CLL is dependent on the intricate convergence of signals for maintenance and growth. In this issue of Blood, Abrams and colleagues tie together 2 of these signaling avenues,1 thereby creating new opportunities to more efficiently target CLL.
Chronic lymphocytic leukemia (CLL) is characterized by the accumulation of mature lymphocytes, which has been ascribed to a defect in apoptosis and a higher proliferation rate in these cells. In recent years, there has been a flurry of papers that address the signaling events that regulate cell death and proliferation in CLL, from the extracellular signals to their intracellular effects. Multiple pathways, most of them originating from the interactions of CLL cells with their microenvironment, have been proposed to be important, and creating a hierarchy of targets that are most likely to be therapeutically useful has become increasingly difficult. The opportunity to link 2 seemingly important pathways and open new avenues for therapeutic exploitation is therefore a welcome prospect from a clinical standpoint.
The British group presenting their work in this issue of Blood had first introduced protein kinase C (PKC)beta in 2007 as a component of the B-cell receptor (BCR) signal in CLL.2 PKCbeta proved robustly up-regulated in CLL, and was involved in determining the signaling strength of the receptor and, thus, the outcome of the signal. In this context, signaling through PKCbeta appears to be implicated in creating survival signals on the one hand, and in limiting the signaling strength of a BCR crosslinking event on the other. The latter may act to limit the proapoptotic potential of the receptor, which is wired to delete autoreactive cells in the case of a strong signal. Indeed, when PKCbeta was deleted from the TCL1A transgenic murine model of CLL, loss of PKCbeta was able to completely abrogate the potential of preleukemic CD5-positive B cells to transform into full-blown CLL.3 This was associated with an exquisite sensitivity of the cells to death signals along the BCR pathway, suggesting that the essential role of PKCbeta is to fine tune the BCR signal in this setting. Further evidence for the importance of PKCbeta stems from observations that the prognostically important ZAP-70 molecule recruits PKCbeta into lipid rafts upon BCR stimulation, where it starts to tweak the Bcl-2 family of cell death regulators in favor of CLL survival.4
Abrams et al now show that an essential factor influencing the up-regulation of the PKCbeta levels they had previously observed seems to be vascular endothelial growth factor (VEGF).
VEGF has been investigated as an auto- and paracrine survival factor in CLL for a number of years. In studies pioneered by a team from the Mayo Clinic, VEGF proved to increase survival of CLL cells by directly modulating components of the cell death machinery (for a short review, see Kay5 ). Other observations support the idea that the rise in VEGF levels contribute to increased angiogenesis. In fact, increased microvessel density and VEGF levels themselves were reported to be prognostic in CLL.6 The Mayo group has also shown that VEGF levels predicted response to chemoimmune-therapeutic strategies,7 and has also determined that the increased production of VEGF in CLL cells is due to the aberrant regulation of the von Hippel-Lindau protein by microRNA 92-1.8 Although it is not entirely clear whether the most important contribution of VEGF to CLL pathogenesis lies in its stimulation of microvessel formation and modulation of the microenvironment, or in its direct effect on the CLL cells, these findings have spawned an interest in targeting the VEGF pathway in CLL. There are a number of studies evaluating the efficacy of the anti-VEGF antibody bevacizumab in CLL. A Mayo Clinic–led trial of bevacizumab monotherapy as salvage therapy for patients with relapsed/refractory CLL was closed early due to lack of efficacy, according to Drs Tait Shanafelt and Neil Kay (personal written communication, March 2010), and our own monotherapy trial for a pretreatment indication CLL showed similar results in a limited set of patients (unpublished data, 2010). However, a number of studies are currently exploring bevacizumab in combination with chemoimmunotherapy, the most interesting of which may be a randomized phase 2 trial comparing the combination of pentostatin, cyclophosphamide, and rituximab with or without bevacizumab that is run by the Mayo Clinic.
How do the new data by Abrams and colleagues impact the field? Interestingly, VEGF levels were previously reported higher in CLL with unmutated Ig sequences (where the BCR seems more active), already suggesting at a correlative level that there is interplay between VEGF and the BCR signal. Of note, this interaction most likely takes place in the active center of the microenvironment and mainly involves the active CD38-positive fraction of the CLL clone (as suggested by work on sorted CD38-positive CLL cells9 ). In addition, VEGF may also be important in limiting CLL cell migration,10 thereby retaining this more active pool in the microenvironmental niche where the CLL cells are most likely to pick up their BCR signals.
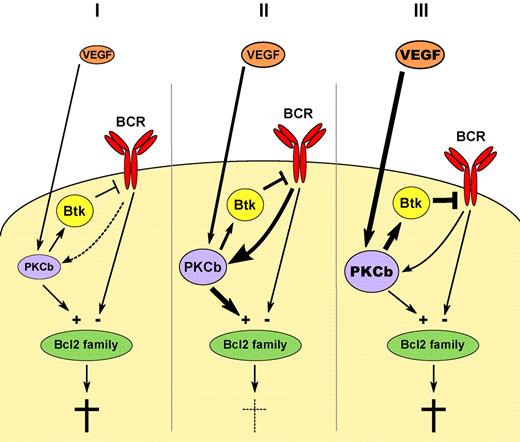
Hypothetical model of the influence of VEGF on BCR signaling in 3 scenarios: (I) Lack of adequate VEGF stimulation leads to low PKCbeta expression and insufficient survival signals along the BCR signaling pathway. (III) A too strong PKCbeta signal may lead to excess negative feedback via Btk and again negatively impact on the critical ratio of prosurvival and death signals likely converging in the Bcl2 family. Only an optimal BCR signaling strength may be able to support CLL survival (II) and VEGF may be an important determinant of the fine tuning.
Hypothetical model of the influence of VEGF on BCR signaling in 3 scenarios: (I) Lack of adequate VEGF stimulation leads to low PKCbeta expression and insufficient survival signals along the BCR signaling pathway. (III) A too strong PKCbeta signal may lead to excess negative feedback via Btk and again negatively impact on the critical ratio of prosurvival and death signals likely converging in the Bcl2 family. Only an optimal BCR signaling strength may be able to support CLL survival (II) and VEGF may be an important determinant of the fine tuning.
The action of the B-cell receptor, although essential for CLL maintenance, seems to be a decidedly 2-edged sword for CLL survival. The data from our murine model, and from the observations of Abrams et al, suggest that a tight window of signaling strength of the BCR is necessary to maintain CLL. Too little a signal and the cells are not supported by survival and proliferation input, and too great a signal and the cells are deleted by a mechanism normally enforcing peripheral tolerance. Abrams et al propose a mechanism by which VEGF may optimize the BCR/PKCbeta signal in this respect. In such an interpretation, the presence of VEGF in the microenvironment boosts the signal above baseline, and the presence of PKCbeta limits it by negative feedback to the BCR via Bruton kinase (Btk). This results in creating a window for optimal signaling strength (see figure scenario II). Intelligent combinations of VEGF targeting with strategies that further modulate the BCR signal may therefore be a very promising avenue in CLL therapy.
Conflict-of-interest disclosure: A.E. has received research funding from Lilly. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal