest NL, as opposed to remote effects or inflammatory processes, include severe pain, asymmetric distribution, and rapid evolution. Painful neuropathy predominated in our series and was common in previously published cases (Table 1). Regardless, the diagnosis is elusive and in 46% of group A patients (identified early in the time period studied), the precise diagnosis was established only at autopsy. In the present case series and in group B from the literature (Table 2), NL was more often detected antemortem with diagnosis at autopsy reported in only 8% and 5% of cases, respectively. This phenomenon of a decrease in the rate of postmortem diagnosis is probably related to the improved resolution of current imaging techniques that can detect affected neural structures with increased precision.
Of all diagnostic tools, imaging studies are of the greatest clinical utility. All our patients were evaluated by one or more imaging techniques, the majority (94%) by MRI. MRI reveals nerve or root enlargement with or without contrast enhancement and often involvement of neural plexus (brachial or lumbar) that is more difficult to detect1,7-14 (Figure 1C). MRI findings are not specific for NL and might sometimes be seen in acute or chronic inflammatory radiculoneuropathies, in neurofibromatosis, in inflammatory pseudotumor, and in malignant tumors of the peripheral nerve sheath. Interpretation of imaging studies in the context of clinical manifestations and laboratory studies is necessary. MRI yields abnormal findings in almost 80% of affected patients (Table 2) and facilitates the diagnosis, particularly when a history of hematologic malignancy is known.
PET-CT appears to be a highly sensitive diagnostic method facilitating identification of NL based on our experience and that reported in the literature (group B, Table 2; Figure 1A,B,F). Altogether, the reported experience is of 40 NL patients evaluated by PET-CT, among whom 87.5% were positive studies. Although the total number of reported cases diagnosed by PET-CT is still small, positive findings are highly suggestive of the diagnosis of NL, particularly in patients with a known history of hematologic malignancy. Together with MRI findings, PET-CT may define the best target for a biopsy, if one is indicated, especially in the instance of primary NL.
Although the majority of our patients were evaluated by multiple diagnostic modalities (Table 2), biopsy of an affected nerve was indicated for pathologic confirmation in 52% of the patients. The diagnostic yield of the biopsy was high (88%; Table 2) and was similar to the rate previously described in the literature (groups A and B, Table 2). Therefore, if imaging and CSF findings are inconclusive, a nerve biopsy presents a reasonable approach if the risk does not outweigh the expected benefit.
Treatment and outcome
There is no known standard treatment for NL; therefore, optimal management is ill defined. Treatment of NL consists of either chemotherapy alone or combined with radiotherapy. To select the appropriate therapy, knowledge of the extent of systemic and nervous system involvement is essential. NL involves roots within, as well as beyond, the borders of the subarachnoid space; thus, intra-CSF chemotherapy and standard craniospinal radiation fields will not treat all of the involved areas. Systemic chemotherapy is critical to address the multiple sites of involvement.
In the current series, 90% of the patients were treated, a rate that appears higher than that reported in the literature (groups A and B, Table 2). This is probably related to the fact that our retrospective chart review specifically requested information on therapeutic management. The information collected from the literature contains inadequate information on clinical management as some of the case reports addressed only the unusual neuroimaging findings.8,11,14-21
In the current series, the majority of patients (70%) were managed by systemic chemotherapy. The most effective regimen is unknown, and the selection is often based on protocols used to treat CNS involvement by malignant lymphoma. Many centers used intravenous high-dose methotrexate, either alone or in combination with other drugs and particularly with high-dose cytarabine. Methotrexate is effective against lymphoma affecting the nervous system and, when given in high doses, can penetrate the blood-brain and blood-nerve barriers. Any other choice of chemotherapy must also meet those criteria. However, in our series, approximately 30% of treated patients did not receive systemic chemotherapy because NL represented relapse of a chemoresistant disease.
Radiotherapy has a limited role in the treatment of NL resulting from involvement of multiple sites, affecting both the CNS and the peripheral nervous system. Extensive radiation fields are poorly tolerated in most patients, but limited-field radiotherapy can be very effective in relieving unremitting neuropathic pain attributed to a particular nerve, plexus, or nerve root.
Clinical improvement (functional recovery, reduction of pain) and radiographic resolution (improvement of nerve root enlargement and enhancement or normalization of FDG-PET uptake) have been observed in 50% to 70% of treated patients (Table 2; Figure 1D-G). Standardized criteria to measure response are not available; therefore, no recommendations can be made regarding treatment response.
There is no previous information on overall survival of patients with NL. The median survival from diagnosis of NL in our series was 10 months with 36-month survival proportion of 24%. These data indicate that an aggressive multimodality therapeutic approach can achieve long-term survival in some patients. The trend toward longer median survival observed in primary NL probably reflects the fact that NL was the presenting manifestation of the malignant disease, unlike in secondary NL. Nonetheless, long-term survival was observed in secondary NL, with 1 in 4 of all patients alive at 3 years.
In conclusion, it appears that NL is more frequently diagnosed in recent years. It is probably related to increased awareness of the disease and an enhanced rate of diagnosis because of the extensive use of contemporary imaging techniques that accurately localize abnormal processes affecting neural structures. Early recognition and treatment of this rare neurologic manifestation of lymphoma and leukemia may improve outcome.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgment
The authors thank Dr Joachim M. Baehring, Department of Neurology, Medicine and Neurosurgery, Yale University School of Medicine, New Haven, CT, for providing information and clarifying data related to his series of patients (group A), which are included in Tables 1 and 2.
Authorship
Contribution: S.G. and B.A. designed the research, collected and analyzed the data, and reviewed the manuscript; T.T.B., M.J.v.d.B., F.B., D.S., O.K., M.C.C., P.R., A.N., E.S., and D.B.-Y. collected data and reviewed the manuscript; T.S. designed research, collected and analyzed data, and wrote the manuscript; and M.C.C. provided imaging studies for Figure 1A through C.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Tali Siegal, Gaffin Center for Neuro-Oncology, Hadassah Hebrew-University Medical Center, Ein Kerem, PO Box 12000, Jerusalem 91120, Israel; e-mail: siegal@hadassah.org.il.
References
Author notes
S.G. and B.A. contributed equally to this study.