Abstract
Ischemia of the heart, brain, and limbs is a leading cause of morbidity and mortality worldwide. Treatment with tissue type plasminogen activator (tPA) can dissolve blood clots and can ameliorate the clinical outcome in ischemic diseases. But the underlying mechanism by which tPA improves ischemic tissue regeneration is not well understood. Bone marrow (BM)–derived myeloid cells facilitate angiogenesis during tissue regeneration. Here, we report that a serpin-resistant form of tPA by activating the extracellular proteases matrix metalloproteinase-9 and plasmin expands the myeloid cell pool and mobilizes CD45+CD11b+ proangiogenic, myeloid cells, a process dependent on vascular endothelial growth factor-A (VEGF-A) and Kit ligand signaling. tPA improves the incorporation of CD11b+ cells into ischemic tissues and increases expression of neoangiogenesis-related genes, including VEGF-A. Remarkably, transplantation of BM-derived tPA-mobilized CD11b+ cells and VEGFR-1+ cells, but not carrier-mobilized cells or CD11b− cells, accelerates neovascularization and ischemic tissue regeneration. Inhibition of VEGF signaling suppresses tPA-induced neovascularization in a model of hind limb ischemia. Thus, tPA mobilizes CD11b+ cells from the BM and increases systemic and local (cellular) VEGF-A, which can locally promote angiogenesis during ischemic recovery. tPA might be useful to induce therapeutic revascularization in the growing field of regenerative medicine.
Introduction
The fibrinolytic system includes a broad spectrum of proteolytic enzymes with physiologic and pathophysiologic functions in several processes such as hemostatic balance, tissue remodeling, tumor invasion, reproduction, and angiogenesis.
The serine protease plasmin is responsible for the degradation of fibrin into soluble degradation products (fibrinolysis). Plasmin is generated through cleavage of the proenzyme plasminogen (Plg) by the urokinase plasminogen activator (uPA) or tissue-type plasminogen activator (tPA). tPA consists of a kringle- and trypsin-like serine protease domain.1 The activity of uPA and tPA is regulated by specific plasminogen activator inhibitors. In the absence of fibrin, tPA displays low activity toward Plg.2 In the presence of fibrin this activity is 2 orders of magnitude higher. The catalytic efficiency of tPA for activation of cell-bound Plg is approximately 10-fold higher than that in solution. Most cells bind Plg through its lysine binding sites with a high capacity but a relatively low affinity.3 Plg receptors such as the integrin αMβ2 play an important role in macrophage motility.4 CD11b/CD18 cells adhere to fibrin, but tPA by its ability to bind to CD11b, has been shown to induce local fibrinolysis and to render adherent cells into migrating cells.5
tPA has been shown to have numerous biologic functions. For example, within the central nervous system (reviewed by Melchor and Strickland6 ) tPA is expressed by neurons and microglial cells (resident macrophages of the brain and spinal cord), where it can generate plasmin to degrade a variety of nonfibrin substrates (eg, β-amyloid), can act as a direct protease without Plg involvement (eg, for the activation of latent platelet-derived growth factor-CC), or can function as a nonproteolytic modulator (eg, of the N-methyl-D-aspartate receptors).
Besides their fibrinolytic activities, plasmin and Plg activators are also implicated in tissue proliferation and cellular adhesion, because they can proteolytically degrade the extracellular matrix (ECM) and regulate the activation of both growth factors and matrix metalloproteinases (MMPs; for review, see Zorio et al7 ). PAs and plasmin generation in specific microenvironments in the bone marrow (BM) may be one of the factors orchestrating hematopoiesis.8,9 Plg activation promotes the release of Kit ligand from BM stromal cells.9,10 Plasmin can cleave thrombopoietin, the master cytokine of megakaryopoiesis and platelet production, thereby decreasing its biologic activity.11 Plasmin can further change the release or activation status of basic fibroblast growth factor, transforming growth factor β, and interleukin-1 β, which either directly or indirectly modulates myelopoiesis by releasing growth factors such as granulocyte colony-stimulating factor (G-CSF) or granulocyte macrophage CSF (GM-CSF), and macrophage CSF.12,13 Thus, paradoxically, plasmin generation can have both a permissive and an inhibitory effect on hematopoiesis.
A number of cell model studies have shown that MMP activation, notably activation of stromelysin-I, MMP-3, and MMP-9, can occur at the cell surface through the uPA/uPAR/Plg cascade for plasmin generation.14 We found increased soluble uPA receptor serum levels in samples from patients treated with G-CSF.15
Endothelial cell invasion is an essential event during angiogenesis, the formation of new blood vessels. This process involves the degradation of the ECM, the basement membrane, and the interstitial stroma and is governed by the activation of MMPs.16 During angiogenesis, endothelial cells stimulated by hypoxia and angiogenic factors start to proliferate and to migrate through the ECM. Endothelial cells are the main source of tPA in the blood circulation. tPA is released after injury or by brain endothelial cells on monocyte interaction.17 Recent studies emphasized a role for BM-derived CD45+ myeloid hematopoietic cells at ongoing angiogenic sites.18–20 Myeloid cells may locally secrete angiogenic factors or MMP-9, which may in turn increase vascular endothelial growth factor (VEGF-A) bioavailability.21 The beneficial effect of recombinant tPA on tissue regeneration in ischemic diseases has been attributed to its ability to degrade fibrin. Here, we report that tPA promotes neoangiogenesis during hind limb (HL) ischemia by mobilizing angiopotent CD45+CD11b+ myeloid cells coexpressing VEGF receptor-1 (VEGFR-1), a process dependent on VEGF signaling. These tPA-mobilized cells showed improved tissue incorporation into the ischemic niche, most probably because of their increased expression of chemokine receptors such as CXCR4 and CCR2, resulting in faster tissue regeneration. tPA not only increased the absolute number of CD11b+ myeloid cells but also enhanced their angiogenic performance, making them a good cellular target for cell-based treatment of ischemic diseases.
Methods
Animals
Plg+/+ and Plg−/− mice and MMP9+/+ and MMP9−/− mice were used after more than 10 back crosses onto a C57BL/6, or CD1 background, respectively.10 C57BL/6 mice were purchased from SLC. We used tPA+/+ and tPA−/− mice and C57BL/6 mice, expressing green fluorescent protein (GFP) under a β-actin promoter. Animal studies were approved by the Animal Review Board of Juntendo University, Tokyo.
Chimeric BM transplantation model
Plg+/+ or Plg−/− animals were lethally irradiated (900 cGy) and 6 hours later were transplanted with 2 × 107 BM-derived mononuclear cells from either Plg+/+ or Plg−/− animals.
Study design
Mutant tPA (Eizai; resuspended in 150 μL of 0.2% bovine serum albumin [BSA]; Sigma Chemical Co) was administered (10 mg/kg body weight) to mice with or without induction of HL ischemia by daily intraperitoneal injections from day 0 to day 2. Control mice received 150 μL of 0.2% BSA or in some experiments G-CSF (200 μg/kg body weight from day 0 to day 2; Kirin Pharma Company). Recombinant murine VEGF-A (PeproTech) was injected intraperitoneally (100 ng/mouse, daily from day 0 to day 2).
C57BL/6 mice were coinjected with tranexamic acid (Daiichi-Sankyo) and tPA intraperitoneally (day 0-2). C57BL/6 mice were treated with uPA (1 mg/kg body weight; Mitsubishi Tanabe Pharma) intraperitoneally from day 0 to day 2.
C57BL/6 mice were treated with a catalytically inactivated tPA (HTPA-ALA; Molecular Innovation) or rtPA (Eizai) at a concentration of 1.32 mg/kg from day 0 to day 2. C57BL/6 mice received daily injection of tPA (day 0-3) and were coinjected with batroxobin (20 BU/kg body weight intraperitoneally) from day −3 until day 5. Peripheral blood was harvested.
Fluorescence-activated cell sorting
Peripheral mononuclear cells (PBMCs) were stained with the following antibodies: CD45-Pacific Blue (clone 30-F11; BioLegend), CD11b-FITC or APC (clone M1/70; PharMingen), F4/80-FITC (clone BM8; BioLegend), CD184-FITC (clone 2B11/CXCR4; PharMingen), and VEGFR-1-PE (clone MF-1; ImClone Systems). Cells were analyzed with the use of a BD FACSAria (Becton Dickinson). CD11b cell populations were isolated, staining with CD45 and CD11b antibodies.
Reverse transcription PCR analysis
Total RNA was extracted with the use of RNA Trizol (Invitrogen). cDNA was generated from the following cell populations isolated from PBMCs of tPA- or BSA-PBS (phosphate-buffered saline)–treated mice: CD11b+ and CD11b− cells. cDNA was amplified by polymerase chain reaction (PCR) with the following specific forward and reverse primer pairs: for VEGF-A, 5′-AATGCTTTCTCCGCTCTGAA-3′ and 5′-CAGGCTGCTGTAACGATGAA-3′; for CXCR-4, 5′-CTTGACTGGCATAGTCGGCAATGGA-3′ and 5′-TGCTGGAATTGAAACACCACCATCC-3′; for CCR2, 5′-AGAGGTCTCGGTTGGGTTGT-3′ and 5′-ATCATAACGTTCTGGGCACC-3′; for GM-CSF, 5′-TAGCTGATAAGGGCCAGGAG-3′; for neuropilin-1, 5′-ACCAAGGAGACCACTGGAAAG-3′ and 3′-AAGAGAGGAAAAAAGGGGGCT-5′; for Tie-2, 5′-GGCTGATTCTTCGGAGATGT-3′ and 3′-GAAAGGCTTTTCCACCATC-5′; and for the β-actin control, 5′-TAAAACGCAGCTCAGTAACAGTCCG-3′ and 5′-TGGAATCCTGTGGCATCCATGAAAC-3′.
Murine ischemic HL mode
Mice were anesthetized with ketamine, 80 mg/kg body weight, and xylazine, 10 mg/kg body weight, given intraperitoneally for operative ligation of 1 femoral artery. Starting from the day of femoral ligation tPA was injected intraperitoneally daily for a period of 3 days. Control groups were similarly injected with 150 μL of 0.2% BSA. A laser Doppler perfusion image analyzer (Moor Instruments) recorded changes in blood flow postoperatively. Blood was collected by retro-orbital bleeding with the use of heparinized capillaries, and white blood cells (WBCs) were counted. Plasma samples were stored at −30°C. Mice were killed 15 or 28 days after resection of the femoral artery.
PBMC transplantation
Blood was collected from GFP mice with or without tPA treatment on days 0, 1, and 2. PBMCs isolated from 400 μL of blood were injected intravenously into HL ischemia–induced mice every day for a period of 3 days.
Isolation and transplantation of PB-derived CD11b+ and VEGFR-1+ cells
C57BL/6 male donor mice were treated with or without tPA from day 0 to day 2. Blood was collected daily from each mouse. Cells were labeled with anti-CD11b magnetic beads (Miltenyi Biotec) or biotinylated VEGFR-1 antibody (clone, MF-1; ImClone Systems), followed by avidin-Microbead isolation by magnetic-activated cell sorting (Miltenyi Biotec). Isolated CD11b+, CD11b− VEGFR-1+, and VEGFR-1− cells were greater than 90% pure by fluorescence-activated cell sorter (FACS) analysis. PB-derived VEGFR-1+ and VEGFR-1− cells (0.3 × 105 cells/injection/d) or CD11b+ and CD11b− cells (1.7 × 105 cells/injection/d) from male donor mice were transplanted daily intravenously into untreated HL ischemia–induced C57BL/6 female mice for a period of 3 days. HL ischemia and cell transplantation was started at the same time.
In vivo blocking experiments
HL ischemic mice, treated with or without rtPA (day 0-2; 10 mg/kg body weight in 150 μL of 0.2% BSA) were coinjected intraperitoneally with 800 μg of anti–mouse VEGFR-1 (clone MF-1), anti–mouse VEGFR-2 (clone DC101), or control immunoglobulin G (IgG) on days 0, 2, and 4. HL ischemic tPA-treated (day 0-2) and nontreated mice were coinjected with 500 μg of anti-CD11b antibody (clone 5C6) on days 0, 2, and 4 in the presence or absence of VEGFR-1 and VEGFR-2 antibodies (see above). tPA-treated (day 0-2) and nontreated mice were coinjected with 50 μg of anti–mouse VEGF-A (R&D Systems) on day 0, or with 125 μg of c-Kit (clone ACK; hybridoma kindly provided by S. Nishikawa, RIKEN, Kobe, Japan) on days 0 and 2. Appropriate isotype antibody controls were included.
ELISA
Plasma samples from tPA-treated and nontreated mice, with or without induction of HL ischemia, were assayed for murine VEGF-A by enzyme-linked immunoabsorbent assay (ELISA; R&D Systems).
In vitro culture supernatants of tPA-mobilized cells
PBMCs (2 × 105) isolated from tPA-treated and nontreated C57BL/6 mice at different time points were cultured in 200 μL of serum-free medium for 24 hours. Supernatant analysis of VEGF-A was performed with ELISA.
Immunohistochemistry
Ischemic muscle tissue samples were cryopreserved with Tissue-Tek OCT Compound (Sakura). Transverse-cut tissue samples of the whole leg were prepared, serum-blocked, and stained with the first overnight at 4°C. Serum-blocked sections were identified with the CD31 antibody (clone, MEC13.3; PharMingen) followed by biotin-conjugated goat anti–rat IgG (Vector) and Cy3-conjugated streptavidin (Alexa 594; Molecular Probes) staining. Myelo-monocytic cells were identified with the VEGFR-1 (clone MF-1; ImClone Systems) and CD11b antibody (clone M1/70; PharMingen) followed by biotin-conjugated goat anti–rat IgG (Vector) and FITC- or Cy3-conjugated streptavidin (Molecular Probes) staining. Nuclei were counterstained with DAPI (Molecular Probes). In addition, tissues were stained with antibodies against murine VEGF-A (clone A-20; Santa Cruz Biotechnology), followed by goat anti–rabbit IgG conjugated with Alexa 594 (Molecular Probes). Immunoreactions were visualized with the use of a Carl Zeiss fluorescence microscope. The capillary density (mean number of capillaries per mm2) of CD31-stained slides was determined as described.22 Ischemic area (mean size per mm2) of H&E-stained slides was determined with the use of computerized image analysis software (KS400; ×40 objective).
Statistical analyses
All data are presented as the mean plus or minus SEM. Intergroup comparisons were performed by paired Student t test or by analysis of variance. Probability values of P less than .05 were interpreted to denote statistical significance.
Results
tPA mobilizes CD11b+ cells into the circulation, a process dependent on plasmin and MMP-9–mediated release of Kit ligand and VEGF-A
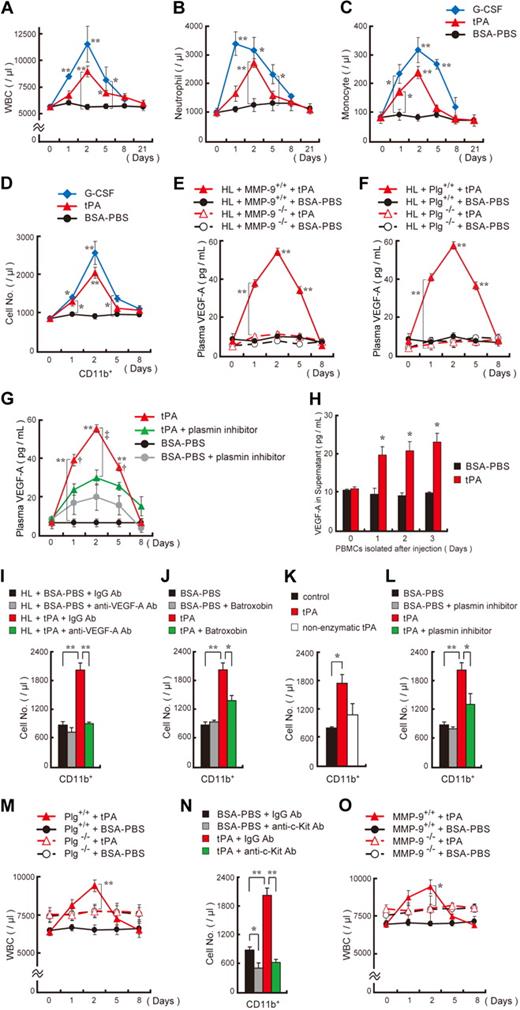
We observed that intraperitoneal or intravenous (data not shown) injection of an engineered, serpin-resistant form of tPA or uPA (data not shown) into C57BL/6 mice increased the number of WBCs (Figure 1A), including neutrophils and monocytes (Figure 1B-C). The absolute number of cells mobilized after tPA treatment was lower than after treatment with the cell-mobilizing cytokine G-CSF. Further study will be needed to understand whether both agents share a common downstream pathway during the mobilization process. No change after tPA administration was observed in the number of CD45− cells (data not shown). Within the CD45+ cell population, tPA augmented the number of CD45+CD11b+ cells (Figure 1D), which were identified as CD45+CD11bhigh neutrophils, CD45+CD11bmed/F4/80med monocytes, and CD45+CD11blow/F4/80− cells (supplemental Figure 1A, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Treatment of mice with anti-CD11b prevented tPA-induced myeloid CD11b+ cell mobilization (data not shown), supporting earlier studies that CD11b is required for monocyte/neutrophil migration through the endothelium.23 CD45+CD11b+ cells isolated from tPA-treated but not BSA-PBS–treated mice showed higher expression of the angiogenesis-associated genes VEGF-A, CD184 (also known as CXCR-4), GM-CSF, CC chemokine receptor 2 (CCR2; a monocyte chemotactic protein-1 receptor), and neuropilin-124 by reverse transcription–PCR (supplemental Figure 1B) and CD184, and VEGFR-1 and c-Kit by FACS analysis (supplemental Table 1). Tie-2 gene expression was only detected on the CD45+CD11b− cell fraction isolated from tPA-treated mice.25
tPA mobilizes hematopoietic myelomonocytic cells into the circulation, a process dependent on plasmin and MMP-9–mediated growth factor release. (A-D) C57BL/6 mice were injected intraperitoneally with recombinant tPA (n = 8 per group) or G-CSF (n = 3) from day 0 until day 2. The total number of WBCs (A), neutrophils (B), and monocytes (C) were counted. (D) FACS analysis of CD45+CD11b+ cells in peripheral blood of tPA-treated, G-CSF–treated, or BSA-PBS–treated mice (n = 3 per group). (E-G) Plasma VEGF-A levels were determined by ELISA in MMP-9−/− (E), Plg−/− (F) and wild-type control mice (n = 6) as well as in mice treated with tranexamic acid, a drug that impairs plasmin activation (n = 3), after tPA administration. Double asterisks indicate a significant difference between tPA-treated MMP-9 or Plg wild-type mice and their matching knockout controls. (H) PBMCs were isolated at the indicated time points from tPA-treated and untreated C57BL/6 mice. Cells were cultured under serum-free conditions for 24 hours. Supernatants were analyzed for VEGF-A by ELISA (n = 6). (I) tPA-treated and untreated C57BL/6 mice were coinjected with antibodies against murine VEGF-A or (J) cotreated with batroxobin intraperitoneally (fibrinogen-reducing agent). (K) Mice were treated with tPA, nonenzymatically active tPA, or vehicle control. (L) tPA- or carrier-injected C57BL/6 mice were coinjected with the plasmin inhibitor tranexamic acid. (I-L) The frequency of circulating CD45+CD11b+ cells was determined on day 2 (n = 3 per group). (M) Plg+/+ and Plg−/− mice received tPA intraperitoneally or BSA-PBS from day 0 to day 2. WBC counts were assessed (n = 5 per group; **P < .001; comparing Plg−/− to Plg+/+ group). (N) C57BL/6 mice were injected with tPA and anti–c-Kit or control antibodies. The frequency of circulating CD45+CD11b+ cells was measured on day 2 (n = 3 per group). (O) WBC counts were determined in MMP-9+/+ and MMP-9−/− mice after tPA administration (n = 4). The asterisk indicates a significant difference between tPA-treated MMP-9−/− and MMP-9+/+ mice. (I-L,N) Asterisks denote a significant difference between the indicated groups. (A-D) Asterisks indicate a significant difference between the BSA-PBS and tPA or G-CSF groups. Single asterisk, P < .05; double asterisk, P < .001. Values represent the mean ± SEM.
tPA mobilizes hematopoietic myelomonocytic cells into the circulation, a process dependent on plasmin and MMP-9–mediated growth factor release. (A-D) C57BL/6 mice were injected intraperitoneally with recombinant tPA (n = 8 per group) or G-CSF (n = 3) from day 0 until day 2. The total number of WBCs (A), neutrophils (B), and monocytes (C) were counted. (D) FACS analysis of CD45+CD11b+ cells in peripheral blood of tPA-treated, G-CSF–treated, or BSA-PBS–treated mice (n = 3 per group). (E-G) Plasma VEGF-A levels were determined by ELISA in MMP-9−/− (E), Plg−/− (F) and wild-type control mice (n = 6) as well as in mice treated with tranexamic acid, a drug that impairs plasmin activation (n = 3), after tPA administration. Double asterisks indicate a significant difference between tPA-treated MMP-9 or Plg wild-type mice and their matching knockout controls. (H) PBMCs were isolated at the indicated time points from tPA-treated and untreated C57BL/6 mice. Cells were cultured under serum-free conditions for 24 hours. Supernatants were analyzed for VEGF-A by ELISA (n = 6). (I) tPA-treated and untreated C57BL/6 mice were coinjected with antibodies against murine VEGF-A or (J) cotreated with batroxobin intraperitoneally (fibrinogen-reducing agent). (K) Mice were treated with tPA, nonenzymatically active tPA, or vehicle control. (L) tPA- or carrier-injected C57BL/6 mice were coinjected with the plasmin inhibitor tranexamic acid. (I-L) The frequency of circulating CD45+CD11b+ cells was determined on day 2 (n = 3 per group). (M) Plg+/+ and Plg−/− mice received tPA intraperitoneally or BSA-PBS from day 0 to day 2. WBC counts were assessed (n = 5 per group; **P < .001; comparing Plg−/− to Plg+/+ group). (N) C57BL/6 mice were injected with tPA and anti–c-Kit or control antibodies. The frequency of circulating CD45+CD11b+ cells was measured on day 2 (n = 3 per group). (O) WBC counts were determined in MMP-9+/+ and MMP-9−/− mice after tPA administration (n = 4). The asterisk indicates a significant difference between tPA-treated MMP-9−/− and MMP-9+/+ mice. (I-L,N) Asterisks denote a significant difference between the indicated groups. (A-D) Asterisks indicate a significant difference between the BSA-PBS and tPA or G-CSF groups. Single asterisk, P < .05; double asterisk, P < .001. Values represent the mean ± SEM.
The angiogenic factor VEGF can be liberated from ECM stores after plasmin or MMP activation.16,26,27 We found that tPA administration augmented plasma VEGF-A levels in wild-type but not in MMP−/− and Plg−/− mice or in mice treated with tranexamic acid, a drug impairing tPA-mediated plasmin activation (Figure 1E-G). Western blotting confirmed that plasma samples of tPA-treated animals expressed 44-kDa biologically active VEGF-A (data not shown). PBMCs isolated from tPA-treated animals at the indicated time points released VEGF-A into culture supernatants (Figure 1H). Anti-VEGF treatment prevented tPA-mediated CD11b+ myeloid cell mobilization (Figure 1I), which is in accordance with previous studies showing that VEGF-A can mobilize or promote migration of hematopoietic VEGFR-1+, endothelial VEGFR-2+28,29 and macrophages.30
We reported that tPA administration increases the number of BM cells, a process partially dependent on KitL signaling.10 To address the question of whether tPA-induced VEGF-A has an effect on BM cell expansion, we coinjected tPA-treated C57BL/6 mice with or without neutralizing antibodies against murine VEGF-A. Blocking VEGF-A signaling with neutralizing antibodies against murine VEGF-A did indeed prevent tPA-mediated BM cell expansion in vivo (data not shown).
CD11b/CD18 (αMβ2, Mac-1) is a receptor for fibrin.31 tPA can induce cell migration by binding to CD11b and degrading fibrin.5 We showed increased fibrinogen staining in BM cells in a model of stress-hematopoiesis, indicating that fibrinogen might play a role in hematopoietic cell retention and mobilization.10 Batroxobin, a drug that reduces circulating fibrinogen, prevented the tPA-mediated WBC (data not shown) and CD11b+ cell increase (Figure 1J). However, further studies will be necessary to fully understand the mechanism by which fibrinogen regulates cell mobilization. So does tPA-mediated cell mobilization depend on plasmin activation? Catalytically inactive tPA did not increase WBC (data not shown) and CD11b+ cell counts (Figure 1K). Similarly, a drug impairing tPA-mediated plasmin activation (Figure 1L) or genetic ablation of Plg (Figure 1M) blocked the tPA-induced WBC (data not shown) and CD11b+ cell increase. We noticed elevated baseline WBC counts in Plg−/− mice compared with Plg+/+ mice, but thedifference was not statistically significant. These data indicate that plasmin activation is required for cell mobilization.
KitL promotes hematopoietic cell survival, proliferation, and mobilization.32 tPA administration can promote myeloid cell expansion by MMP-9–mediated release of KitL from stromal/niche cells.10 Indeed, antibodies against c-Kit blocked tPA-mediated CD11b+ cell mobilization (Figure 1N), and tPA-mediated cell mobilization did not occur in MMP−/− mice (Figure 1O). These data indicate that c-Kit signaling is required for tPA-mediated cell mobilization.
tPA administration accelerates ischemic revascularization
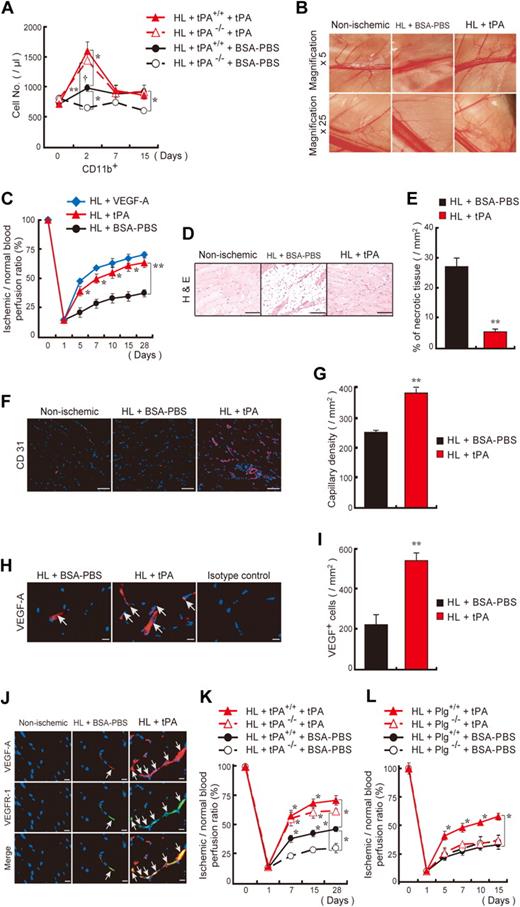
tPA is secreted after vascular injury. We exploited a HL ischemic model to show that tPA was required for CD11b+ myeloid cell mobilization, neoangiogenesis, and ischemic muscle tissue regeneration. Even though tPA−/− mice showed normal CD11b+ cell counts at baseline, kinetic studies showed enhanced numbers of circulating CD11b+ cells in tPA+/+ but not in tPA−/− mice during the natural course of HL ischemic recovery (Figure 2A). Administration of tPA restored CD11b+ cell mobilization in HL ischemic tPA−/− mice similar to wild-type levels. Next, we studied whether tPA accelerates HL ischemic recovery. Faster collateral vessel growth (Figure 2B) and blood flow recovery (Figure 2C) were seen in the ischemic limb of tPA-treated than in BSA-PBS–treated mice. Ischemic tissue sections from tPA-treated wild-type mice showed less fatty degeneration (Figure 2D), smaller areas of necrotic tissue (Figure 2E), and higher capillary density (Figure 2F-G) than controls. tPA augmented the number of ischemic muscle-residing VEGF-A+ cells coexpressing VEGFR-1 (Figure 2H-J) in HL ischemia–induced mice compared with controls. tPA improved blood flow recovery and increased capillary density (data not shown) in tPA−/− mice (Figure 2K) but was ineffective in Plg−/− (Figure 2L) or MMP-9−/− mice (data not shown). In summary these data showed that endogenous tPA plays a role during the natural course of ischemic recovery, which coincided with impaired CD11b+ cell mobilization. In addition, exogenous tPA administration accelerates ischemic tissue regeneration and neoangiogenesis.
tPA administration accelerates revascularization in a HL ischemia model. (A) tPA or vehicle was injected intraperitoneally daily from day 0 to day 2 into tPA+/+ and tPA−/− mice after HL ischemia induction. The frequency of circulating CD45+CD11b+ cells was measured (n = 3 per group). The single dagger (†) symbol denotes a significant difference (P < .05) between day 0 and day 2 in HL tPA+/+ mice. (B) C57BL/6 mice were treated with tPA or vehicle. Macroscopic pictures of the lower limb region were taken on day 15 after HL induction. Images were acquired with 0.5× and 2.5×/0.1 numeric aperture (NA) lenses (corresponding to 5× and 25× magnification), an Olympus SZX7 camera, and Adobe Photoshop CS3. (C) The ischemic/normal blood flow ratio, determined by analysis of the whole cross-section of the limb by laser Doppler perfusion imaging analysis, is shown as a function of time. (D-G) Evaluation of necrotic areas (D-E, after 15 days; bar = 200 μm) and capillary density in sections (F-G, after 28 days; bar = 50 μm) of HL-induced mice treated with or without tPA (n = 6 per group). Panels D and F were acquired with a 20×/0.50 NA and a Carl Zeiss KS400 camera, and analyzed using Axio Vision (Carl Zeiss). (H-I) Quantification of VEGF-A+ cells (arrows) in ischemic tissues (n = 6 per group, after 15 days; bar = 20 μm). Images were acquired with a 40×/0.75 NA and a Carl Zeiss KS400 camera, and analyzed using Axio Vision (Carl Zeiss). (J) Immunohistochemical staining of VEGFR-1 and VEGF-A of ischemic muscle tissues derived from vehicle- or tPA-treated HL ischemic mice and nonischemic mice. Arrows indicate VEGFR-1+VEGF-A+ cells (bar = 20 μm). Images were acquired with a 40×/0.75 NA and a Carl Zeiss KS400 camera, and analyzed using Axio Vision (Carl Zeiss). (K-L) tPA or vehicle was injected intraperitoneally daily into tPA (K) or Plg (L) wild-type and deficient mice after induction of HL ischemia. Functional perfusion measurements of the collateral region were performed (n = 3 per group). A significant difference between tPA+/+ and tPA−/− mice treated with BSA-PBS is shown. If not otherwise mentioned, asterisks denote significant differences between BSA-PBS– and tPA-treated groups. *P < .05; **P < .001; values represent the mean ± SEM.
tPA administration accelerates revascularization in a HL ischemia model. (A) tPA or vehicle was injected intraperitoneally daily from day 0 to day 2 into tPA+/+ and tPA−/− mice after HL ischemia induction. The frequency of circulating CD45+CD11b+ cells was measured (n = 3 per group). The single dagger (†) symbol denotes a significant difference (P < .05) between day 0 and day 2 in HL tPA+/+ mice. (B) C57BL/6 mice were treated with tPA or vehicle. Macroscopic pictures of the lower limb region were taken on day 15 after HL induction. Images were acquired with 0.5× and 2.5×/0.1 numeric aperture (NA) lenses (corresponding to 5× and 25× magnification), an Olympus SZX7 camera, and Adobe Photoshop CS3. (C) The ischemic/normal blood flow ratio, determined by analysis of the whole cross-section of the limb by laser Doppler perfusion imaging analysis, is shown as a function of time. (D-G) Evaluation of necrotic areas (D-E, after 15 days; bar = 200 μm) and capillary density in sections (F-G, after 28 days; bar = 50 μm) of HL-induced mice treated with or without tPA (n = 6 per group). Panels D and F were acquired with a 20×/0.50 NA and a Carl Zeiss KS400 camera, and analyzed using Axio Vision (Carl Zeiss). (H-I) Quantification of VEGF-A+ cells (arrows) in ischemic tissues (n = 6 per group, after 15 days; bar = 20 μm). Images were acquired with a 40×/0.75 NA and a Carl Zeiss KS400 camera, and analyzed using Axio Vision (Carl Zeiss). (J) Immunohistochemical staining of VEGFR-1 and VEGF-A of ischemic muscle tissues derived from vehicle- or tPA-treated HL ischemic mice and nonischemic mice. Arrows indicate VEGFR-1+VEGF-A+ cells (bar = 20 μm). Images were acquired with a 40×/0.75 NA and a Carl Zeiss KS400 camera, and analyzed using Axio Vision (Carl Zeiss). (K-L) tPA or vehicle was injected intraperitoneally daily into tPA (K) or Plg (L) wild-type and deficient mice after induction of HL ischemia. Functional perfusion measurements of the collateral region were performed (n = 3 per group). A significant difference between tPA+/+ and tPA−/− mice treated with BSA-PBS is shown. If not otherwise mentioned, asterisks denote significant differences between BSA-PBS– and tPA-treated groups. *P < .05; **P < .001; values represent the mean ± SEM.
Ex vivo tPA-treated CD11b+ and VEGFR-1+ cells improve neoangiogenesis
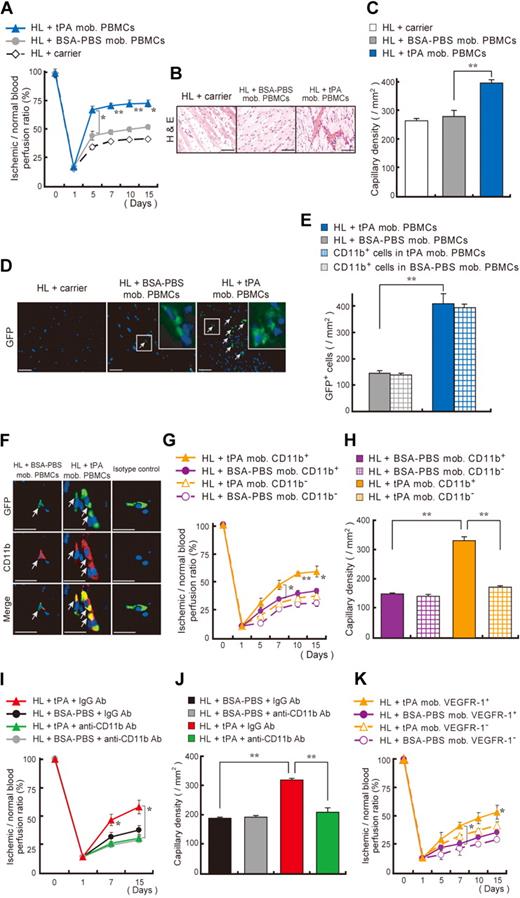
To determine the proangiogenic potential of tPA-mobilized cells, we isolated PBMCs from GFP donor mice receiving tPA or vehicle and transplanted them daily into HL ischemic recipients. Mice that received a transplant with tPA-mobilized PBMCs showed faster blood flow recovery (Figure 3A), smaller areas of fatty degeneration (Figure 3B), and increased capillary density (Figure 3C) compared with mice injected with BSA-PBS–stimulated PBMCs. Nearly all donor-derived GFP+ cells coexpressed CD11b (Figure 3D-F) and were detectable in ischemic muscle tissues of mice injected with tPA-mobilized PBMCs but not in tissues of mice injected with control cells.
Transplantation of tPA-treated CD11b+ and VEGFR-1+ myeloid cells improve neoangiogenesis. (A-F) tPA- or BSA-PBS–mobilized PBMCs from GFP mice or carrier (no cells) were injected intravenously into HL ischemia–induced recipients. (A) Blood flow was determined. (B-F) Ischemic tissues of lower limbs isolated 15 days after injection and HL ischemia induction were stained with (B) H&E and (C) antibodies against CD31 for capillary density evaluation (n = 3 per group). Images were acquired with 20×/0.50 NA and a Carl Zeiss KS400 camera, and analyzed using Axio Vision (Carl Zeiss). (D-E) Within ischemic muscle tissues GFP+ donor cells (arrows) were quantified. Inserts depict a magnified view. Images were acquired with a 20×/0.50 NA and a 63×/1.3 oil objective using a Carl Zeiss KS400 camera. (E) Quantification of the number of GFP+ cells and GFP+ cells coexpressing CD11b in ischemic tissues (n = 8 per group). (F) CD11b (Mac-1) staining of lower limb ischemic tissue from mice receiving carrier injections, from mice that received a transplant with PBMCs, or from BSA-PBS–treated or tPA-treated donor GFP mice. Arrows indicate transplanted GFP+CD11b+ cells in ischemic tissues (bar = 20 μm). Images were acquired with 40×/0.75 NA and a Carl Zeiss KS400 camera, and analyzed using Axio Vision (Carl Zeiss). (G-H) PB-derived CD11b+ or CD11b− cells isolated from tPA- or BSA-PBS–treated donors were transplanted into HL ischemia–induced recipients for 3 days (n = 4 per group). (G) Blood flow was determined. (H) Capillary density was evaluated. (I-J) HL ischemic C57BL/6 mice were treated with tPA and coinjected with anti-CD11b or control monoclonal antibodies. (I) Blood flow was analyzed by laser Doppler analysis. (J) Capillary density was evaluated. (K) PB-derived VEGFR-1+ or VEGFR-1− cells, isolated from tPA- or BSA-PBS–treated donors, were injected into HL ischemia–induced recipients, and blood flow was examined (n = 4 per group). Single asterisk (P < .05) and double asterisk (P < .001) indicate a significant difference between the groups. Values represent mean ± SEM.
Transplantation of tPA-treated CD11b+ and VEGFR-1+ myeloid cells improve neoangiogenesis. (A-F) tPA- or BSA-PBS–mobilized PBMCs from GFP mice or carrier (no cells) were injected intravenously into HL ischemia–induced recipients. (A) Blood flow was determined. (B-F) Ischemic tissues of lower limbs isolated 15 days after injection and HL ischemia induction were stained with (B) H&E and (C) antibodies against CD31 for capillary density evaluation (n = 3 per group). Images were acquired with 20×/0.50 NA and a Carl Zeiss KS400 camera, and analyzed using Axio Vision (Carl Zeiss). (D-E) Within ischemic muscle tissues GFP+ donor cells (arrows) were quantified. Inserts depict a magnified view. Images were acquired with a 20×/0.50 NA and a 63×/1.3 oil objective using a Carl Zeiss KS400 camera. (E) Quantification of the number of GFP+ cells and GFP+ cells coexpressing CD11b in ischemic tissues (n = 8 per group). (F) CD11b (Mac-1) staining of lower limb ischemic tissue from mice receiving carrier injections, from mice that received a transplant with PBMCs, or from BSA-PBS–treated or tPA-treated donor GFP mice. Arrows indicate transplanted GFP+CD11b+ cells in ischemic tissues (bar = 20 μm). Images were acquired with 40×/0.75 NA and a Carl Zeiss KS400 camera, and analyzed using Axio Vision (Carl Zeiss). (G-H) PB-derived CD11b+ or CD11b− cells isolated from tPA- or BSA-PBS–treated donors were transplanted into HL ischemia–induced recipients for 3 days (n = 4 per group). (G) Blood flow was determined. (H) Capillary density was evaluated. (I-J) HL ischemic C57BL/6 mice were treated with tPA and coinjected with anti-CD11b or control monoclonal antibodies. (I) Blood flow was analyzed by laser Doppler analysis. (J) Capillary density was evaluated. (K) PB-derived VEGFR-1+ or VEGFR-1− cells, isolated from tPA- or BSA-PBS–treated donors, were injected into HL ischemia–induced recipients, and blood flow was examined (n = 4 per group). Single asterisk (P < .05) and double asterisk (P < .001) indicate a significant difference between the groups. Values represent mean ± SEM.
To examine whether CD11b+ cells, known to enhance both normal and malignant neoangiogenesis,33 were the effector cells for tPA-driven neoangiogenesis, we transplanted equal numbers of tPA-mobilized CD11b+ cells into HL recipient mice. tPA-mobilized but not BSA-PBS–mobilized CD11b+ cells accelerated ischemic reperfusion (Figure 3G) and increased capillary density in ischemic tissues of HL ischemic recipients (Figure 3H). Antibodies against CD11b prevented tPA-mediated HL ischemic tissue recovery and blocked tPA-driven vessel formation (Figure 3I-J). tPA not only augmented the absolute number of circulating CD11b+ cells (supplemental Table 2), but tPA-stimulated cells on a cellular basis also improved tissue regeneration better than BSA-PBS–stimulated CD11b+ cells. Because approximately 50% of CD11b+ cells coexpressed VEGFR-1 (supplemental Table 1), and the frequency of CXCR4+VEGFR-1+ cells/hemangiocytes was augmented after tPA therapy compared with controls (1.08% ± 0% versus 0.195% ± 0.005%, respectively; n = 3 per group; P < .05), we examined the effect of tPA on VEGFR-1–driven neoangiogenesis. Transplanted tPA-stimulated VEGFR-1+ cells improved tissue ischemic regeneration (Figure 3K). Ischemic tissue revascularization was achieved with a 5-fold lower numbers of tPA-stimulated VEGFR-1+ cells than tPA-stimulated CD11b+ cells. These data suggest that tPA-mediated neoangiogenesis is driven by a CD11b+VEGFR-1+ cell population.
Inhibition of VEGF-A signaling prevents tPA-mediated cell mobilization and tissue neoangiogenesis
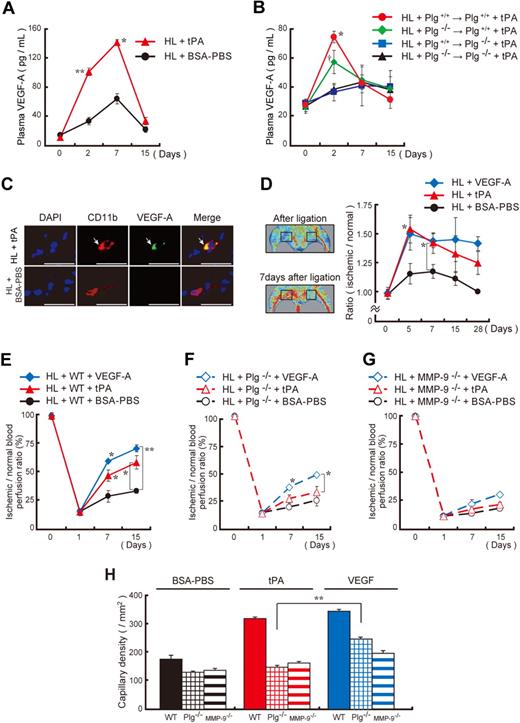
Plasmin can release VEGF-A from ECM stores.26 tPA administration in HL ischemic animals resulted in VEGF-A plasma elevation (Figure 4A). We performed reciprocal transplants of wild-type hematopoietic cells into lethally irradiated Plg−/− mice or vice versa (Plg+/+ BM cell transplantation into Plg−/− recipients or Plg−/− BM cell transplantation into Plg+/+ recipients) followed by induction of HL ischemia. We show that tPA treatment increased plasma VEGF-A levels in HL Plg wild-type mice with or without transplantation of Plg wild-type BM cells (Figure 4B). Plasma VEGF-A levels were impaired in HL ischemic Plg-deficient recipient mice. These data implied that plasmin activation within the nonhematopoietic environment was required for tPA-mediated systemic VEGF-A elevation (see also Figure 1E-G). Within ischemic tissues, we found VEGF-A staining mainly in CD11b+ cell by immunohistochemistry (Figure 4C). VEGF-A administration partially rescued impaired angiogenesis observed in Plg−/− HL ischemic mice, as reported,34 but did not in MMP-9−/−mice (Figure 4D-H).
Administration of recombinant VEGF-A partially rescues the angiogenic defect observed in Plg- and MMP-9–deficient mice. (A-B) Plasma VEGF-A levels were analyzed in tPA-treated and untreated HL-induced C57BL/6 (A) and chimeric (B) mice (n = 3 per group). Chimerism was achieved as follows: Lethally irradiated Plg+/+ or Plg−/− mice received a transplant with Plg+/+ or Plg−/− donor BM cells. Six weeks later, HL ischemia–induced chimeric mice were treated intraperitoneally with/without tPA. Asterisks and single dagger indicate a significant difference between tPA-treated and BSA-PBS–treated groups. (C) Immunohistochemical staining for CD11b and VEGF-A of ischemic muscle tissue derived from tPA-treated and vehicle-treated HL ischemic mice. Arrows indicate CD11b+ VEGF-A+ cells (bar = 25 μm). Images were acquired with 40×/0.75 NA and a Carl Zeiss KS400 camera, and analyzed using Axio Vision (Carl Zeiss). (D-H) HL-induced wild-type control (D-E,H), Plg−/− (F,H), and MMP-9−/− (G,H) mice were injected intraperitoneally daily with tPA, mVEGF-A, or BSA-PBS from day 0 until day 2. (D) Functional perfusion measurements of the collateral region with the use of a laser scan at the indicated time points in wild-type animals. Representative laser Doppler perfusion images are shown of the regions analyzed. Blood perfusion (red to yellow) was augmented in tPA-treated mice compared with vehicle-injected controls (green to blue = reduced perfusion signal). (E-G) Blood flow recovery was determined by laser Doppler perfusion imaging in the ischemic limbs (n = 3; *P < .05, **P < .001). (H) Capillary density was evaluated on CD31-stained sections 15 days after ligation (n = 3; **P < .001). Asterisks indicate a significant difference between the indicated groups. Error bars represent SEM.
Administration of recombinant VEGF-A partially rescues the angiogenic defect observed in Plg- and MMP-9–deficient mice. (A-B) Plasma VEGF-A levels were analyzed in tPA-treated and untreated HL-induced C57BL/6 (A) and chimeric (B) mice (n = 3 per group). Chimerism was achieved as follows: Lethally irradiated Plg+/+ or Plg−/− mice received a transplant with Plg+/+ or Plg−/− donor BM cells. Six weeks later, HL ischemia–induced chimeric mice were treated intraperitoneally with/without tPA. Asterisks and single dagger indicate a significant difference between tPA-treated and BSA-PBS–treated groups. (C) Immunohistochemical staining for CD11b and VEGF-A of ischemic muscle tissue derived from tPA-treated and vehicle-treated HL ischemic mice. Arrows indicate CD11b+ VEGF-A+ cells (bar = 25 μm). Images were acquired with 40×/0.75 NA and a Carl Zeiss KS400 camera, and analyzed using Axio Vision (Carl Zeiss). (D-H) HL-induced wild-type control (D-E,H), Plg−/− (F,H), and MMP-9−/− (G,H) mice were injected intraperitoneally daily with tPA, mVEGF-A, or BSA-PBS from day 0 until day 2. (D) Functional perfusion measurements of the collateral region with the use of a laser scan at the indicated time points in wild-type animals. Representative laser Doppler perfusion images are shown of the regions analyzed. Blood perfusion (red to yellow) was augmented in tPA-treated mice compared with vehicle-injected controls (green to blue = reduced perfusion signal). (E-G) Blood flow recovery was determined by laser Doppler perfusion imaging in the ischemic limbs (n = 3; *P < .05, **P < .001). (H) Capillary density was evaluated on CD31-stained sections 15 days after ligation (n = 3; **P < .001). Asterisks indicate a significant difference between the indicated groups. Error bars represent SEM.
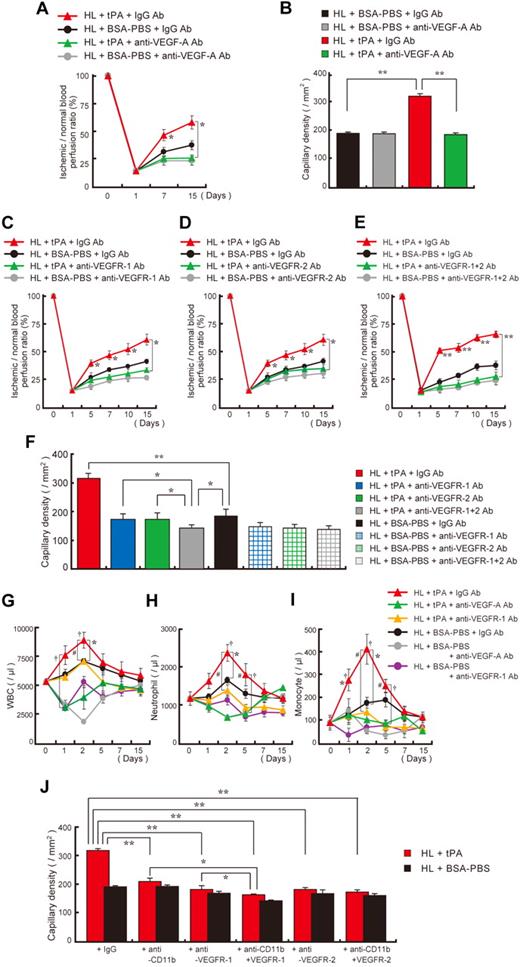
But is VEGF-A signaling required for tPA-mediated tissue neoangiogenesis? Blockade of VEGF signaling with antibodies against murine VEGF-A (Figure 5A-B) or VEGF (Figure 5C-F) receptors inhibited tPA-mediated ischemic tissue recovery and neoangiogenesis as well as myeloid cell mobilization by VEGFR-1 (Figure 5G-I) in an HL ischemic model. Similarly, tPA did not improve neoangiogenesis in myeloid cell–depleted mice (Figure 5J). Coinjection of anti–VEGFR-1 antibodies into myeloid cell–depleted mice further reduced neoangiogenesis both in BSA-PBS– and tPA-treated mice. These data indicate that CD11b+ hematopoietic cells are the main “angiogenic effector cells” for tPA-mediated cell-driven neovascularization. However, it is possible that some non-CD11b/VEGFR-1+ cells (eg, endothelial cells) might contribute to the tPA-induced angiogenic response.
Inhibition of VEGF signaling prevented tPA-mediated ischemic tissue recovery. (A-B) C57BL/6 mice were treated with tPA and coinjected with anti–VEGF-A or control monoclonal antibodies. (A) Ischemic recovery was quantified by laser Doppler (n = 3 per group). Asterisks indicate a significant difference between tPA-treated mice coinjected with IgG or anti–VEGF-A antibody. (B) Capillary density was quantified 15 days after ligation (n = 3 per group). HL ischemic mice treated with or without tPA were coinjected intraperitoneally with neutralizing doses of VEGFR-1 (C), VEGFR-2 (D), a combination of VEGFR-1 and VEGFR-2 (E), or IgG antibodies at 2-day intervals, starting from day 0, and functional perfusion measurements of the collateral region were performed in each group with the use of a laser scan (n = 3; *P < .05,**P < .001). (F) Quantification of the capillary density in tissue sections taken 15 days after artery ligation by CD31 staining of sections from HL-induced mice treated with or without tPA (n = 3; *P < .05; **P < .001 versus vehicle). (G-I) Blood cell counts were determined at the indicated time points. (G) WBC, (H) neutrophil, and (I) monocyte counts (*P < .05 comparison of the tPA plus IgG-treated HL group with the BSA-PBS plus IgG-treated group; †P < .05 comparison of the tPA plus IgG-treated HL group with the tPA plus anti–VEGF-A group; #P < .05 comparison of the tPA plus IgG-treated HL group with the tPA plus anti–VEGFR-1 group). Error bars represent SEM. (J) HL-induced C57BL/6 mice treated with tPA or BSA-PBS and antibodies against CD11b were cotreated with antibodies against VEGFR-1 or VEGFR-2. Capillary density was evaluated (n = 3 per group). If not otherwise indicated, a single asterisk (P < .05) and double asterisk (P < .001) indicate a significant difference between groups. Values represent the mean ± SEM.
Inhibition of VEGF signaling prevented tPA-mediated ischemic tissue recovery. (A-B) C57BL/6 mice were treated with tPA and coinjected with anti–VEGF-A or control monoclonal antibodies. (A) Ischemic recovery was quantified by laser Doppler (n = 3 per group). Asterisks indicate a significant difference between tPA-treated mice coinjected with IgG or anti–VEGF-A antibody. (B) Capillary density was quantified 15 days after ligation (n = 3 per group). HL ischemic mice treated with or without tPA were coinjected intraperitoneally with neutralizing doses of VEGFR-1 (C), VEGFR-2 (D), a combination of VEGFR-1 and VEGFR-2 (E), or IgG antibodies at 2-day intervals, starting from day 0, and functional perfusion measurements of the collateral region were performed in each group with the use of a laser scan (n = 3; *P < .05,**P < .001). (F) Quantification of the capillary density in tissue sections taken 15 days after artery ligation by CD31 staining of sections from HL-induced mice treated with or without tPA (n = 3; *P < .05; **P < .001 versus vehicle). (G-I) Blood cell counts were determined at the indicated time points. (G) WBC, (H) neutrophil, and (I) monocyte counts (*P < .05 comparison of the tPA plus IgG-treated HL group with the BSA-PBS plus IgG-treated group; †P < .05 comparison of the tPA plus IgG-treated HL group with the tPA plus anti–VEGF-A group; #P < .05 comparison of the tPA plus IgG-treated HL group with the tPA plus anti–VEGFR-1 group). Error bars represent SEM. (J) HL-induced C57BL/6 mice treated with tPA or BSA-PBS and antibodies against CD11b were cotreated with antibodies against VEGFR-1 or VEGFR-2. Capillary density was evaluated (n = 3 per group). If not otherwise indicated, a single asterisk (P < .05) and double asterisk (P < .001) indicate a significant difference between groups. Values represent the mean ± SEM.
Discussion
There is convincing evidence that a small number of angiogenic cells are present in the blood of most mammals, including humans. In the present study, we demonstrate that plasmin activation through administration of serpin-resistant mutant tPA alone was sufficient to mobilize BM-derived hematopoietic, angiopotent cells into the circulation. Our findings support a mechanism whereby tPA-generated plasmin mobilizes myeloid cells through the release of VEGF-A and KitL, processes that are dependent on MMP-9 activation and, possibly, on that of other MMPs.
The number of circulating cells increasingly used for autologous and allogeneic transplants and tissue regeneration can be significantly improved with cytokines such as G-CSF.22 Stress-induced hematopoietic cell egress from the BM is a multistep process, involving activities of cytokines and chemokines, as well as proteolytic enzymes. Proliferation and/or differentiation in the BM usually precedes enhanced hematopoietic cell egress.35 We recently demonstrated that tPA alone had no effect on myeloid precursor cell expansion in vitro, but increased BM cell numbers in vivo/in the presence of stromal cells a process partially dependent on c-Kit/KitL signaling.10 Here, we report that the tPA-mediated increase in BM cells (myeloid cells) also requires VEGF-A signaling, because neutralizing antibodies against VEGF-A prevented tPA-mediated BM cell (including CD11b+) cell expansion (data not shown). VEGF-A is a principal regulator of blood vessel formation and hematopoiesis and is capable of mobilizing hematopoietic progenitors and differentiated myeloid cells.28,36 In addition, the c-Kit/KitL signaling pathway is also implicated in cell migration/mobilization.37 Here, we demonstrate that tPA administration increases circulating proangiogenic CD45+CD11b+ cells coexpressing both VEGFR-1 and c-Kit, a process which could be blocked with the use of antibodies against VEGF-A and c-Kit. Interestingly, intraventricular leukocytosis has been observed in rats with intraventricular hemorrhage/hematoma that were treated with high doses of tPA.38 Streptokinase administration in patients with acute myocardial infarction caused a marked increase in circulating WBCs.39
MMP-9 has been shown to be required for macrophage migration and is induced in premetastatic lung endothelial and BM cells, including macrophages after VEGF-A treatment involving VEGFR-1.29,40 Here, we report that tPA-mediated CD11b+ cell mobilization requires the presence of MMP-9. MMP-9, which is produced in the BM, eg, after treatment with, eg, G-CSF,37 is able to degrade occludin, one of the components that comprise the endothelial tight junctions.41 Monocytes can induce tPA release in brain endothelial cells, which subsequently activates extracellular signal–regulated kinase 1/2. Both tPA and extracellular signal–regulated kinase 1/2 have been shown to control the breakdown of the tight junction protein occludin.17 Determination of the extent to which the integral plasma membrane protein occludin mediates some of the tPA-induced myeloid cell mobilization by modulating the BM endothelial barrier awaits further studies.
The mechanism by which tPA stimulates angiogenesis is unclear. The angiogenic effect of tPA was seen as early as 5 to 7 days after surgery. These data suggest that the kinetics of angiogenic cell mobilization by tPA may mirror that observed for hematopoietic cell mobilization peaking on day 2. Here, we demonstrate that the angiogenic effect of tPA can be adoptively transferred by blood mononuclear cells. Of interest, tPA administration increased not only the absolute number of these angiopotent cells, but also more importantly tPA-stimulated cells showed qualitatively improved angiogenic performance/potential compared with BSA-PBS–treated controls in an HL ischemic murine model. This is in contrast to the reported proangiogenic effects of other mobilizing agents such as G-CSF, which cause an increase in the basal numbers of circulating mononuclear cells rather than inducing the mobilization of a unique angiogenic cell population.42 Within the mobilized cell population, we identified a tPA-responsive, hematopoietic CD45+CD11b+VEGFR-1+, angiopotent and readily transplantable cell population, which may serve as a basis for tPA therapy in patients with ischemia. tPA-mobilized CD45+CD11b+ cells also showed higher gene expression of neuropilin-1, but not Tie-2, which has been shown to indicate a more angiogenic/arteriogenic cell population within the mononuclear cell fraction.24,25 Monocytes are key contributors to angiogenesis at sites of ischemic injury because they can secrete proangiogenic cytokines, including VEGF-A, and basic fibroblast growth factor.43
VEGF-A is a critical survival factor for vascular endothelium.44 The role of plasmin in the ischemic, proteolytic microenvironment can function as a determinant controlling VEGF-A–mediated activities. VEGF189 or VEGF206, proteins bound to extracellular heparin-containing proteoglycans, can be released by plasmin, and the released 34-kDa dimeric species can function as endothelial cell mitogens.26 However, recent data have shown that the angiogenic potential of matrix-associated VEGF-A isoforms is superior to soluble isoforms.45 We showed that tPA administration results in systemic VEGF-A plasma elevation, which required plasmin activation, because genetic ablation of Plg and pharmacologic inhibition of plasmin activation reduced systemic VEGF-A elevation. We showed that the extent of revascularization after tPA administration correlates with absolute plasma VEGF-A levels and increased numbers of tissue-residing CD11b+ cells coexpressing VEGF-A. Neutralizing antibodies against VEGF receptors or VEGF-A in vivo abolished tPA-mediated tissue regeneration.
Proteolysis of fibrin matrices by endothelial cells plays essential roles in the migratory and morphogenic differentiation processes underlying angiogenesis. Because fibrin is part of the provisional matrix needed to support vascular cell responses for repair, the binding of growth factors and cytokines, such as VEGF-A, will localize them to the site of injury so that they can enhance the cellular responses needed for angiogenesis.46
Thus, activation of the fibrinolytic cascade promotes both mobilization of hematopoietic cells and their incorporation into the ischemic tissue. This hematopoietic cell recruitment is a critical step in regulating the supply of local and systemic angiogenic factors, which finally accelerates ischemic revascularization. Because VEGF-A also increases the expression and activity of PAs, especially tPA in endothelial cells,47 based on our data, we propose that tPA production in endothelial cells is required for the recruitment of hematopoietic cells to neoangiogenic sites, including peripheral ischemic sites. These data are consistent with our findings that disruption of the tPA gene reduced the number of CD11b+ cells during the natural course of HL ischemic recovery and with data of other groups showing impaired macrophage migration in tPA−/− mice during peritonitis and sciatic nerve injury.48,49 Macrophage migration during inflammation has been shown to depend on CD11b/Mac-1 recognition of a binary complex consisting of fibrin within the provisional matrix and tPA.5 Cao et al5 elegantly demonstrated that subsequent neutralization of tPA by its inhibitor plasminogen activator inhibitor-1 enhanced binding of the integrin-protease-inhibitor complex to the endocytic receptor lipoprotein receptor–related protein, triggering a switch from cell adhesion to cell detachment.
Our combined data show that a serpin-resistant form of tPA induces leukocyte mobilization and promotes neoangiogenesis. Our findings have implications for regenerative medicine: Administration of tPA alone or in combination with other growth factors might be a novel strategy to increase the efficiency of hematopoietic, angiopotent cell harvests for cell-based therapy of ischemic diseases. These data set forth the novel concept that plasmin activation, apart from controlling coagulation, controls myeloid cell–driven neoangiogenesis during tissue regeneration.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Dr A. Furuhata and S. Nakamura from Juntendo University and the FACS core facility for their help, and ImClone (NY) for providing antibodies against murine VEGF receptors. Human recombinant tPA was a generous gift from Eisai Corporation (Japan). Pauline O'Grady, a medical writer, provided editorial assistance to the authors during the preparation of this manuscript.
This work was supported by research grants from the Japan Society for the Promotion of Science and Grants-in-Aid for Scientific Research from Ministry of Education, Culture, Sports, Science and Technology (K.H. and B.H.); in part by research grants from the Ministry of Health, Labour and Welfare (K.H.), Mitsubishi Pharma Research Foundation (K.H.), Kyowa Hakko Kirin Co Ltd. (K.H,), Japan Leukaemia Research Fund (K.H,), Novartis Foundation for the Promotion of Science (B.H.), the Sagawa Foundation for Promotion of Cancer Research (B.H.), Kato Memorial Bioscience Foundation (B.H.), Astellas Foundation for Research on Metabolic disease (B.H.); by grants from the Lundbeck Foundation (L.R.L.) and the National Cancer Institute (Z.W.). This work was supported by a Program for Improvement of the Research Environment for Young Researcher (B.H.) funded by the Special Coordination Funds for Promoting Science and Technology of the Ministry of Education, Culture, Sports, Science and Technology, Japan.
Authorship
Contribution: Y.O., M.O., L.R.L, H.N., K.H., Z.W., and B.H. wrote the manuscript; L.R.L, Z.Z., H.O., Z.W., K.O., H.Y, and A.N. supplied the reagents; and K.Y., Y.O., M.O., C.N., M.I., Y.T., K.H., H.A., H.K. and B.H. designed and performed experiments, and analyzed data.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Koichi Hattori, Center for Stem Cell Biology and Regenerative Medicine, Institute of Medical Science, University of Tokyo, 4-6-1, Shirokanedai, Minato-ku, Tokyo 108-8639, Japan; e-mail: khattori@ims.u-tokyo.ac.jp.
References
Author notes
M.O. and Y.O. contributed equally to this study.
B.H. and K.H. share senior authorship.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal