Abstract
Chronic lymphocytic leukemia (CLL) is a malignant disease of mature B lymphocytes. We have previously shown that a characteristic feature of CLL cells are high levels of expression and activity of protein kinase CβII (PKCβII), and that this might influence disease progression by modulating signaling in response to B-cell receptor engagement. The aim of the present work was to investigate the factors involved in stimulating PKCβII expression in CLL cells. Here we show that the activation of PKCβII in CLL cells stimulated with vascular endothelial growth factor (VEGF) can drive expression of the gene for PKCβ, PRKCB1. We found that this effect of VEGF on PRKCB1 transcription is paralleled by high expression of PKCβII protein and therefore probably contributes to the malignant phenotype of CLL cells. Taken together, the data presented in this study demonstrate that VEGF, in addition to its role in providing prosurvival signals, also plays a role in overexpression of PKCβII, an enzyme with a specific pathophysiologic role in CLL.
Introduction
Chronic lymphocytic leukemia (CLL) is a disease that results from a clonal expansion of antigen-experienced B cells that are developmentally blocked and resistant to apoptosis.1 This disease is important because it is one of the most prevalent forms of leukemia and because it is also associated with significant morbidity and mortality.1 CLL is a heterogeneous disease that can follow a course that is either indolent or aggressive, with the difference probably depending on the ability of the malignant cells to respond to engagement of the B-cell receptor (BCR) by increasing proliferation and survival.2 Nevertheless, the molecular basis of CLL is still poorly understood, and this disease remains so far incurable. Therefore, identification of the factors that contribute to CLL pathophysiology may lead to the discovery of agents that are effective in the treatment of this disease.
We have previously shown that a prominent feature of the malignant CLL cells is overexpression of protein kinase CβII (PKCβII).3 Furthermore, we have also determined that a possible pathophysiologic role for this isozyme in CLL cells is in the modulation of BCR signaling, resulting in the increase of cell survival.3 PKCβII is a classic isoform of the PKC family of proteins and is typified by its requirement for diacylglycerol, Ca2+, and phosphatidylserine to achieve full enzymatic activity within a cell.4 PKCβ plays a key role in BCR signaling,5,6 and it is known that expression of this protein is important during B-cell development.7 Considering that the presence of PKCβ may be essential for the development of CLL,8 the aim of the present study was to investigate the factors that contribute to the overexpression of this enzyme in the malignant cells of this disease.
The human gene that encodes PKCβII, PRKCB1, is located on chromosome 16,9 and the promoter region within this gene has been identified and partially characterized.10,11 Preliminary investigations of PRKCB1 regulation show that expression of this gene can be stimulated by active PKCβ.11-13 It has also been reported that such PKCβ-stimulated PRKCB1 transcription has importance with respect to particular stimuli.12 The example here is the critical role PKCβ expression and activation play in the cell differentiation that follows cytokine stimulation of monocytes.
CLL cells are exposed to a variety of stimuli provided in the microenvironment that are important to their pathophysiology.14 Many of these stimuli are known to activate PKCs, and some are known to have a direct role in the activation of PKCβ. In particular, BCR signaling,5,15 B cell–activating factor of tumor necrosis factor family (BAFF) receptor engagement,16,17 and interaction with vascular endothelial growth factor (VEGF)18-22 all play an important role in CLL cell survival and are known to directly stimulate PKCβ activity.
In the present study, we examined the role of active PKCβ in the regulation of PRKCB1 transcription in CLL cells. We found that activation of PKCβII and transcription of its gene are mediated specifically by stimulation of the malignant cells in CLL with VEGF. We also found that VEGF-mediated PRKCB1 transcription in CLL cells is dependent on the relatively high levels of PKCβII that are present within these cells. Thus, we define a new role for VEGF in CLL, ie, in stimulating gene transcription and maintenance of PKCβII in the malignant cells of this disease.
Methods
Materials
Antibodies against PKCβII and Flk1 (A-3 and C-1158) and horseradish peroxidase-conjugated anti–mouse, anti–rabbit, immunoglobulin (Ig) antibodies were purchased from Santa Cruz Biotechnology (Insight Biotechnology). Rabbit antineuropilin-1, mouse anti–pS180-Bruton tyrosine kinase (Btk), and rabbit anti-Btk were purchased from Cell Signaling Technology (New England Biolabs). Mouse anti–β-actin and mithramycin were purchased from Sigma-Aldrich. SU5416 was from Merck Biosciences. Purified recombinant PKCβII protein was purchased from Merck Biosciences. Recombinant VEGF, basic fibroblast growth factor (bFGF), BAFF, interferon-γ, and interleukin-4 were purchased from R&D Systems Europe. Alexa Fluor 633 anti–mouse and Alexa Fluor 680 anti–rabbit antibodies and protein G Sepharose were purchased from Invitrogen. polyHEMA was from Sigma-Aldrich. Immobilon membranes were from Millipore. The PKCβII-specific inhibitor LY379196 was kindly provided by Lilly Research Laboratories.
Purification of cells
Diagnosis of CLL was based on standard morphologic, immunophenotypic, and cytogenetic criteria.23 CLL cells were obtained from the peripheral blood of patients by informed consent and with the approval of the Liverpool Research Ethics Committee in accordance with the Declaration of Helsinki. The CLL-cell cases used in the present study were chosen randomly from stored samples within the University of Liverpool Leukemia Tissue Bank and are listed in supplemental Table 1 (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
The cells used in this study were cryopreserved and stored within the tissue bank. When required, the cells were thawed, resuspended in culture media (RPMI 1640 + 0.5% bovine serum albumin, penicillin/streptomycin, l-glutamine), and equilibrated at 37°C as described before.24 We found that the response of cryopreserved cells to our experimental conditions, and in particular to VEGF, was very similar to that of freshly isolated cells with regard to the regulation of PKCβ mRNA levels (supplemental Figure 1). Thus, the use of cryopreserved cells for the purposes of this study is justified.
The CLL cases used in this study contained cell populations where the majority of cells (> 90%) were CD19+. However, for some cases, the cells were further purified using a B-cell isolation kit within a MiniMacs system (Miltenyi Biotec) according to the manufacturer's instructions. Cell purity was assessed by fluorescence-activated cell sorter using CD19 antibodies This was done to investigate whether the other cell types (such as monocytes, T cells, and NK cells) found in the unpurified CLL cell preparations contributed to our measurement of PKCβ mRNA levels. We found that there was essentially no difference in PKCβ mRNA levels between CD19-purified and unpurified CLL cells from the same case (supplemental Figure 2). This indicates that it is highly doubtful that the PKCβ mRNA levels we observe come from cells that are not CLL cells. Therefore, for the rest of the study, we relied on cells from unpurified cases.
Normal B cells were purified from buffy coats obtained from the British Transfusion Service. This was done using a negative isolation kit, which removes monocytes, T cells, and NK cells but leaves the CD19+ cells behind. Cell purity was assessed by flow cytometry using a BD Biosciences FACSCalibur and was not less than 90% B cells for all the cases used.
Quantitation of PKCβII in cells
PKCβII protein levels in CLL cells were quantitated as described.3 Briefly, CLL cells were lysed with buffer A (125mM Tris, pH 6.8, 5mM ethylenediaminetetraacetic acid, 1% sodium dodecyl sulfate [SDS], 10% glycerol), sonicated to disrupt released DNA, and incubated for 10 minutes at 95°C. The protein concentration in the cell lysates was quantitated using a Bio-Rad DC protein assay kit. A total of 10 μg of protein was loaded onto 10% SDS-polyacrylamide gel electrophoresis gels together with known amounts of purified recombinant PKCβII protein, and the separated proteins then electroblotted to Immobilon membranes. The membranes were probed with anti-PKCβII antibodies followed by second-layer antibodies conjugated with Alexa Fluor 633 fluorochrome. Results were read with a Fujifilm FLA-5000 imager for measurement of immunofluorescence. Specific protein quantitation was achieved by comparing the immunofluorescence of PKCβII bands within CLL cell lysates with that of recombinant protein bands used as standards on the same Western blot.
RT-PCR analysis
Total RNA was extracted from B cells using an RNeasy mini kit (QIAGEN) according to the manufacturer's instructions; 1 μg of purified RNA was reverse transcribed using Moloney murine leukemia virus reverse transcriptase (Promega) and an oligo(dT)15 primer.
For real-time amplification of PKCβ mRNA, purified cDNA was mixed with DyNAmo SYBR Green I qPCR master mix (Finnzymes), a consensus PKCβ forward primer (5′-TGGGGTGACAACCAAGACATTC-3′), and PKCβ reverse primer (5′-GCTGGATCTCTTTGCGTTCAAG-3′). We used β-actin as an internal control, and this was amplified from each cDNA sample using the forward primer (5′-CCTCGCCTTTGCCGATCC-3′) and reverse primer (5′-GGATCTTCATGAGGTAGTCAGTC-3′). All polymerase chain reaction (PCR) reactions were performed on a Stratagene Mx3005P quantitative reverse-transcription (RT)–PCR machine under optimized, identical cycling conditions consisting of a 10-minute initial denaturing step at 95°C, followed by 45 cycles of amplification (denature at 94°C for 30 seconds, anneal at 58°C for 30 seconds, extension at 72°C for 30 seconds, and collect fluorescence data at 80°C). A melting curve was then measured from 65°C to 98°C. The PCR products were subjected to an additional 72°C extension for 10 minutes if they were to be analyzed on agarose gels. The specificity of each of these PCR products was confirmed as a single band with the expected molecular size on agarose gels and as a narrow peak that appeared in the first-order negative derivative melting curve when temperatures rose higher than 80°C. The transcript levels of PKCβII were obtained using the ΔΔCt method, normalized against β-actin. In some cases, PKCβII transcript (in femtograms [fg]) was measured against standard amounts of PKCβII plasmid DNA (a kind gift from Dr Y. Hannun, Medical University of South Carolina, Charleston, SC).
Amplification of VEGF-R2 mRNA in normal B, CLL, andEAhy926 cells was performed using primers and a protocol that has been published previously by this department.18
Analysis of Btk phosphorylation in CLL cells
Analysis of S180 phosphorylation in Btk was performed according to a previously published protocol.3 Briefly, radioimmunoprecipitation assay buffer (25mM Tris, pH 8.0, 150mM NaCl, 25mM sodium pyrophosphate, 50mM sodium glycerophosphate, 50mM NaF, 2mM ethylenediaminetetraacetic acid, 2mM ethyleneglycoltetraacetic acid, 10% glycerol, 1% Triton X-100, 1% sodium deoxycholate, 0.1% SDS) lysates of CLL cells were immunoprecipitated with anti–phospho-S180-Btk antibodies. Proteins within immunoprecipitates and whole-cell lysates were separated by SDS-polyacrylamide gel electrophoresis, and Western blots were probed with a rabbit anti-Btk antibody.
Measurement of intracellular calcium
For the intracellular Ca2+, measurements were performed using Fura-2AM. Cells were loaded with 2.5μM Fura-2AM for 30 minutes and then washed into Ca2+-free phosphate-buffered saline. [Ca2+]i was measured with a Hitachi F-7000 fluorescence spectrometer, measuring fluorescence emission at 510 nm during excitation at 340 nm and 380 nm according to a published protocol,25 and using the software provided with the instrument. Ca2+ release was stimulated in CLL cells with the addition of 20 μg/mL goat anti–human IgM antibodies (Jackson ImmunoResearch Laboratories).
Measurement of PKCβ promoter activity
For these experiments, we used the endothelial cell line EAhy926 (supplied to us at low passage number by Dr M. Perez-Casal, University of Liverpool, Liverpool, United Kingdom). We chose this cell line because it has low expression of PKCβII and because it expresses the VEGF receptor. EAhy926 cells were maintained in Iscove modified Dulbecco medium, which was supplemented with 10% fetal calf serum, pen/strep, and l-glutamine, under standard culture conditions. Stable cell lines expressing green fluorescent protein (GFP)–PKCβII and GFP were established by transfecting GFP-pcDNA and PKCβII-GFP-pcDNA (a kind gift from Yusuf A. Hannun, Medical University of South Carolina, Charleston, SC) plasmids into the EAhy926 cells using nucleofection (nucleofection solution V, program O-17), and selecting the transfected cells with 500 μg/mL G418. To measure the effect of added VEGF on PKCβ promoter activity, the GFP- and GFP-PKCβII–expressing EAhy926 cells were transfected with 5 μg of plasmid containing the PKCβ promoter coupled to a firefly luciferase coding sequence, and with 100 ng of a plasmid containing the coding sequence for Renilla luciferase using nucleofection as discussed earlier in this paragraph. The transfected cells were cultured for 24 hours and then stimulated for a further 24 hours with 200 ng/mL VEGF. Luciferase activity was measured using a Dual-Luciferase Reporter Assay System (Promega) according to the manufacturer's instructions.
Statistical analysis
Datasets were compared for statistical significance using either Student t test or Mann-Whitney U test. The comparisons were performed by computer with Microsoft Excel and SPSS, Version 15.0 software, respectively.
Results
PKCβII activity regulates PKCβ mRNA levels in CLL cells
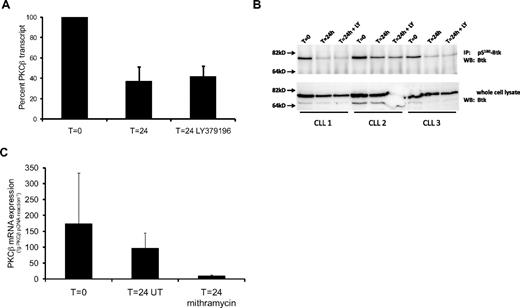
Several studies have demonstrated that PKCβ activity stimulates PRKCB1 expression.11-13 Considering that PKCβII can show considerable levels of activity in CLL cells,3 we investigated whether the inhibition of this activity affected PKCβ mRNA in these cells. In these experiments, we used LY379196, a compound that is a highly specific PKCβ inhibitor when used at concentrations less than or equal to 100nM.26-28 Figure 1A shows that PKCβ mRNA levels in CLL cells cultured for 24 hours decreased to approximately 50% of the levels present in the cells at the beginning of the experiment (T = 0). Figure 1A also shows that the presence of 100nM LY379196 had no effect on the decrease of PKCβ mRNA levels in the cultured CLL cells.
PKCβII gene transcription and protein kinase activity in CLL cells decrease after 24-hour culture. (A) Quantitative RT-PCR analysis of PKCβ mRNA levels in CLL cells showing the effect of in vitro culture in the presence and absence of 100nM LY379196 on PKCβ expression. A 24-hour incubation of CLL cells resulted in a significant drop in PKCβ mRNA levels (P = .002, n = 9). Results are reported as a percentage of PKCβ mRNA levels in CLL cells at T = 0. (B) Western blot analysis of Btk in anti–pS180-Btk immunoprecipitates from CLL cells (top panel), and in whole-cell lysates (bottom panel). Where CLL cells were incubated with LY379196, a concentration of 100nM was used. (C) Quantitative RT-PCR analysis of PKCβ mRNA levels in CLL cells exposed to 200nM mithramycin for 24 hours. Treatment of CLL cells with mithramycin resulted in a significant reduction in PKCβ mRNA levels compared with untreated (UT) cells (P = .013, n = 5). PKCβ mRNA amounts are reported in femtograms (fg) of PKCβ plasmid DNA equivalents.
PKCβII gene transcription and protein kinase activity in CLL cells decrease after 24-hour culture. (A) Quantitative RT-PCR analysis of PKCβ mRNA levels in CLL cells showing the effect of in vitro culture in the presence and absence of 100nM LY379196 on PKCβ expression. A 24-hour incubation of CLL cells resulted in a significant drop in PKCβ mRNA levels (P = .002, n = 9). Results are reported as a percentage of PKCβ mRNA levels in CLL cells at T = 0. (B) Western blot analysis of Btk in anti–pS180-Btk immunoprecipitates from CLL cells (top panel), and in whole-cell lysates (bottom panel). Where CLL cells were incubated with LY379196, a concentration of 100nM was used. (C) Quantitative RT-PCR analysis of PKCβ mRNA levels in CLL cells exposed to 200nM mithramycin for 24 hours. Treatment of CLL cells with mithramycin resulted in a significant reduction in PKCβ mRNA levels compared with untreated (UT) cells (P = .013, n = 5). PKCβ mRNA amounts are reported in femtograms (fg) of PKCβ plasmid DNA equivalents.
Because LY379196 had no effect on PKCβ mRNA levels and because overnight culture of the cells had no significant effect on CLL cell viability (supplemental Figure 3), the decrease in PKCβ mRNA levels that we observe in the untreated CLL cells may reflect a spontaneous decrease in PKCβII activity levels. To investigate this possibility, we examined the phosphorylation of S180 in Btk. We used S180 phosphorylation as a surrogate marker of PKCβII activity in CLL cells because this residue in Btk is a known substrate of PKCβII in B cells.5 Figure 1B shows that 24-hour culture of CLL cells in the presence or absence of 100nM LY379196 resulted in similar strong reductions in the level of pS180-Btk, indicating that PKCβII activity levels decrease in cultured CLL cells. Taken together with the results presented in Figure 1A, these data suggest that a proportion of PRKCB1 transcription in CLL cells is dependent on PKCβII activity.
To examine whether the levels of PKCβ mRNA that remained in 24-hour cultured CLL cells resulted from transcription of the gene or from stabilization of the transcript, we used mithramycin, which is an inhibitor of Sp-1–mediated transcription.29 Figure 1C shows that the presence of 200nM mithramycin in cultures of CLL cells resulted in a quantitative reduction of PKCβ mRNA levels compared with untreated cells. This reduction was not the result of changes in cell viability because the presence of mithramycin was not cytotoxic to CLL cells over the time frame of the experiment. Indeed, the presence of 200nM mithramycin did not largely affect CLL cell viability even after 144 hours of culture (supplemental Figure 4C). Thus, taken together with the data using the PKCβ inhibitor LY379196, these results indicate that a proportion of PRKCB1 transcription is independent of PKCβII activity.
We next investigated the role of external stimuli in regulating PKCβ mRNA levels in CLL cells.
VEGF stimulates PKCβ gene expression in CLL cells
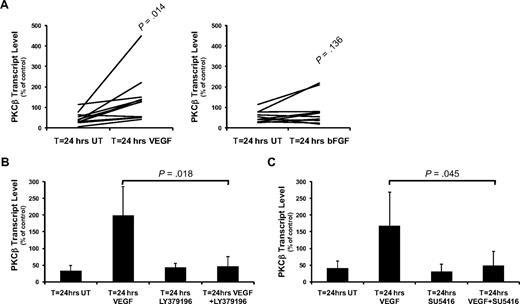
To investigate the nature of the stimulus controlling transcription of the PKCβ gene, we used a range of stimuli that are known to be important to CLL pathophysiology. These stimuli included BCR crosslinking,15 interleukin-4,30 interferon-γ,31 lipopolysaccharide, BAFF,16 CD40 ligation,32 VEGF,19 and bFGF,33 and they were used at concentrations previously described to induce a response in CLL cells. We found that, of these stimuli, only the presence of VEGF resulted in significant (P = .014) PKCβ mRNA production in all CLL cases tested (Figure 2A; supplemental Figure 5). In our experiments, a concentration of 100ng/mL VEGF was chosen as the optimal concentration for CLL cell stimulation based on its usage within previous reports.19,22 With respect to bFGF, significant induction of PKCβ mRNA was only seen in a proportion of cases (Figure 2A; supplemental Table 1), and the magnitude of response (1.45 ± 0.80-fold induction in response to bFGF) was less than that of CLL cells responding to VEGF (3.51 ± 2.03-fold induction). CD40 ligation induced a small increase in PKCβ mRNA, but this was most probably the result of the induction of VEGF secretion as we have described elsewhere.21
VEGF stimulates PKCβ mRNA expression in CLL cells. Quantitative RT-PCR analysis of PKCβ mRNA levels in CLL cells reported as a percentage of PKCβ mRNA levels in control (T = 0) cells. (A) The effect of 24-hour treatment of CLL cells with 100 ng/mL VEGF (n = 11) or with 100 ng/mL bFGF (n = 10) on PKCβ mRNA levels. (B) Effect of 100nM LY379196 on VEGF-induced PKCβ mRNA expression in CLL cells (n = 4). (C) Effect of 10μM SU5416 on VEGF-induced PKCβ mRNA expression in CLL cells (n = 4). (B-C) Error bars represent SD.
VEGF stimulates PKCβ mRNA expression in CLL cells. Quantitative RT-PCR analysis of PKCβ mRNA levels in CLL cells reported as a percentage of PKCβ mRNA levels in control (T = 0) cells. (A) The effect of 24-hour treatment of CLL cells with 100 ng/mL VEGF (n = 11) or with 100 ng/mL bFGF (n = 10) on PKCβ mRNA levels. (B) Effect of 100nM LY379196 on VEGF-induced PKCβ mRNA expression in CLL cells (n = 4). (C) Effect of 10μM SU5416 on VEGF-induced PKCβ mRNA expression in CLL cells (n = 4). (B-C) Error bars represent SD.
We next investigated the role of PKCβ in VEGF-stimulated induction of PRKCB1 transcription. Pretreatment of CLL cells with 100nM LY379196 inhibited the increase in PKCβ mRNA levels that is induced by VEGF (Figure 2B), indicating that VEGF-stimulated gene expression through a PKCβ-mediated mechanism. Incubation of CLL cells with 10μM SU5416, an inhibitor of VEGF receptor kinase activity,34 also blocked VEGF-stimulated PKCβ gene expression (Figure 2C), demonstrating that induction of PRKCB1 transcription by VEGF is mediated by activation of its receptor.
Taken together, these results suggest that VEGF stimulates PRKCB1 transcription in CLL cells through a mechanism involving receptor-mediated activation of PKCβII.
VEGF stimulates PKCβII activity in CLL cells
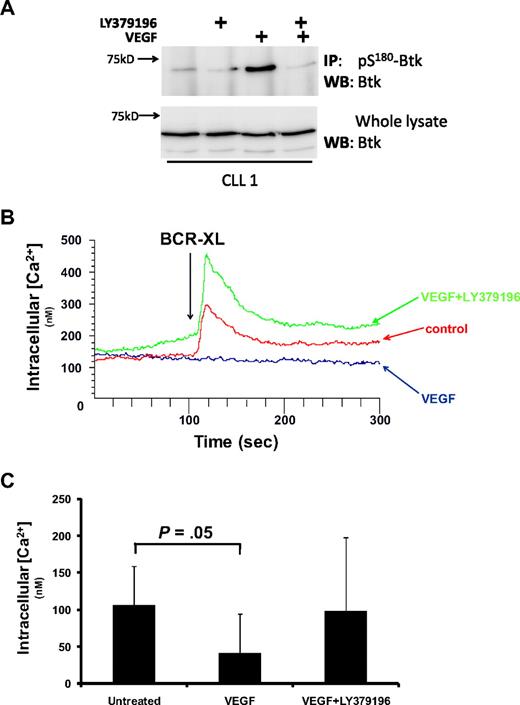
To show that VEGF stimulates PKCβII activity, we examined whether this cytokine stimulated an increase in pS180-Btk levels in CLL cells. Figure 3A shows that the treatment of CLL cells with VEGF stimulated an increase in the levels of pS180-Btk and that preincubation of the cells with LY379196 inhibited this increase. These results indicate that VEGF stimulates increased PKCβII activity in CLL cells.
VEGF stimulates PKCβII activity in CLL cells. (A) Western blot analysis of Btk in whole-cell lysates and in anti–pS180-Btk immunoprecipitates from CLL cells treated with 100 ng/mL VEGF in the presence and absence of 100nM LY379196. This experiment is representative of 3 using different CLL cases. (B) Effect of 100 ng/mL VEGF on BCR-induced intracellular Ca2+ release in CLL cells. Fura-2-loaded CLL cells were stimulated with 20 μg/mL anti-IgM antibody (BCR-XL) in the presence and absence of VEGF. LY379196 (100nM) was used to reverse the effects of VEGF. (C) Data presented in panel B, highlighting that the incubation of CLL cells with VEGF induced significant inhibition of intracellular Ca2+ release (P = .05, n = 6).
VEGF stimulates PKCβII activity in CLL cells. (A) Western blot analysis of Btk in whole-cell lysates and in anti–pS180-Btk immunoprecipitates from CLL cells treated with 100 ng/mL VEGF in the presence and absence of 100nM LY379196. This experiment is representative of 3 using different CLL cases. (B) Effect of 100 ng/mL VEGF on BCR-induced intracellular Ca2+ release in CLL cells. Fura-2-loaded CLL cells were stimulated with 20 μg/mL anti-IgM antibody (BCR-XL) in the presence and absence of VEGF. LY379196 (100nM) was used to reverse the effects of VEGF. (C) Data presented in panel B, highlighting that the incubation of CLL cells with VEGF induced significant inhibition of intracellular Ca2+ release (P = .05, n = 6).
The consequence of Btk phosphorylation by PKCβ is that Btk is no longer able to activate phospholipase Cγ2, thereby attenuating the induction of intracellular Ca2+ release during antigen receptor stimulation of B cells. Figure 3B and C shows that treatment of CLL cells with VEGF reduces the level of BCR-induced Ca2+ release. Moreover, these figures also show that the presence of LY379196 completely inhibits the effect of VEGF on BCR-induced Ca2+ flux. These data confirm the role of VEGF in stimulating PKCβII-mediated Btk phosphorylation in CLL cells, and, taken together with the experiments presented in Figure 2B, also strongly support the notion that VEGF-induced increases in PKCβ mRNA levels in CLL cells results from the activation of PKCβII.
VEGF does not stimulate PKCβ gene expression in normal peripheral B cells
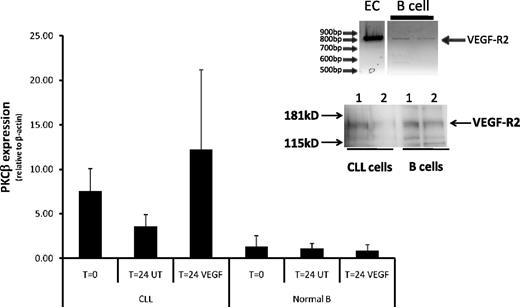
CLL cells and normal B cells express receptors for VEGF,19,22,35 and it is therefore possible that VEGF is able to stimulate PKCβ mRNA production in both cell types. Figure 4 shows quantitative RT-PCR analysis of PKCβ mRNA expression in CLL and CD19+ normal B cells. VEGF strongly stimulated PKCβ mRNA production in CLL cells but had no effect on the production of PKCβ mRNA in normal B cells. This lack of effect of VEGF on normal B cells is not the result of the absence of receptors for VEGF because we could detect expression of VEGF-R1, VEGF-R2, and neuropilin-1 on these cells (Figure 4 inset; supplemental Figure 6). Thus, VEGF-mediated PRKCB1 expression appears to be specific to the CLL cells used in this study.
VEGF does not stimulate PKCβ gene expression in normal B cells. Quantitative RT-PCR analysis of PKCβ mRNA levels in CLL (n = 6) and normal peripheral B cells (n = 3) incubated in the presence and absence of 100 ng/mL VEGF for 24 hours and compared with control uncultured cells. The PKCβ mRNA levels are reported relative to β-actin mRNA levels in the same cell in arbitrary units. (Inset) RT-PCR analysis of VEGF-R2 expression in normal B cells and in an endothelial cell line (EAhy926; top panel) and Western blot analysis of VEGF-R2 protein expression in CLL- and normal B-cell lysates (equal amounts of protein were loaded onto the gel).
VEGF does not stimulate PKCβ gene expression in normal B cells. Quantitative RT-PCR analysis of PKCβ mRNA levels in CLL (n = 6) and normal peripheral B cells (n = 3) incubated in the presence and absence of 100 ng/mL VEGF for 24 hours and compared with control uncultured cells. The PKCβ mRNA levels are reported relative to β-actin mRNA levels in the same cell in arbitrary units. (Inset) RT-PCR analysis of VEGF-R2 expression in normal B cells and in an endothelial cell line (EAhy926; top panel) and Western blot analysis of VEGF-R2 protein expression in CLL- and normal B-cell lysates (equal amounts of protein were loaded onto the gel).
Role of overexpressed PKCβII in VEGF stimulated PRKCB1 expression
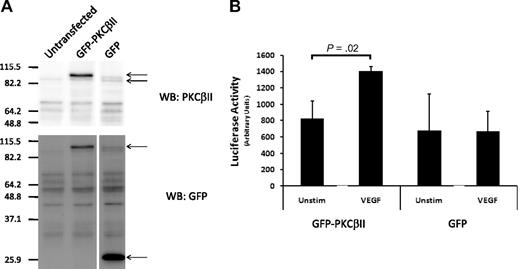
Previous work from this department demonstrated that CLL cells differ from normal B cells with respect to overexpression of PKCβII.3 Because VEGF stimulates PRKCB1 expression in CLL cells, but not in normal B cells despite being able to signal in both cell types, it follows that the overexpression of PKCβII may be required for VEGF to induce expression of the gene. To investigate this possibility, we transfected wild-type PKCβII, which was coupled to GFP, or GFP alone into an endothelial cell line (EAhy926), and selected the cells that overexpressed these proteins (Figure 5A). We then used a luciferase gene coupled to the PRKCB1 promoter to measure the effect of VEGF stimulation on PKCβ expression in these cells. Figure 5B shows that VEGF stimulated increased promoter activity in the EAhy926 cells that overexpressed GFP-PKCβII, but not in the cells that overexpressed GFP. This strongly suggests that the overexpression of PKCβII in CLL cells is responsible for driving VEGF stimulation of PRKCB1 expression.
Role of overexpressed PKCβII in VEGF-stimulated induction of PKCβ mRNA expression. (A) Western blot analysis of PKCβII and GFP expression in an endothelial cell line (EAhy926) transfected to overexpress GFP-PKCβII or GFP. (B) Effect of 100 ng/mL VEGF on PKCβ promoter-driven luciferase activity in GFP-PKCβII- and GFP-transfected endothelial cells (n = 4).
Role of overexpressed PKCβII in VEGF-stimulated induction of PKCβ mRNA expression. (A) Western blot analysis of PKCβII and GFP expression in an endothelial cell line (EAhy926) transfected to overexpress GFP-PKCβII or GFP. (B) Effect of 100 ng/mL VEGF on PKCβ promoter-driven luciferase activity in GFP-PKCβII- and GFP-transfected endothelial cells (n = 4).
Discussion
The present study focuses on the identification of factors that contribute to the high expression of PKCβII in CLL cells. We found that VEGF contributes to high levels of PKCβII expression in CLL cells by stimulating activation-induced transcription of the gene. Our previous work suggested that overexpressed PKCβII has a pathophysiologic role in the regulation of BCR signaling in CLL cells.3 Moreover, the work of others has demonstrated that PKCβ is essential for the development of CLL in a Tcl1-transgenic mouse model of this disease.8 If we additionally consider that VEGF is present in the hemic tissues where CLL cells proliferate,18 that VEGF is also important for providing prosurvival signals,21,22 and that both VEGF and PKCβII are reported to show increasing levels of expression in relation to disease stage and tumor burden,3,36,37 then the data of the present study suggest that VEGF may be central to the natural history of the disease through its effect on the regulation of PKCβII expression.
In other cell types, PKCβII is reported to stimulate transcription of the PRKCB1 gene through apparent constitutive PKC signals that result from overexpression of the protein.12,13 However, in CLL cells we find that, although PKCβII is overexpressed, enzyme activity-driven transcription of the PRKCB1 gene is stimulation-dependent rather than the result of constitutive PKC signaling. Thus, we demonstrate that CLL cells rely on VEGF-induced activation of overexpressed PKCβII to maintain high levels of the PKCβ transcript. In the absence of such stimulation, we find that PKCβ mRNA and activity levels spontaneously decrease in cultured CLL cells. These findings are supported by our observation that the presence of LY379196 has no effect on this spontaneous decrease.
In vivo, CLL cells are exposed to a variety of stimuli, which are known to be important in their survival. The signals induced by many of these stimuli will involve activation of PKCs, but signals provided by VEGF,20 bFGF,38 BCR crosslinking,5 CD40L,39 interferon-γ,40 and BAFF17 are all known to specifically involve activation of PKCβ. However, in our experiments, we found that only VEGF, bFGF, and CD40 ligation were able to stimulate increases in PKCβ mRNA levels. The reasons for this selectivity are unclear; case selection may play a role because of the heterogeneity observed in the response of different CLL cases to bFGF. It could be that our analysis of the other stimuli on PKCβ gene expression is flawed because we did not look at the response in enough cases. However, the selectivity in CLL cell response to particular signals may also be the result of the context of these signals. VEGF and bFGF signal through receptor tyrosine kinases, and the response to CD40 ligation is probably the result of the release of autocrine VEGF, which then acts on its receptor as we have described earlier.21 That only some of the CLL cases used in this study responded to bFGF whereas most responded to VEGF can be explained in terms of receptor expression. CLL cells generally express receptors for VEGF,21,41 but not always the receptor and coreceptors that are required for bFGF.42,43 Previous work from this department has shown a similar heterogeneity in malignant cell response to bFGF between different CLL clones.44 Thus, a combination of low receptor and low cofactor expression probably plays a role in the response of CLL cells to bFGF in terms of regulating PRKCB1 transcription. Whether the ability of CLL cells to respond to bFGF correlates with disease severity remains to be seen; however, in the cohort of CLL cases used in the present study, this ability was not associated with markers of poor disease prognosis, such as IgVH mutation or CD38 expression (supplemental Table 1).
We show that VEGF regulates BCR responsiveness in CLL cells, probably through a mechanism involving PKCβ-catalyzed phosphorylation of Btk on S180. This notion is supported by published work demonstrating that such phosphorylation of Btk results in the down-regulation of BCR-induced signaling in normal B cells.5 The ability of VEGF to regulate BCR responsiveness in a PKCβII-dependent fashion has an interesting consequence in the light of our previous work showing that PKCβII activity regulates BCR signaling and its outcomes in CLL cells.3 From these data, it is easy to hypothesize that VEGF must also influence the outcome of BCR signaling in CLL cells. Considering that VEGF is produced by CLL cells and acts in an autocrine fashion to increase CLL cell survival18,19,21,22,37 and that elevated levels of this cytokine are found in the nodes/proliferation centers of CLL patients,18 it is therefore probable that VEGF contributes to the expansion of the malignant clone by influencing the effects of BCR engagement. In this setting, VEGF would play a major role in CLL pathogenesis through its ability to stimulate PKCβII activity and down-regulate potentially proapoptotic BCR signals. Whether VEGF-induced activation of PKCβII would play an additional direct role in the prosurvival effects of this cytokine cannot be inferred from the data presented in this study. The malignant cells from the CLL cases used maintained a high degree of viability over the culture period(s) used, and addition of VEGF to these cultures did not have a significant effect on this viability. Thus, the cytoprotective effects of VEGF on CLL cells that have been described by others18,19,21,22,37 were not observed in this study.
Are the effects of VEGF on PKCβ expression specific for CLL cells? This question is important because B cell–derived VEGF is required for angiogenesis within lymph nodes during immune stimulation45 and because VEGF is present in the lymph nodes of CLL patients.18 Thus, the ability of CLL but not normal B cells to respond to VEGF by up-regulating PKCβ expression may be related to the pathology of the disease. The present work shows that VEGF does not affect PKCβ mRNA levels in normal B cells. This absence of response is not the result of a lack of VEGF-receptor expression on normal B cells because we found that these cells express VEGF-R1 and VEGF-R2, as well as neuropilin-1, which helps in facilitating the binding of VEGF to its receptor.46 Thus, our work confirms the work of others19,22,35 and shows that B and CLL cells express similar levels of VEGF-R2. Taken together, this information suggests that normal B cells respond to VEGF stimulation differently than do CLL cells. More work will be needed to fully characterize the signaling mechanisms stimulated by VEGF in both cell types.
Considering that one of the main differences between normal B and CLL cells is the overexpression of PKCβII,3 it is therefore probable that this is the factor determining VEGF responsiveness in terms of PRKCB1 transcriptional regulation in CLL cells. Indeed, our experiments support this idea and show that VEGF stimulated PKCβ promoter activity in an endothelial cell line that overexpressed PKCβII, but not in a similar cell line that overexpressed GFP. This leaves the question of what is initially responsible for the overexpression of PKCβII in CLL cells during development of the disease. The experiments with mithramycin in the present study indicate that CLL cells possess additional factors to VEGF-induced PKC activation for the regulation of PKCβ expression. Mithramycin intercalates into G-C rich regions of DNA to inhibit the binding of transcription factors, such as Sp-1.29 That PKCβ mRNA levels are reduced in mithramycin-treated CLL cells suggests that Sp-1 or Sp-1-like transcription factors may be responsible for the high levels of PKCβII in these cells. A role for Sp-1 in PRKCB1 transcription is supported by studies showing that binding sites for Sp-1 as well as for E47 and Oct1 are present within the promoter region of this gene.10,11,47 Other factors that may also contribute to PKCβII overexpression in CLL cells include changes in gene methylation because the promoter region contains CpG islands11 and because gene expression in CLL can be affected by both hypomethylation48 and hypermethylation.49 Finally, the promoter region of PRKCB1 is also known to contain single nucleotide polymorphisms, which can affect transcription of the gene.50-53
Our use of mithramycin also revealed an interesting aspect of CLL cells in terms of the protein stability of PKCβII within these cells. We found that, despite the quantitative reduction in PKCβ mRNA levels caused by mithramycin, even for extended periods of time, there were significant levels of PKCβII protein expression that remained in the treated CLL cells (supplemental Figure 4A-B). This result demonstrates that PKCβII protein turnover is probably very slow in CLL cells and suggests that agents, such as PKCβ-specific siRNA, will not be useful for studying the pathophysiology of this enzyme in this disease. This slow turnover of PKCβII protein in CLL cells could result from deregulation of the phosphatases, such as PH domain and leucine-rich repeat protein phosphatase, which are responsible for its degradation54 ; CLL cells show reduced expression of this phosphatase, particularly in cases with 13q14 deletions.55 Thus, high PKCβII protein levels in CLL cells may be the result of an inability of these cells to efficiently degrade this protein.
In conclusion, the findings of the present study show that VEGF is involved in the regulation of PKCβ expression in CLL cells. Therapeutically, this may be important because of the recent demonstration that the level of PKCβ expression in the malignant cells of the Tcl1 mouse model of CLL determines both disease development and progression.8 Control of PKCβ expression is therefore probably an important contributor to CLL pathogenesis, and VEGF, because of its role in stimulating PKCβ expression, is therefore also implicated in this process. Thus, our study provides further evidence to support the importance of VEGF in CLL, and suggests that this cytokine and its signaling pathway are probably good therapeutic targets for the treatment of this disease.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr M. Glenn and Dr N. Kalakonda of the University of Liverpool Division of Haematology for critical reading of the manuscript.
This work was supported by the Northwest Cancer Research Fund and Leukaemia Research Fund, United Kingdom (J.R.S.).
Authorship
Contribution: S.T.A. and B.R.B.B. designed and performed the research and analyzed the data; and M.Z. and J.R.S. designed the research, analyzed the data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
M.Z. passed away during the preparation of this manuscript.
Correspondence: Joseph R. Slupsky, PhD, Division of Haematology, University of Liverpool, Third Fl, Duncan Bldg, Daulby St, Liverpool, United Kingdom, L69 3GA; e-mail: jslupsky@liverpool.ac.uk.
References
Author notes
S.T.A. and B.R.B.B. contributed equally to this study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal