Abstract
Src homology 2 domain-containing inositol 5-phosphatase (SHIP−/−) animals display an age-related increase in interleukin-6 (IL-6), a decrease in B lymphopoiesis, and an elevation in myelopoiesis. We investigated the origin of the IL-6 production and show that it is largely produced by peritoneal and splenic macrophages. IL-6 production by these macrophages is not a direct result of the loss of SHIP: IL-6 production is not spontaneous, is absent from bone marrow-derived macrophages, declines with prolonged culture of macrophages, and requires a stimulus present in vivo. The IL-6–rich peritoneal cavity of SHIP−/− mice shows more than 700-fold more immunoglobulin G (IgG) than wild-type, approximately 20% of which is aggregated or in an immune complex and contains B220+ cells that secrete IgG. The SHIP-deficient peritoneal macrophages show evidence of IgG receptor stimulation. Animals lacking both the signal-transducing γ-chain of IgG receptors and SHIP or Ig and SHIP produce less IL-6. The data indicate a feed-forward process in which peripheral macrophages, responding through IgG receptors to secreted IgG, produce IL-6, to support further B-cell production of IgG. Because of the proinflammatory phenotype of SHIP−/− animals, these findings emphasize the importance of IL-6–neutralizing strategies in autoimmune and proinflammatory diseases.
Introduction
Interleukin 6 (IL-6) is a multifunctional cytokine that is produced in a variety of clinical situations associated with inflammation.1 IL-6 was originally isolated and cloned as a B-cell differentiation factor that induced terminal B-cell differentiation and supported the production of immunoglobulin G (IgG).2 Indeed, IL-6 transgenic mice eventually die of a fatal plasmacytoma.3 Serum levels of IL-6 correlate with disease activity in several chronic inflammatory diseases, such as systemic lupus erythematosis (SLE),4 rheumatoid arthritis,5 and multiple myeloma.6 Animals that express a mutated form of the IL-6 receptor causing constitutive IL-6 signal transduction develop an autoimmune disease resembling arthritis.7 Neutralizing antibodies to IL-6 improve the clinical features of such diseases in animal models8 and in patients,9 showing an important role of IL-6 in the progression of autoimmune diseases. Various types of hematopoietic and nonhematopoietic cells can produce IL-6, and the cellular source of IL-6 in these diseases varies from endothelial cells10 to macrophages11 to B lymphocytes.12
Phosphatidylinositol 3-kinase (PI3K) is a lipid kinase with 3 catalytic isoforms13 that generates phosphatidylinositol-3,4,5-trisphosphate (PIP3). The γ-isoform of PI3K catalytic subunit is predominant in innate immune cells.14 PI3K and its products regulate signal transduction events involving antigen15 and Toll-like receptors.16 Immunoglobulin G (IgG) receptors (FcγR) through their activating and associated γ-chain17 bind to IgG-containing immune complexes or IgG-opsonized particles and likewise use PI3K to function in phagocytosis and cytokine production.18,19 Accordingly, PI3K is required for numerous immunologic responses, including proliferation, migration, and cytokine production.20 Because of the prominent role of PI3K in activation of innate immune cells, PI3K has a crucial role in the progression and maintenance of chronic inflammation. Thus, studies have shown that inhibition or deletion of the gamma isoform of PI3K protects animals from progression of autoimmune diseases,21,22 and new small molecule inhibitors of PI3K-γ are being tested on patients.23
Because PI3K and PIP3 contribute to autoimmune disease progression, the loss of negative regulators of PI3K is associated with a proinflammatory phenotype. Src homology 2 domain-containing inositol 5-phosphatase (SHIP) functions as a negative regulator of PI3K and is directly recruited to the tyrosine-phosphorylated cytoplasmic tail of several IgG receptors.24,25 Animals lacking SHIP display a wide range of hematologic abnormalities, including hyper-responsive macrophages,25 mast cells26 and B cells,27 a myeloproliferative syndrome, and multiple defects in lymphopoiesis.28-31 Macrophages32 and B cells27 of SHIP-deficient mice show elevated levels of PIP3, the lipid substrate for SHIP. SHIP-deficient mice have elevated M2 (healing) macrophages, expressing high levels of arginase I and Ym1.33-35 Deletion of the related lipid phosphatase, phosphatase and tensing homolog, evokes an SLE-like disease.36 The SLE-like syndrome of phosphatase and tensing homolog−/− mice is enhanced when combined with a deficiency in SHIP.37
SHIP−/−mice display elevated IL-6 level in their serum38 and increased serum IgG.31 We have shown that the increased IL-6 causes suppression of B lymphopoiesis and elevates myelopoiesis.28,39 The cellular target for IL-6–mediated alterations in hematopoiesis appears to be an early multipotent progenitor.29 The cellular source of IL-6 in SHIP−/− animals, and the mechanism by which it is produced is not known. It is possible that IL-6 production is spontaneous and occurs once SHIP, a negative regulator of many signaling pathways in hematopoietic cells, is deleted. We investigated the issue here and show that peritoneal and splenic macrophages in SHIP−/− animals generate the bulk of circulating IL-6 in the SHIP−/− animal. However, IL-6 production is not spontaneous and declines on culture of the peritoneal macrophages and is absent from SHIP-deficient macrophages raised in vitro. The IL-6 production results in part from the fact that SHIP−/− animals display increased amounts of aggregated IgG or IgG in immune complexes in the peritoneum and in part because the SHIP-deficient macrophages are hyper-responsive to FcγR stimulation. We provide evidence that these 2 features combine to produce dramatically elevated IL-6 levels and a proinflammatory phenotype in the animal.
Methods
Mice
The SHIP−/− mice on the C57BL/6 background were bred at our facility and originally provided by Dr G. Krystal (Terry Fox Laboratory, British Columbia Cancer Agency, Vancouver, BC). Heterozygous mice were bred, and animals were genotyped at day 10 after birth. Fc-γ-chain−/− and μMT mice on the C57BL/6 background were purchased from Taconic Farms. SHIP−/−, Fc γ-chain40 and SHIP, μMT41 double-knockout mice were obtained by interbreeding. Littermate wild-type (WT) C57BL/6 mice were used as controls. All studies were approved by the Oklahoma Medical Research Foundation Institutional Animal Care and Use Committee.
Reagents
Rabbit polyclonal antibody to phosphorylated Vav was purchased from Santa Cruz Biotechnology. Rabbit polyclonal antibody to phosphorylated JNK, total JNK, and total Akt and mouse monoclonal antibody to phosphorylated Akt were purchased from Cell Signaling Technology. Horseradish peroxidase (HRP)–labeled anti–mouse IgG antibody was purchased from KPL. Fluorochrome- or biotin-conjugated antibodies to Ly-6C, Gr-1, Mac-1/CD11b, and F4/80 were purchased from eBioscience. Streptavidin-conjugated and anti–Mac-1 magnetic microbeads were purchased from Miltenyi Biotec. The PI3K inhibitor LY294002 and the JNK inhibitor SP60025 were purchased from Calbiochem.
Isolation of macrophages
Single-cell suspensions prepared from liver were resuspended in 40% Percoll, layered more than 70% Percoll, and centrifuged for 25 minutes. Lungs were isolated with an intact trachea, and the lung cells were collected by lavaging the organs with 2.5 mL phosphate-buffered saline (PBS). The peritoneum was lavaged with 2.5 mL PBS. Total bone marrow mononuclear cells were obtained by flushing tibias and femurs with 2 to 5 mL of PBS. Peripheral blood was collected by cardiac puncture, and red blood cells were removed by lysis in ammonium chloride-containing buffer. The mononuclear cells from all organs were analyzed for surface markers and intracellular IL-6 as described in the paragraph below. Alternatively, mature macrophages were purified by positive selection with anti-CD11b/Mac-1–coated microbeads or with biotin-conjugated F4/80 antibody followed by streptavidin-coated microbeads. After incubation, the cells and microbeads were washed in magnetic-activated cell sorting (MACS) buffer (PBS containing 0.5% bovine serum albumin and 2mM ethylenediaminetetraacetic acid) and the macrophages were collected by MACS LS+ positive selection column on a MACS separation system (Miltenyi Biotec). In some experiments, peritoneal macrophages were also enriched by adherence to plastic plates and collected after repeated washing to remove nonbinding cells. Macrophage purity was routinely 80% to 90%, as assessed by flow cytometry for Mac-1 and F4/80. In some experiments, the cell-free lavage fluid was concentrated 10-fold (to 250 μL) by ultrafiltration using Millipore-Ultrafree centrifuge tubes and a 10 000-kDa filter. An aliquot of the concentrated material was incubated for 1 hour on ice with 20 μL of a 50% suspension of Sepharose beads conjugated to protein A or glutathione-S-transferase (GST) per 100 μL of fluid.
Flow cytometry for surface antigen and intracellular staining
Stained cells were analyzed by LSR-II or FACS calibur (BD Biosciences), analyzed with FlowJo software (TreeStar) as previously described,39 using 0.05% saponin for permeabilization to determine cytoplasmic IL-6. The primary gate was drawn on the total population of viable cells and then gated based on surface Mac1 and F4/80 expression. Intracellular IL-6 levels were determined from the Mac1-F4/80 double-positive population for each of the different organs tested.
Western blotting
Peritoneal macrophages were lysed in TN1 buffer (50mM Tris, pH 8.0, 10mM ethylenediaminetetraacetic acid, 10mM Na4P2O7, 10mM NaF, 1% Triton X-100, 125mM NaCl, 10mM Na3VO4, 10 μg/mL each aprotinin and leupeptin). Proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, transferred to nitrocellulose membranes, and probed with the antibody of interest and appropriate HRP-conjugated secondary antibodies. The filters were developed by enhanced chemiluminescence. The bands were quantitated by measuring individual chemiluminescence using a Lumi-Imager F1 workstation (Roche Molecular Biochemicals). In most cases, the signal present in the control lane was defined as 1.0, and the signal present in the experimental lane was an amount of signal relative to the unstimulated level.
ELISA
For detection of IL-6, cells were cultured for various time points in the presence or absence of heat-aggregated IgG (10 μg/mL) or concentrated peritoneal lavage fluid (50 μL), with or without inhibitors. Cell supernatants were collected and centrifuged to remove dead cells. The supernatants were analyzed by enzyme-linked immunosorbent assay (ELISA) using IL-6 specific kits from eBioscience. For detection of serum level IL-6, blood was collected from cardiac puncture and serum was collected by centrifugation. For detection of IgG, 96-well plates were coated with serially diluted peritoneal lavage fluid or supernatants of cultured cells. After washing, the plates were incubated with HRP-labeled anti–mouse IgG antibody, purchased from KPL. The presence of HRP was detected by the addition of 3,3′,5,5′ tetramethylbenzidine substrate solution (eBioscience), and the optical density was determined at 405 nm. Mouse IgG (Lampire Biological Laboratories) was used as a standard. Data were analyzed using a paired Student t test.
Preparation of heat-aggregated IgG
Heat-aggregated IgG was prepared according to methods described previously.42 In brief, purified mouse IgG at a concentration of 10 mg/mL was heated at 62°C for 30 minutes, washed once in Hanks balanced salt solution, and used to stimulate cells.
Results
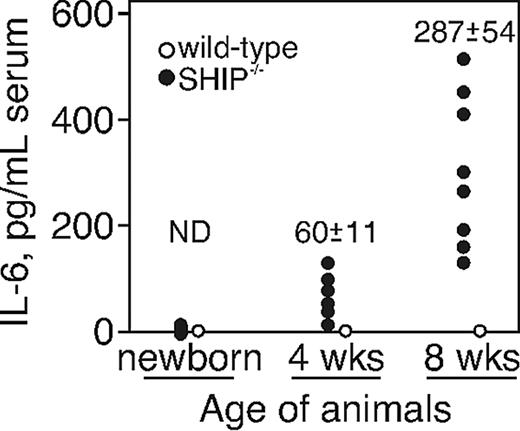
SHIP−/− mice display an age-dependent change in hematopoiesis,28 which is the result of increased IL-6 production,29 and IL-6 has been detected in the sera of SHIP-deficient animals.38 We first asked at what age serum IL-6 levels were elevated in SHIP−/− mice. We collected serum samples by cardiac puncture from newborn mice and animals that were 4 or 8 weeks old. IL-6 levels were tested by a capture ELISA. We found that at birth, neither WT nor SHIP−/− mice had detectable IL-6 in serum. However, in SHIP−/−, but not in WT, IL-6 was detectable by 4 weeks of age (60 ± 11 pg/mL) and continued increasing as the animals aged (287 ± 54 pg/mL; Figure 1). The elevations in IL-6 in SHIP−/− mice paralleled the changes in B lymphopoiesis that were documented in our earlier report.28 WT mice showed no detectable IL-6 at any age.
Serum IL-6 level increases with age in SHIP−/− mice. Serum IL-6 levels determined by ELISA of individual WT and SHIP−/− mice at the age of 4 and 8 weeks are shown. ND indicates not detected.
Serum IL-6 level increases with age in SHIP−/− mice. Serum IL-6 levels determined by ELISA of individual WT and SHIP−/− mice at the age of 4 and 8 weeks are shown. ND indicates not detected.
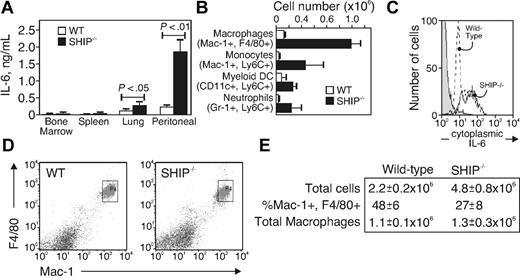
We were interested in identifying the cellular source of the IL-6 production. Our earlier report showed that Mac-1+ cells derived from SHIP−/− mice appeared to produce IL-6 spontaneously.28 Therefore, we collected cells from bone marrow, blood, spleen, liver, lung, and peritoneum of WT and SHIP−/− mice and analyzed IL-6 production by intracellular staining in mature macrophages (Mac-1, F4/80 double-positive). We found IL-6+ macrophages in all organs of SHIP−/− mice, with the greatest total cell numbers in the peritoneum, lung, and spleen (Table 1). As an additional test, we isolated cells from the same organs and enriched myeloid-derived cells with anti–Mac-1 magnetic beads. The preparations were cultured without any exogenous stimulus in media for 6 hours, and the supernatants were collected. We found (Figure 2A) dramatic and unprovoked IL-6 production (1800 pg/mL) in the cultured peritoneal Mac-1+ cells from SHIP−/− mice, relative to the other tissue sources. Surprisingly, although a considerable percentage of the cells displayed cytoplasmic IL-6, the splenic macrophages of SHIP−/− mice did not produce IL-6 on culture (Figure 2A). The alveolar Mac-1+ cells from SHIP−/− mice produced IL-6 at a level higher than that of WT (280 pg/mL, SHIP−/−; and 95 pg/mL, WT), but the production was less than the SHIP-deficient peritoneal Mac-1+ cells. There was barely detectable IL-6 production from Mac-1+ cells of other organs derived from both WT and SHIP−/− mice.
Total number of Mac-1+, F4/80+, and cytoplasmic IL-6+ cells in various organs
| Organ . | Mac1+, F4/80+, ×106 . | Mac1+,F4/80+, cytoplasmic IL-6+, ×103 . | ||
|---|---|---|---|---|
| WT . | SHIP−/− . | WT . | SHIP−/− . | |
| Spleen | 4.7 ± 2 | 14.6 ± 4 | 2700 ± 700 | 9500 ± 4200 |
| Bone marrow | 0.6 ± 0.2 | 0.5 ± 0.1 | 260 ± 45 | 100 ± 10 |
| Liver | 0.26 ± 0.2 | 0.33 ± 0.2 | 150 ± 50 | 200 ± 76 |
| Peritoneum | 1.3 ± 0.3 | 1.7 ± 1.2 | 106 ± 9 | 1200 ± 226 |
| Blood | 0.7 ± 0.5 | 2.3 ± 0.8 | 300 ± 18 | 210 ± 10 |
| Lung | 0.5 ± 0.2 | 3.4 ± 0.9 | 125 ± 7 | 450 ± 69 |
| Organ . | Mac1+, F4/80+, ×106 . | Mac1+,F4/80+, cytoplasmic IL-6+, ×103 . | ||
|---|---|---|---|---|
| WT . | SHIP−/− . | WT . | SHIP−/− . | |
| Spleen | 4.7 ± 2 | 14.6 ± 4 | 2700 ± 700 | 9500 ± 4200 |
| Bone marrow | 0.6 ± 0.2 | 0.5 ± 0.1 | 260 ± 45 | 100 ± 10 |
| Liver | 0.26 ± 0.2 | 0.33 ± 0.2 | 150 ± 50 | 200 ± 76 |
| Peritoneum | 1.3 ± 0.3 | 1.7 ± 1.2 | 106 ± 9 | 1200 ± 226 |
| Blood | 0.7 ± 0.5 | 2.3 ± 0.8 | 300 ± 18 | 210 ± 10 |
| Lung | 0.5 ± 0.2 | 3.4 ± 0.9 | 125 ± 7 | 450 ± 69 |
Values are from 3 or 4 animals of each strain at 7 to 8 weeks of age.
WT indicates wild-type; and SHIP, Src homology 2 domain-containing inositol 5-phosphatase.
Peritoneal macrophages from SHIP−/− mice produce IL-6. (A) IL-6 production of freshly isolated and purified macrophages from bone marrow, spleen, lung, and peritoneum of WT and SHIP−/− mice, analyzed by ELISA after a 6-hour culture. Data are mean ± SE of 3 identical experiments. (B) Subpopulations of peritoneal cells were identified by flow cytometry as macrophages (Mac-1+, F4/80+), monocytes (Mac-1+, F4/80−, Ly-6Chi), myeloid dendritic cells (CD11c+, Ly-6C+), and neutrophils (Mac-1+, Gr-1+, Ly-6C+) and analyzed for intracellular IL-6. Shown is the total number of each population that expressed intracellular IL-6. Data are mean ± SE of 3 animals. (C) IL-6 histogram of peritoneal macrophages (defined as Mac-1+, F4/80+) from WT (dashed line) or SHIP−/− (solid line) mice. Unstained cells are shown in gray. (D) Peritoneal macrophages identified with anti–Mac-1 and anti-F4/80 antibodies. (E) Total cells from 7 separate animals.
Peritoneal macrophages from SHIP−/− mice produce IL-6. (A) IL-6 production of freshly isolated and purified macrophages from bone marrow, spleen, lung, and peritoneum of WT and SHIP−/− mice, analyzed by ELISA after a 6-hour culture. Data are mean ± SE of 3 identical experiments. (B) Subpopulations of peritoneal cells were identified by flow cytometry as macrophages (Mac-1+, F4/80+), monocytes (Mac-1+, F4/80−, Ly-6Chi), myeloid dendritic cells (CD11c+, Ly-6C+), and neutrophils (Mac-1+, Gr-1+, Ly-6C+) and analyzed for intracellular IL-6. Shown is the total number of each population that expressed intracellular IL-6. Data are mean ± SE of 3 animals. (C) IL-6 histogram of peritoneal macrophages (defined as Mac-1+, F4/80+) from WT (dashed line) or SHIP−/− (solid line) mice. Unstained cells are shown in gray. (D) Peritoneal macrophages identified with anti–Mac-1 and anti-F4/80 antibodies. (E) Total cells from 7 separate animals.
To better resolve the Mac-1+ fraction in SHIP−/− mice, we isolated cells from the peritoneum, stained them with antibodies to Mac-1, F4/80, CD11c, Gr-1, and Ly6C to resolve mature macrophages (Mac-1+, F4/80+), monocytes (Mac-1+, F4/80−, Ly6Chi), myeloid dendritic cells (CD11c+, Ly6C+), and neutrophils (Mac-1+, Gr-1+, Ly6C+43,44 ). After surface staining, the cells were fixed, permeabilized, and stained for intracellular IL-6. Figure 2B shows a summary of 3 individual WT and SHIP−/− mice. The data are expressed as the total number of cells positive for cytoplasmic IL-6 and having the phenotype shown in Figure 2B. We found higher numbers of IL-6–expressing cells in all the peritoneal subpopulations from SHIP-deficient relative to WT mice, but the Mac-1, F4/80-expressing mature macrophages were the most significant population, showing an average of approximately 1 × 106 total cytoplasmic IL-6+ cells. A histogram of cytoplasmic IL-6 level in the Mac-1, F4/80 subpopulations of WT and SHIP−/− mice is shown in Figure 2C. The majority of the cells from the SHIP-deficient mice are cytoplasmic IL-6+. These results suggest that mature peritoneal macrophages are the major source of the high circulating IL-6 found in the sera of SHIP−/− mice. The production might be spontaneous and caused by the lack of the negative regulator, SHIP.
An alternate possibility is that IL-6 production occurs at a similar rate between WT and SHIP−/− macrophages, but the total numbers of peritoneal macrophages are increased in SHIP−/− mice. To test this possibility, we examined the population of peritoneal macrophages in both WT and SHIP−/− mice. Resident peritoneal macrophages were defined as F4/80+ and Mac-1+.44 We found (Figure 2D-E) that SHIP−/− mice had more than twice the total cell number in the peritoneum at the age of 8 weeks (WT, 2.2 × 106; SHIP−/− mice, 4.8 × 106). However, the percentage of peritoneal F4/80+, Mac-1+ macrophages among the total peritoneal cells in SHIP−/− mice (27%) was lower than that of WT (48%); therefore, the total number of peritoneal macrophages was approximately the same in both strains (1.1 × 106 in WT; 1.3 × 106 in SHIP−/−). The data in Figure 2B and C indicate that nearly all the SHIP-deficient peritoneal macrophages are positive for cytoplasmic IL-6. Thus, the increased serum IL-6 present in the SHIP−/− mouse is not the result of increased numbers of macrophages in the peritoneum; rather, it stems from increased IL-6 production by resident mature macrophages.
We found that continued culture of the peritoneal macrophages beyond 6 hours caused a dramatic decline in the production of IL-6 (not shown). Furthermore, SHIP-deficient bone marrow-derived macrophages generated by culture of total bone marrow with colony-stimulating factor-1 (CSF-1), as described,45 did not produce IL-6 at any point in the culture (not shown). These observations suggest that the IL-6 production is not an intrinsic property of the SHIP-deficient macrophages. Rather, the data suggest that there is an unidentified stimulus present in the peritoneum of SHIP−/− mice that is removed on prolonged culture and absent in CSF-1 cultures of bone marrow cells. The production of IL-6 by the unknown stimulant may be negatively regulated by SHIP, or the stimulus may be more abundant in the peritoneum of SHIP−/− mice.
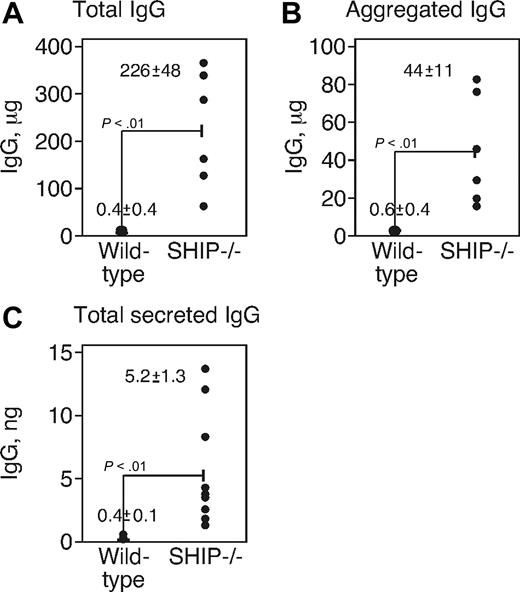
We tested the latter possibility by measuring IgG levels (which could stimulate IgG receptors to cause IL-6 production) in the peritoneal cavities of WT and SHIP−/− mice. Peritoneal lavage was performed by injecting 2.5 mL of PBS into WT and SHIP−/− mice and IgG in the lavage fluid was measured by ELISA. We found (Figure 3A) that total IgG was 750-fold higher in the lavage fluid of SHIP−/− mice (WT, 0.4 mg; SHIP−/− mice, 226 mg). Next, we tested whether the IgG was aggregated by centrifuging the lavage fluid (55 000g) and resuspending the insoluble protein in the pellet for analysis by ELISA for IgG. We found (Figure 3B) that the material collected by centrifugation showed the presence of IgG and that the amount in the lavage fluid of SHIP−/− mice in the pellet was 70-fold greater than that of WT (WT, 0.6 mg; SHIP−/− mice, 44 mg). Lastly, we measured the production of IgG by culturing for 24 hours the peritoneal cells from which the macrophages had been removed by magnetic bead selection. The resulting population is largely CD19+, B220+ B cells (not shown). We found (Figure 3C) that peritoneal cells from SHIP−/− mice secreted notably higher amounts of IgG (WT, 0.4 ng; SHIP−/− mice, 5.2 ng). These data show that the peritoneum of SHIP-deficient mice contains more IgG, more IgG-producing cells, and more aggregated IgG than WT mice.
SHIP−/− mice had a high level of total and aggregated IgG in peritoneum. (A) Total IgG obtained by ELISA in peritoneal lavages of WT and SHIP−/− mice. (B) The lavage fluid was centrifuged 55 000g, and IgG (defined as aggregated IgG) was measured in the pellet. (A-B) The data were obtained from 6 individual mice, and each point represents a single animal. (C) IgG production from peritoneal cells was determined by ELISA. The F4/80+ macrophages were removed, and the remaining cells were cultured for 24 hours. Cells were collected, and secreted IgG was measured by ELISA from 9 individual mice.
SHIP−/− mice had a high level of total and aggregated IgG in peritoneum. (A) Total IgG obtained by ELISA in peritoneal lavages of WT and SHIP−/− mice. (B) The lavage fluid was centrifuged 55 000g, and IgG (defined as aggregated IgG) was measured in the pellet. (A-B) The data were obtained from 6 individual mice, and each point represents a single animal. (C) IgG production from peritoneal cells was determined by ELISA. The F4/80+ macrophages were removed, and the remaining cells were cultured for 24 hours. Cells were collected, and secreted IgG was measured by ELISA from 9 individual mice.
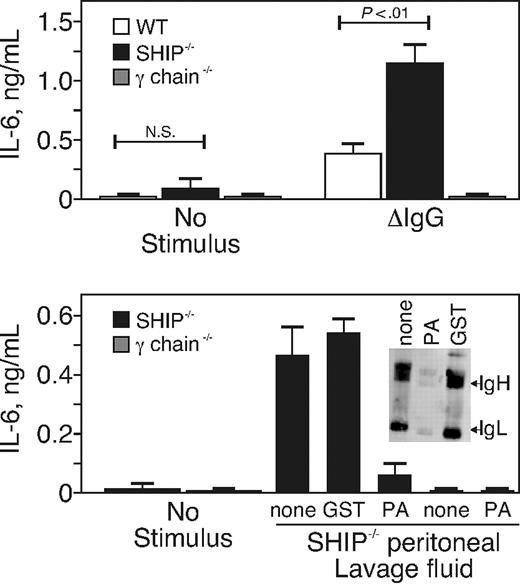
We also addressed the alternate possibility that the SHIP-deficient mice produce more IL-6 than their WT counterparts in response to the same stimulus. We therefore compared the ability of heat-aggregated IgG to stimulate IL-6 production from the peritoneal macrophages of WT and SHIP−/−, using macrophages from mice lacking the signal-transducing γ-chain (γ-chain−/−) of the FcγR40 as a negative control. For this experiment, we cultured peritoneal macrophages that were purified by adherence for 24 hours so that the in vivo-derived IL-6 production diminished to background levels. After 24 hours of culture, the macrophages were stimulated with purified, heat-aggregated mouse IgG for 12 hours, and the supernatants were tested for IL-6 by ELISA. We found (Figure 4A) that peritoneal macrophages from SHIP−/− mice secreted 2-fold more IL-6 relative to that made by peritoneal macrophages from WT mice. These data show that IL-6 production stimulated by FcγR engagement in peritoneal macrophages is negatively regulated by SHIP.
SHIP negatively regulated IL-6 production caused by FcγR stimulation. (A) Peritoneal macrophages were purified from WT, SHIP−/−, or γ-chain−/− mice by adherence and cultured 24 hours. The cells were stimulated with nothing (no stimulus) or with 10 μg/mL of heat-aggregated IgG (ΔIgG) and incubated for an additional 12 hours before supernatants were collected. IL-6 production stimulated by FcγR engagement of peritoneal macrophages was determined by ELISA. Results are from 3 separate experiments and are the mean ± SE of IL-6 in nanograms per milliliter. N.S. indicates not significant. (B) Peritoneal lavage fluid was collected from SHIP−/− mice and concentrated 10-fold by ultrafiltration (to 250 μL). The concentrated lavage material was incubated with nothing or with Sepharose-conjugated protein A (PA) or GST as indicated. Bone marrow–derived macrophages of SHIP−/− or γ-chain−/− mice were then treated with 50 μL of the concentrated lavage fluid and incubated for 12 hours. The supernatants were measured by IL-6 ELISA. Results are from 2 separate experiments and are the mean ± SE of IL-6 in nanograms per milliliter. (Inset) Western blot of 50 μL of lavaged material probed with rabbit anti–mouse Ig.
SHIP negatively regulated IL-6 production caused by FcγR stimulation. (A) Peritoneal macrophages were purified from WT, SHIP−/−, or γ-chain−/− mice by adherence and cultured 24 hours. The cells were stimulated with nothing (no stimulus) or with 10 μg/mL of heat-aggregated IgG (ΔIgG) and incubated for an additional 12 hours before supernatants were collected. IL-6 production stimulated by FcγR engagement of peritoneal macrophages was determined by ELISA. Results are from 3 separate experiments and are the mean ± SE of IL-6 in nanograms per milliliter. N.S. indicates not significant. (B) Peritoneal lavage fluid was collected from SHIP−/− mice and concentrated 10-fold by ultrafiltration (to 250 μL). The concentrated lavage material was incubated with nothing or with Sepharose-conjugated protein A (PA) or GST as indicated. Bone marrow–derived macrophages of SHIP−/− or γ-chain−/− mice were then treated with 50 μL of the concentrated lavage fluid and incubated for 12 hours. The supernatants were measured by IL-6 ELISA. Results are from 2 separate experiments and are the mean ± SE of IL-6 in nanograms per milliliter. (Inset) Western blot of 50 μL of lavaged material probed with rabbit anti–mouse Ig.
Because we found increased IgG in the peritoneal lavage of SHIP−/− mice, we tested the ability of the lavaged material to engage IgG receptors and induce IL-6 production. We lavaged the peritoneum, concentrated the fluid by ultrafiltration 10-fold, and applied the concentrated lavage fluid to macrophages of SHIP−/− or γ-chain−/− mice. In some cases, the concentrated lavage fluid was adsorbed with Sepharose beads conjugated to protein A or to GST as a negative control. We found (Figure 4B) that the peritoneal lavage fluid of SHIP−/− mice stimulated IL-6 production by macrophages of SHIP- but not γ-chain-deficient mice. The IL-6–inducing activity was removed by protein A- but not GST-Sepharose. The Figure 4 inset shows the presence of IgG in the lavage fluid after adsorption with nothing, protein A, or GST-Sepharose. Together, the data suggest that the elevated amount of IgG in the peritoneum of SHIP−/− animals is capable of stimulating Fc receptors. IL-6 is produced in the animal because of the presence of this stimulant and because the cells are hyper-responsive to FcγR stimulation.
Based on this hypothesis, we predicted that signaling molecules involved in FcγR stimulation would show a high level of activity when derived from the peritoneal macrophages of SHIP−/− mice. To test this prediction, we measured the levels of phosphorylation of Jnk,46 Vav,47 and Akt,42 all of which act distal to FcγR stimulation. For these experiments, we used freshly isolated peritoneal macrophages derived from WT and SHIP−/− or WT and SHIP/γ-chain double-knockout mice as a negative control. We lysed the cells immediately after macrophage enrichment by positive selection with magnetic beads. The lysates were tested by Western blots with antibodies to the activated forms of the various proteins involved in FcγR signal transduction. We found that phosphorylation of Vav (Figure 5A), Akt (Figure 5B), and Jnk (Figure 5C) was present in lysates of unstimulated macrophages from SHIP-deficient mice but not from WT or SHIP−/−, γ-chain double-deficient mice. We quantitated the degree to which these proteins were activated by determining the ratio of phosphorylated to total protein from WT and SHIP−/− mice. The data in Figure 5D show the fold increase of each of these signaling proteins in peritoneal macrophages of SHIP−/− relative to that of WT macrophages. The ratio was significantly (P < .05) higher in each case and varied from 2.2-fold greater (pJNK/JNK) to a high of 3.4-fold greater (pVav/Vav) in SHIP-deficient macrophages. These data indicate that, in peritoneal macrophages from SHIP−/− mice, the signaling pathway initiated by FcγR stimulation is activated. The observation is consistent with the notion that the cells have received a signal in vivo from FcγR engagement and consistent with the stimulus being aggregated IgG.
Signal transduction proteins of the FcγR pathway are activated in ex vivo peritoneal macrophages of SHIP−/− mice. Freshly purified peritoneal macrophages were lysed without any treatment. Western blots were used to determine levels of tyrosine-phosphorylated Vav (pVav) and total Vav (A), serine-phosphorylated Akt (pAkt) and total Akt (B), and serine- and tyrosine-phosphorylated SAPK/JNK (pJNK) and total JNK (C). (D) The ratio of the signal of phosphoprotein to total of the SHIP-deficient sample to the WT sample. Data are representative of 3 independent experiments. (E) Freshly isolated peritoneal macrophages from SHIP−/− mice were cultured with the PI3K inhibitor (LY294002) or the JNK inhibitor (SP60025) or dimethyl sulfoxide for 6 hours. Supernatants were collected, and IL-6 levels were determined by ELISA. Results from 3 separate experiments are mean ± SE of IL-6 in picograms per milliliter.
Signal transduction proteins of the FcγR pathway are activated in ex vivo peritoneal macrophages of SHIP−/− mice. Freshly purified peritoneal macrophages were lysed without any treatment. Western blots were used to determine levels of tyrosine-phosphorylated Vav (pVav) and total Vav (A), serine-phosphorylated Akt (pAkt) and total Akt (B), and serine- and tyrosine-phosphorylated SAPK/JNK (pJNK) and total JNK (C). (D) The ratio of the signal of phosphoprotein to total of the SHIP-deficient sample to the WT sample. Data are representative of 3 independent experiments. (E) Freshly isolated peritoneal macrophages from SHIP−/− mice were cultured with the PI3K inhibitor (LY294002) or the JNK inhibitor (SP60025) or dimethyl sulfoxide for 6 hours. Supernatants were collected, and IL-6 levels were determined by ELISA. Results from 3 separate experiments are mean ± SE of IL-6 in picograms per milliliter.
As an additional test of this hypothesis, we used chemical inhibitors to block PI3K and Jnk and examined the effect on IL-6 production. For these experiments, we treated freshly isolated peritoneal macrophages from SHIP−/− mice with the PI3K inhibitor (LY294002) or the JNK inhibitor (SP60025) or vehicle (dimethyl sulfoxide) and measured the IL-6 production by ELISA after a 6-hour culture. We found (Figure 5E) that both the PI3K inhibitor and JNK inhibitor reduced IL-6 production from the freshly isolated peritoneal macrophages of SHIP−/− mice. These findings are consistent with our hypothesis that IL-6 arises in SHIP-deficient mice from in vivo stimulation by aggregated IgG, to activate PI3K and Jnk via FcγR.
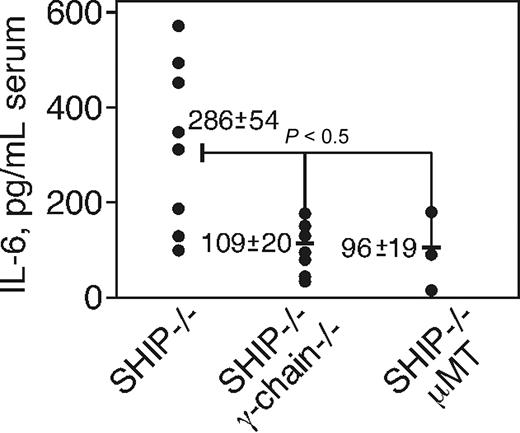
If the high level of aggregated IgG binding to activating FcγR contributes to IL-6 production, the spontaneous IL-6 production should be reduced in γ-chain- or Ig-deficient animals. To test this prediction, we mated SHIP+/− animals with γ-chain−/− or with mice having a targeted mutation in the transmembrane region of IgM (μMT mice) that are B cell-deficient and hence lack IgG.41 We then compared the IL-6 present in the sera of SHIP−/−, γ-chain−/−, or SHIP−/−, μMT double-deficient offspring to parental SHIP−/− mice. Sera from individual animals of both strains of mice were tested at 8 weeks of age for IL-6 by ELISA. As in WT mice, we did not detect any IL-6 in animals lacking the γ-chain (not shown). We found (Figure 6) that the IL-6 burden in SHIP−/− mice (n = 8) was approximately 300 pg/mL, whereas in both double-knockout strains of animals (n = 8, n = 3) the IL-6 level was reduced to approximately 100 pg/mL. Although IL-6 production was not completely eliminated, the fact that the loss of the γ-chain or of IgG greatly reduced IL-6 shows that the bulk of IL-6 in the SHIP deficiency comes via a pathway involving IgG engagement of activating FcγR.
The loss of Fc γ-chain reduces the IL-6 level in the sera of SHIP−/− mice. Serum IL-6 levels of SHIP−/−, SHIP, μMT, and SHIP, γ-chain double-knockout mice at the age of 8 weeks. The serum was obtained by cardiac puncture, and IL-6 level was determined by ELISA. Results from 8 mice of SHIP−/− and SHIP, γ-chain double-knockout and 3 mice of SHIP, μMT double-knockout each strain are indicated as pictograms per milliliter.
The loss of Fc γ-chain reduces the IL-6 level in the sera of SHIP−/− mice. Serum IL-6 levels of SHIP−/−, SHIP, μMT, and SHIP, γ-chain double-knockout mice at the age of 8 weeks. The serum was obtained by cardiac puncture, and IL-6 level was determined by ELISA. Results from 8 mice of SHIP−/− and SHIP, γ-chain double-knockout and 3 mice of SHIP, μMT double-knockout each strain are indicated as pictograms per milliliter.
Discussion
Here we describe a sequence of events that accounts for the progressive proinflammatory phenotype of SHIP−/− mice and reveal the importance of IL-6 neutralization strategies in proinflammatory diseases. Elevated IL-6,which accompanies many chronic inflammatory conditions, can exacerbate the pathology associated with autoimmune diseases in several ways: First, IL-6 increases myelopoiesis to increase numbers of peripheral effector cells in autoimmune pathology.28,29,39 Second, IL-6 promotes the production of self-reactive antibodies and/or aggregated immunoglobulin by B cells.48 Finally, engagement of FcγR on macrophages or dendritic cells induces synthesis of still more IL-6. This feed-forward process, which occurs in many forms of inflammation, requires intervention with therapies that neutralize IL-6 or block responses to it.9 Animals lacking SHIP display high serum levels of IL-6, which causes increased myelopoiesis and reduced B lymphopoiesis in bone marrow.28,29 The results of the developmental changes are in part responsible for the increased myeloid lineage cells in select target organs lung and spleen in the SHIP−/− animals.30 SHIP-deficient myeloid cells are also hyper-responsive to cytokines,32,38 chemokines,49 lipopolysaccharide,50 and FcγR responding to IgG-containing immune complexes.19,25 The developmentally induced changes in peripheral cells caused by IL-6 might be responsible for similar changes in the makeup and activation of peripheral macrophages in inflamed joints of patients with rheumatoid arthritis,51 SLE,52 and other autoimmune diseases.53
Once IL-6 is produced, it acts as a potent stimulant for B-cell proliferation,54 plasma cell survival,55 and antibody production.56 IL-6 alone can drive further IgG production from plasma cells, including anti-ssDNA antibodies.57 In autoimmune disease, IL-6 can elevate the production of pathogenic antibodies. For example, it was shown that coculture of peritoneal macrophages derived from an animal model of SLE with host B cells stimulated the production of pathogenic IgG molecules having anti-DNA activity.48 The IgG production stimulated by the peritoneal macrophages was relieved with the addition of neutralizing anti–IL-6 antibodies. Thus, IL-6 production can exacerbate the pathology accompanying autoimmune disease.
To stimulate IL-6 production by FcγR, IgG must act as a multimer by opsonizing pathogens or (artificially) by heat aggregation.58 The aggregation of IgG provides a crosslinking function, which is needed for FcγR to signal,59 as it is needed for other immune receptors.60 The peritoneal lavage fluid from SHIP-deficient animals had a higher amount of total IgG and a higher amount of aggregated IgG, defined by IgG that was subject to ultracentrifugation. Although myelopoiesis is elevated in SHIP−/− mice,28,29,39 we did not find an increase in the total number of peritoneal macrophages. We did observe an increase in peritoneal B cells expressing CD19 and B220, and these cells secreted IgG. The nature of the aggregated IgG is not clear but might arise from spontaneous aggregation of the increased IgG monomers present in this compartment. However, we have observed a low-level production of antinuclear antibodies in older (> 10 weeks) SHIP−/− mice. Thus, some of the IgG in the peritoneum may be in the form of immune complexes rather than aggregates, which are also capable of stimulating FcγR.
Given these features of IL-6, it is remarkable that the SHIP-deficient animals do not develop overt signs of autoimmune disease. One reason may be the short life span of the animals, as mentioned in the paragraph above.30 SHIP−/− mice live to an age of approximately 10 weeks and eventually develop severe lung pathology, probably because of the massive infiltration of myeloid cells.30 However, despite this short life span, the SHIP−/− mice have some similarities in hematopoietic development with other mouse models of autoimmune disease. Lyn−/− mice likewise show elevated and unexplained myelopoiesis,61 elevated serum Ig levels, and increased IL-6 production from cultured dendritic cells.62 In contrast to SHIP−/− animals, the Lyn−/− mice go on to develop full autoimmune disease.63 Thus, the loss of SHIP may cause a propensity toward autoimmune disease, but the animals probably do not live long enough to develop clinical features of autoimmune disease. The loss of SHIP in the B-cell compartment may contribute to elevated B-cell survival in the peritoneum because B cells lacking SHIP show increased activation of the survival kinase Akt.64 Nevertheless, the ability of the SHIP-deficient peritoneal macrophages to produce IL-6 was extremely high on a per-cell basis and relative to other organ-associated macrophage populations. Lung and spleen macrophages could be another significant source of IL-6 in SHIP−/− mice, but the ability of macrophages from these organs of SHIP−/− mice to produce IL-6 was not high as peritoneal macrophages on a per-cell basis. This may be because the high IgG levels found in serum of SHIP−/− mice31 are not aggregated or present in an immune complex while in circulation.
Even when FcγR signaling was abrogated, by deletion of the γ-chain gene or by deletion of Ig, both present in the SHIP-deficient background, the IL-6 level did not completely revert to normal background levels. We found that bone marrow-derived SHIP-deficient macrophages do not make IL-6 in the absence of a stimulus and that the IL-6 production of in vivo-derived macrophages eventually ceases over a 4-hour period of culture. We interpret these observations as indicating that the IL-6–stimulating activity must come from an in vivo source. It is probable that IgG activation of FcγR is not the sole route for IL-6 production in SHIP−/− mice. For example, the SHIP−/− mice may have an increase in circulation of an endogenous stimulus for a Toll-like receptor.65
Although neutralization of circulating IL-666 and IL-6 receptors67 produces dramatic results of relieving morbidity and mortality in animal models of autoimmune disease, the mechanism by which IL-6 promotes pathology is not clear. Our previous reports28,29,39 established that IL-6 decreases lymphopoiesis and elevates myelopoiesis. The effect of these alterations in hematopoiesis results in an increased number of circulating myeloid cells. Earlier studies have shown that animals lacking cytokines that control myeloid cell output (granulocyte colony-stimulating factor68 and granulocyte-macrophage colony-stimulating factor69 ) are resistant to various forms of autoimmune disease. Although IL-6 is not needed for myelopoiesis, IL-6 is clearly a cytokine that likewise skews hematopoiesis toward a myeloid output but also elevates IgG production from B lymphocytes.
Besides a block in B lymphopoiesis, the SHIP−/− mouse shows an elevation in myelopoiesis that is not IL-6 dependent,29 suggesting that IL-6 itself is not responsible for increased production of monocytes, macrophages, and neutrophils. However, this feature is unique to the SHIP deficiency: we earlier showed that the addition of recombinant IL-6 raised by 10-fold the output of Mac-1+ cells from WT primitive progenitors.28 Likewise, we found that adding recombinant IL-6 to WT progenitors caused a doubling of the myeloid output, from 31% to 77% in single-cell OP9 cultures and from 20% to 94% in serum-free, stroma-free single-cell cultures.29 Lastly, in an in vivo approach, we showed that the total number of Mac-1+ cells in the bone marrow of an autoimmune-prone B6.Sle1.Yaa mouse strain was restored to WT levels when IL-6 was removed by crossing with an IL-6−/− mouse.39 There is no SHIP deficiency in this mouse, indicating that the elevated myelopoiesis in the B6.Sle1.Yaa autoimmune-prone mouse is entirely IL-6 dependent. It is probable that, under the unusual conditions of the SHIP−/− animal, the elevated myelopoiesis is the result of increased signaling by any of several myeloid-producing cytokine receptors (CSF-1, IL-3, and granulocyte-macrophage colony-stimulating factor).70
The causes of autoimmune disease are not clear and are hotly debated. Hypotheses include failures of central or peripheral B or T lymphoid tolerance, hyperactivity of Toll-like receptors, “molecular mimicry” of antipathogen host responses, and many others. Indeed, there may not be a single unifying cause for the multiple forms of human autoimmune disease. However, elevated IL-6 accompanies almost every form of autoimmunity. Thus, although perhaps not causally related to autoimmune disease, IL-6 most probably contributes to autoimmune pathology in the ways described here and earlier: by increasing autoimmune IgG production and by skewing hematopoiesis toward the production of the offending myeloid cells.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: K.M. designed and performed the work, analyzed all the data, and wrote the paper; H.M. designed and performed the studies shown in Figures 2, 5, and 6, analyzed all the data, and helped write the paper; D.A.D. gave advice about myeloid subpopulations in the peritoneal cavity and their markers, helped design the work in Figure 2, and edited the paper; and K.M.C. designed experiments, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: K. Mark Coggeshall, Oklahoma Medical Research Foundation, Program in Immunobiology & Cancer, 825 NE 13th St, Oklahoma City, OK 73104; e-mail: mark-coggeshall@omrf.org.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal