P-selectin expression has been shown in Helicobacter pylori–infected persons, an infection that has been clinically associated with platelet-related diseases, such as idiopathic thrombocytopenic purpura. However, the role of P-selectin expression during H pylori infection remains unclear. In this study, we hypothesized that P-selectin expression was associated with platelet aggregation during H pylori infection. Using flow cytometry, we examined the levels of adhesion between H pylori and platelets as well as the levels of P-selectin expression and platelet phosphatidylserine (PS) expression during H pylori infection. Significantly high levels of adhesion between pro-aggregatory bacteria and platelets were observed. We identified that H pylori IgG is required for bacteria to induce P-selectin expression and that a significant release of P-selectin is essential for H pylori to induce aggregation. In addition, cellular apoptotic signs, such as membrane blebbing, were observed in platelet aggregates. PS expression was also detected in platelets during infection with both pro-aggrogatory and nonaggregatory strains of H pylori. These results suggest that the decrease in platelet counts seen during H pylori infection is the result of P-selection–dependent platelet aggregation and PS expression induced by the bacteria.
Introduction
Many diseases associated with platelet aggregation have been described as being related to Helicobacter pylori infection. For example, H pylori–infected persons have a tendency toward suffering from myocardial infarctions,1,–3 coronary heart disease,4,5 and stroke.6 It has also been suggested that H pylori may trigger the formation of thrombotic thrombocytopenic purpura (TTP) by inducing platelet aggregation through an interaction with the von Willebrand factor (VWF).7 There have also been implications that chronic H pylori infection may be associated with idiopathic thrombocytopenic purpura (ITP), as eradication of the bacteria from gastric mucosa has shown improvement in some ITP patients.8,,,,,,,–16 There is ongoing interest in identifying the various H pylori virulence factors that may predict the risk of developing symptoms of ITP. Studies have primarily focused on 2 groups of putative bacterial virulence factors, the cag pathogenicity island (for which CagA is a marker) and the vacuolating cytotoxin, such as VacA17,18 ; however, CagA and VacA have not been suggested to be causes of H pylori–induced platelet aggregation.19
There has been evidence showing that associations exist between H pylori and platelet aggregation in vivo. Platelet aggregation was observed in rat gastric mucosal microcirculation in vivo after H pylori administration.20 An increase in arterial thrombosis was also found in chronic H pylori–infected mice.17 However, the mechanisms of how H pylori induces platelet aggregation are not clearly understood. Byrne et al proposed that the H pylori strain 60190 (ATCC 49503) induces platelet aggregation through interactions between H pylori, its antibody, and the platelet receptor FcγRIIA (CD32), as well as VWF and its receptor glycoprotein (GP) Ib/IX.19 VWF found in blood plasma is produced in the endothelium (in Weibel-Palade bodies) and megakaryocytes (α granules of platelets).21 Evidence suggests that VWF is one of the essential elements involved in H pylori–induced platelet aggregation.19 Recent work has shown that the D′-D3 domains of VWF can interact with the integral membrane protein P-selectin (CD62P) in Weibel-Palade bodies.21
P-selectin is a member of the selectin family of cell surface receptors, which primarily mediates tethering and rolling of leucocytes.22,–24 Platelets are known to become activated when brought into contact with activators, including arachidonic acid (AA), adenosine diphosphate (ADP), collagen, and epinephrine. Once activated, platelets release several different coagulation factors and platelet-activating factors,25 such as P-selectin, usually found in the membrane of the platelet-secretory granules (α granules).26,27 This is known as degranulation, during which redistribution of these factors from the membrane of the granules to the plasma membrane occurs.22 H pylori infection may be associated with the increase of P-selectin expression; however, the function of P-selectin in this situation remains unclear. In this study, we explored the functions of P-selectin in the induction of platelet aggregation during H pylori infection.
Not all H pylori strains induce platelet aggregation, but H pylori infections are known to induce apoptosis in AGS cells of the human gastric epithelial cell line, AGS cells.28 Platelet apoptosis has also been reported.29,30 We therefore hypothesized that, aside from inducing platelet aggregation, H pylori may also induce platelet apoptosis, which may explain the decrease in platelet levels seen in some TTP and ITP patients. Interestingly, these patients show improved platelet counts after eradication of the bacteria.
Methods
Study subjects
Fifteen healthy study subjects with platelet counts ranging between 150 000 and 400 000/mm3 were enrolled into this study. H pylori (Hp) infection status was assessed by the capsule 13C-urea breath test (UBT; INER-Hp C-tester) and anti-Hp antibody test using Hp Rapid Test Strip (ACON Laboratories). UBT was performed according to previous study protocols.31 Six of 15 study subjects tested positive for both UBT and anti-Hp antibodies and were grouped as Hp IgG (+); the remaining 9 subjects, who tested negative for both UBT and anti-Hp antibodies, were grouped as Hp IgG (−). Informed consent was obtained from all study subjects in accordance with the Declaration of Helsinki, and study protocols were approved by the Institutional Review Board of the Kaohsiung Municipal Hsiao-Kang Hospital (KMHK-IRB no. A96040270).
H pylori culture and LPS extraction
H pylori strains ATCC 49503, 51932, and 43504 were cultured on CDC ANA blood agar (BD Biosciences) at 37°C in a microanaerobic chamber for 3 days. H pylori was scraped from the agar, resuspended in phosphate-buffered saline (PBS), and used within 4 hours. This is true for all experiments apart from those assessing the effect of bacteria storage time, where bacteria were kept at room temperature for up to 72 hours. The amount of H pylori cultured was determined by optical disturbance (OD) at 600 nm, where 1 OD was equivalent to 1 × 108 colony-forming units (CFUs)/mL H pylori. H pylori lipopolysaccharide (LPS) extraction was performed using the LPS extraction kit (iNtRON Biotechnology) according to the manufacturer's instructions. Finally, the extracted LPS was spun at 3000g for 15 minutes and then resuspended in PBS.
Platelet preparation
Platelets were isolated from blood collected in blood collection tubes containing 3.2% (0.109M) sodium citrate of both Hp IgG− and Hp IgG+ study subjects. Platelet-rich plasma (PRP) was obtained by spinning blood samples at 1000g for 10 minutes, whereas platelet-poor plasma, used as reference for the aggregation assays, was obtained by further spinning at 3000g for 15 minutes. Washed platelets were prepared by adding one-sixth volume of acid-citrate-dextrose solution (2.5% trisodium citrate, 1.4% citric acid, and 2% D (+)-glucose) to PRP. The platelets were spun down (3500g, 8 minutes) and the pellets resuspended in N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid buffer (10mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid, 137mM NaCl, 2.68mM KCl, 0.42mM NaH2PO4, 1.7mM MgCl2, 1mM CaCl2, 5mM D(+)-glucose, pH 7.35). The platelet count was adjusted to the original concentration of the blood donor (∼ 250 000/mL).32 Platelets were kept in a sealed, air-permeable bag and oscillated in a flat-bottomed shaking device at the constant speed of 70 cycles per minute at room temperature for 24 hours. These platelets were then used for the stored platelet P-selectin activation and aggregation assays.33
Platelet aggregation assays
The aggregation assay was applied by incubating a PRP (100 × 106 platelets) and 0.4 OD H pylori (resuspended in PBS; 4 × 106 bacteria) mixture (platelet/bacteria ratio = 25:1) at 37°C for 5 minutes and assessed by a Platelet Aggregometer (Chrono-Log). Aggregation was expressed as a variation in light transmission, using the light transmitted through platelet-poor plasma as a baseline.19 The ability for platelet aggregation was analyzed before each individual experiment using platelet activators, such as 0.5 mg/mL AA, 20μM ADP, 10 μg/mL collagen, and 0.3mM epinephrine (Helena Laboratories), as positive controls. Pooled immunoglobulins (Igs) were obtained from the serum of subjects with positive anti–H pylori antibody test and purified. The serum was mixed with an equal amount of wash/binding buffer (0.1M sodium phosphate, 0.15M NaCl, pH 7.4) and then incubated with protein A/G beads at 4°C for 1 hour, after which they were collected. The pooled Igs were then eluted with elution buffer (0.2M glycine, pH 3); after this, they were concentrated with Amicon Ultra Centrifugal Filter Devices (Millipore) and then adjusted to the final concentration of 10 mg/mL34 in each sample solution.
Adhesion of H pylori to platelet assays
To avoid aggregational depletion of platelets induced by H pylori infection (platelet/bacteria ratio = 25:1), an H pylori concentration of 0.1 OD (platelet/bacteria ratio = 100:1) was chosen for this experiment. This allows a low level of aggregation to occur while still permitting the use of flow cytometry to analyze the Hp-conjugated platelets. After incubation with H pylori, stained with a Bacteria Counting Kit (Invitrogen), platelets were labeled with phycoerythrin (PE)–conjugated mouse anti-CD41 IgG (BD Biosciences). The association between H pylori and platelets was examined by flow cytometry, and 1 × 104 platelets were analyzed. An association was considered present when the platelets stained positive for both H pylori (FL1) and PE (FL2).
Polymerase chain reaction amplification of H pylori urease
DNA extraction was performed on the H pylori, PRP, and aggregate mixtures by spinning down at 3000g for 15 minutes. The primers used were derived from the internal 412-bp fragment of the urease A gene35 : 5′-GCCAATGGTAAATTAGTT-3′ and 5′-CTCCT-TAATTGTTTTTAC-3′. Polymerase chain reaction was performed, and the products were analyzed on a 2% agarose electrophoresis gel stained with ethidium bromide.
Determination of VWF levels
After platelet aggregation assays, the supernatant was collected and the VWF antigen determined by an immunoturbidimetric assay with the STA Liatest VWF kit (Diagnostica Stago) on a Sysmex CA-1500 analyzer (Sysmex).36
P-selectin expression analysis using flow cytometry
The expression of P-selectin on platelets was determined by flow cytometry with monoclonal antibodies. PE-conjugated mouse anti-CD62P IgG (BD Biosciences) was used to assess α granule degranulation. Fluorescein isothiocyanate (FITC)-conjugated mouse anti-CD41 IgG (BD Biosciences) was used as an independent marker for the activation of platelets.37 Anti–CD62P-PE antibodies were added to the resuspended platelets, which were then collected by spinning the 1% formaldehyde prefixed PRP and H pylori mixture (platelet/bacteria ratio = 100:1) at 3000g for 15 minutes. After incubation in the dark at room temperature for 30 minutes, anti-CD-FITC antibodies were added and incubated in the dark at room temperature for a further 30 minutes. The fluorescence intensity was analyzed on the EPICS XL-MCL flow cytometer (Beckman Coulter). The level of platelets expressing P-selectin was defined as a fraction of the 10 000 platelets sorted exhibiting specific binding (ie, CD62p+) minus that exhibiting nonspecific binding (ie, the percentage defined with IgG-FITC conjugate).38 Each experiment was repeated at least 3 times. The mean level of P-selectin expression and fold increase in H pylori–infected samples, compared with noninfected control samples, was recorded.
Examining apoptotic membrane blebbing using scanning electron microscopy
Platelet aggregates were collected after incubating PRP with H pylori for 5 minutes (platelet/bacteria ratio = 25:1). The reaction was terminated with 2.5% glutaraldehyde (Sigma-Aldrich) in PBS after 2 hours. The aggregates were then rinsed with PBS 3 times and fixed with 1.33% osmium tetroxide (Fluka) in PBS for 1 hour. The aggregates were again rinsed with PBS 3 times before undergoing a dehydration process using series concentrations of ethanol (Sigma-Aldrich). After dehydration, the aggregates were fixed onto metal discs and viewed with a FEI Quanta 400 F (FEI) environmental scanning electron microscope under secondary electron imaging mode.
Detection of surface exposure of PS using flow cytometry
Platelet-surface exposure of phosphatidylserine (PS) was determined using flow cytometry with the Annexin V-FITC Apoptosis Detection Kit (BioVision) according to the manufacturer's instructions. Platelets were labeled with PE-conjugated mouse anti-CD41 IgG and mixed with annexin V–FITC after incubation with H pylori (platelet/bacteria ratio = 100:1). Platelet apoptosis was determined by flow cytometry where both annexin V-FITC (FL1) and PE-conjugated mouse anti-CD41 IgG (FL2) stains were positive.
Results
Limitations of H pylori to induce platelet aggregation
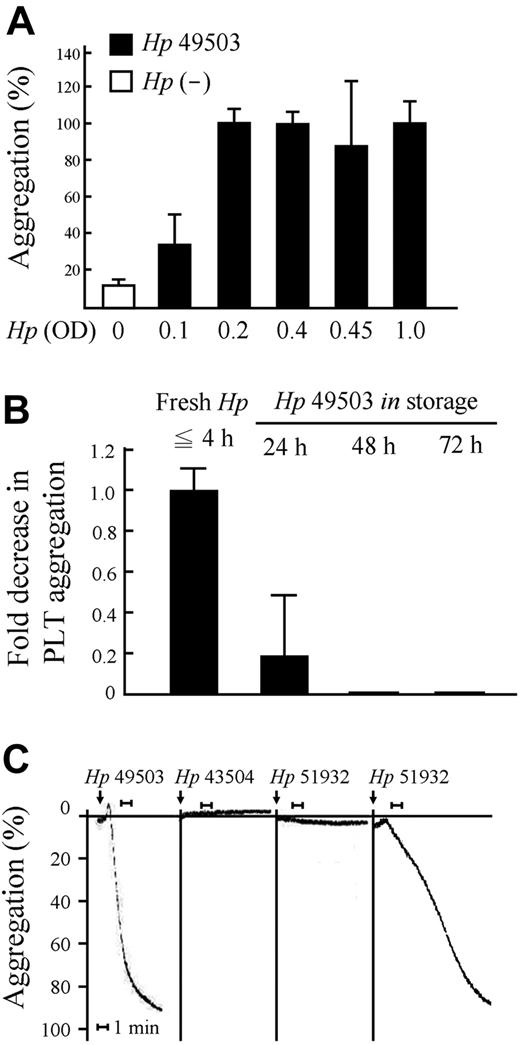
Dramatically high percentages of platelet aggregation were found when 0.2 OD pro-aggregatory H pylori strain ATCC 49503 (Hp 49503; 20 × 106 CFU/mL) was applied (platelet/bacteria ratio = 50:1) to the assay. This trend continued for concentrations up to 1.0 OD H pylori (platelet/bacteria ratio = 10:1; Figure 1A). These results indicate that the concentration threshold for H pylori–induced platelet aggregation is where platelet/bacteria ratio is 50:1. Levels of H pylori–induced platelet aggregation decreased with increasing bacteria storage time (Figure 1B). In accord with these results, double the threshold concentration of H pylori (0.4 OD; platelet/bacteria ratio = 25:1) and fresh H pylori (≦ 4 hours) were used in the following experiments. Platelet aggregation occurred in response to infection with Hp 49503 (CagA+/VacA+); this strain was therefore used as the pro-aggregatory H pylori strain. Aggregation was occasionally observed in response to the H pylori strain ATCC 51932 (Hp 51932; CagA−/VacA−), and no aggregation was seen with the H pylori strain ATCC 43504 (Hp 43504; CagA+/VacA+). The latter was therefore used as the nonaggregatory H pylori strain (Figure 1C).
Limitations for platelet aggregation induced by H pylori. PRP (100 × 106 platelets) and Helicobacter pylori (OD 0.1 to OD 1.0, equivalent to 1 × 106 CFU to 10 × 106 CFU) mixtures (platelet/bacteria ratio from 100:1 to 10:1, respectively) were used for aggregation assay. (A-B) Platelet aggregation occurs in a bacteria concentration- and storage time-dependent manner. The threshold for aggregation is H pylori more than or equal to OD 0.2 (platelet/bacteria ratio = 50:1) with a bacteria storage time of more than or equal to 4 hours. (C) Platelet aggregation occurred in some H pylori strains (ATCC 49503 and occasionally ATCC 51932).
Limitations for platelet aggregation induced by H pylori. PRP (100 × 106 platelets) and Helicobacter pylori (OD 0.1 to OD 1.0, equivalent to 1 × 106 CFU to 10 × 106 CFU) mixtures (platelet/bacteria ratio from 100:1 to 10:1, respectively) were used for aggregation assay. (A-B) Platelet aggregation occurs in a bacteria concentration- and storage time-dependent manner. The threshold for aggregation is H pylori more than or equal to OD 0.2 (platelet/bacteria ratio = 50:1) with a bacteria storage time of more than or equal to 4 hours. (C) Platelet aggregation occurred in some H pylori strains (ATCC 49503 and occasionally ATCC 51932).
Adhesion of H pylori to platelets induces platelet aggregation
A significantly higher binding ability using flow cytometric assays between platelets and bacteria was observed in pro-aggregatory Hp 49503 compared with that of nonaggregatory Hp 43504 and that of Hp 51932 (Figure 2A). The firm adhesion between platelets and H pylori was further demonstrated by amplifying the H pylori specific urease A gene fragment extracted from both the aggregates and the PRP mixtures. A 412-bp of DNA fragment was observed in the Hp 49503 aggregates (Figure 2B lanes 2 and 3); however, none was observed from the nonaggregatory Hp 43504 PRP reaction mixture (Figure 2B lanes 4 and 5). AA-induced platelet aggregates were used as a negative control (Figure 2B lane 1), and DNA fragments amplified from bacterial DNA extracts were used as positive indicators (Figure 2B lanes 6 and 7). These results provide new evidence with regards to the significance of adhesion between the pro-aggregatory H pylori and platelets in initiating platelet aggregation.
H pylori adheres to platelets in both PRP and platelet aggregates. PRP (100 × 106 platelets) and H pylori (1 × 106 CFU) mixture (platelet/bacteria ratio = 100:1) were analyzed for Hp-conjugated platelets using flow cytometry. A total of 1 × 104 platelets were examined. (A) Binding was significantly higher in pro-aggregatory Hp 49503 compared with nonaggregatory Hp 43504 and Hp 51932. (B) The binding between H pylori and platelets in aggregates was examined by amplifying H pylori–specific urease A gene fragment (412-bp) DNA extracted from the aggregates and from nonaggregatory PRP mixtures. A 412-bp of DNA fragment was observed in the Hp 49503-induced platelet aggregates, but none was found in the AA-induced aggregates or the nonaggregatory PRP mixture.
H pylori adheres to platelets in both PRP and platelet aggregates. PRP (100 × 106 platelets) and H pylori (1 × 106 CFU) mixture (platelet/bacteria ratio = 100:1) were analyzed for Hp-conjugated platelets using flow cytometry. A total of 1 × 104 platelets were examined. (A) Binding was significantly higher in pro-aggregatory Hp 49503 compared with nonaggregatory Hp 43504 and Hp 51932. (B) The binding between H pylori and platelets in aggregates was examined by amplifying H pylori–specific urease A gene fragment (412-bp) DNA extracted from the aggregates and from nonaggregatory PRP mixtures. A 412-bp of DNA fragment was observed in the Hp 49503-induced platelet aggregates, but none was found in the AA-induced aggregates or the nonaggregatory PRP mixture.
P-selectin activation is essential during platelet aggregation induced by H pylori infection
LPSs extracted from these H pylori strains (Hp 49503, Hp 43504, and Hp 51932) showed no signs of aggregation induction (Figure 3A) and were therefore used as negative controls, and AA was used as a positive control for P-selectin activation assays. Results showed that significant P-selectin activation occurred only in response to the pro-aggregatory Hp 49503 (Figure 3B). Neither Hp 43504 nor Hp 51932 was able to induce platelet activation. P-selectin activation seen with pro-aggregatory Hp 49503 appeared to occur in a concentration-dependent manner. However, no increase in P-selectin was seen with Hp 43504, despite raising the bacterial concentration to 1.0 OD (Figure 3C). The comparisons of P-selectin expression between Hp IgG− and Hp IgG+ during H pylori infection are shown in Figure 3D. No significant difference in P-selectin expression was observed between the 2 groups (Figure 3D bar 1 vs bar 3). No change in P-selectin expression was observed in Hp IgG− in response to H pylori infection (Figure 3D bar 2 vs bar 1), although a significant increase was observed in Hp IgG+ (Figure 3D bar 4 vs bar 3). These results indicate that Hp IgG may have an essential role in P-selectin expression during the bacteria infection.
The pro-aggregatory strain of H pylori stimulates the expression of P-selectin. Platelets were fixed with formaldehyde and labeled with anti-CD41 IgG and P-selectin, as well as the anti-CD62P antibody. They were then analyzed using flow cytometry. (A) No aggregation occurred in response to LPSs extracted from H pylori. These were therefore used as negative controls. (B) P-selectin activation (platelet/bacteria ratio = 100:1) was found to occur only in response to pro-aggregatory Hp 49503. Hp 43504, Hp 51932, or any LPSs extracted from H pylori did not induce P-selectin expression. (C) The activation was found to occur in a concentration-dependent manner in pro-aggregatory Hp 49503 infection. (D) A significant increase in P-selectin expression was observed only in Hp IgG+ in response to H pylori infection.
The pro-aggregatory strain of H pylori stimulates the expression of P-selectin. Platelets were fixed with formaldehyde and labeled with anti-CD41 IgG and P-selectin, as well as the anti-CD62P antibody. They were then analyzed using flow cytometry. (A) No aggregation occurred in response to LPSs extracted from H pylori. These were therefore used as negative controls. (B) P-selectin activation (platelet/bacteria ratio = 100:1) was found to occur only in response to pro-aggregatory Hp 49503. Hp 43504, Hp 51932, or any LPSs extracted from H pylori did not induce P-selectin expression. (C) The activation was found to occur in a concentration-dependent manner in pro-aggregatory Hp 49503 infection. (D) A significant increase in P-selectin expression was observed only in Hp IgG+ in response to H pylori infection.
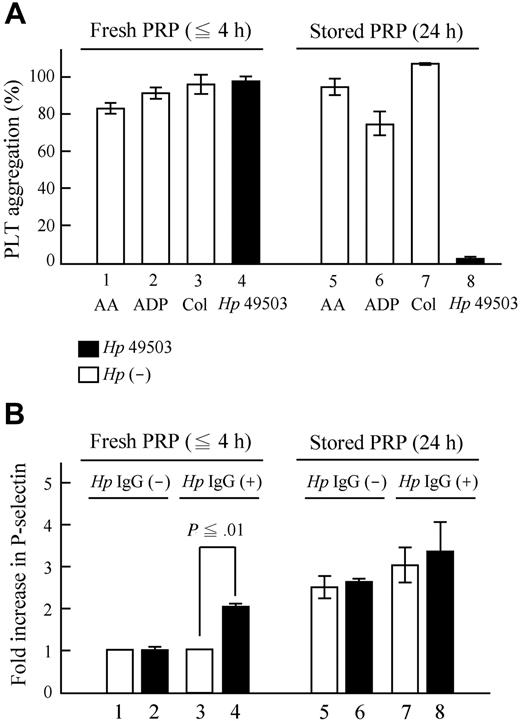
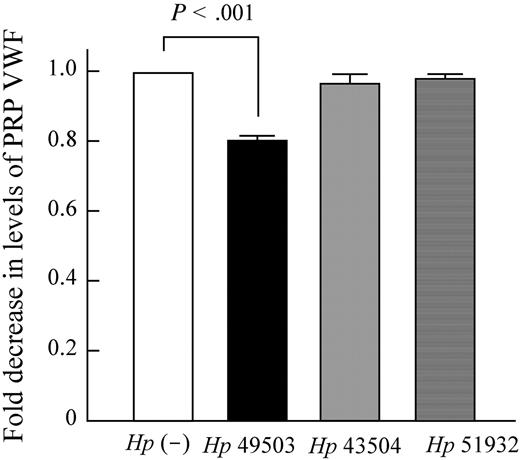
The aggregation induced by pro-aggregatory Hp 49503 is inhibited when anti-CD62P was applied (Figure 4A bar 10 vs bar 9). It was not only observed with Hp 49503 infection but also with other stimuli, such as AA (Figure 4A bar 2 vs bar 1) and epinephrine (Figure 4A bar 8 vs bar 7). However, among these assays, full inhibition was only observed where Hp 49503 applied. Despite being a pro-aggregatory Hp strain, Hp 49503 was unable to induce platelet aggregation in the washed platelet assays (Figure 4B bar 3 vs bar 1). There was also no further decrease in the levels of aggregation when anti-CD62P was added (Figure 4B bar 4 vs bar 3), thus suggesting that washed platelets are not activated by Hp 49503. Platelet aggregation resumed when pooled Igs of Hp IgG+ samples were added (Figure 4B bar 5 vs bar 3) and were again inhibited with the addition of anti-CD62P (Figure 4B bar 6 vs bar 5). No aggregation was observed in stored Hp IgG+ PRP infected with Hp 49503 (platelet/bacteria ratio = 25:1; Figure 5A bar 8), although standard agonists (AA, ADP, and collagen shown in Figure 5A bars 5-7, respectively) were able to induce aggregation. No significant increase in P-selectin expression in the stored PRP (Figure 5B bar 8 vs bar 7) was observed during H pyroli infection (platelet/bacteria ratio = 100:1). Furthermore, Figure 6 showed that VWF depletion occurs only in Hp 49503-induced platelet aggregation mixtures.
Anti–P-selectin antibodies completely inhibit H pylori–induced platelet aggregation. Before the addition of H pylori (platelet/bacteria ratio = 25:1) and aggregation activators, anti-CD62P antibodies were incubated with PRP for 1 minute. (A) Addition of anti-CD62P antibodies was found to inhibit aggregations induced by pro-aggregatory Hp 49503, AA, and epinephrine. PRP, washed platelets, and pooled Igs were obtained from the anti-Hp antibody-positive samples. Washed platelets were prepared by resuspending the pellets of PRP mixture in N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid buffer and were then adjusted to the original concentration (∼ 250 000/mL). Pooled Igs purified with protein A/G beads were adjusted to the final concentration of 10 mg/mL. (B) Platelet aggregation induced by Hp 49503 was inhibited in PRP and in washed platelets with pooled Igs where anti-CD62P antibodies applied.
Anti–P-selectin antibodies completely inhibit H pylori–induced platelet aggregation. Before the addition of H pylori (platelet/bacteria ratio = 25:1) and aggregation activators, anti-CD62P antibodies were incubated with PRP for 1 minute. (A) Addition of anti-CD62P antibodies was found to inhibit aggregations induced by pro-aggregatory Hp 49503, AA, and epinephrine. PRP, washed platelets, and pooled Igs were obtained from the anti-Hp antibody-positive samples. Washed platelets were prepared by resuspending the pellets of PRP mixture in N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid buffer and were then adjusted to the original concentration (∼ 250 000/mL). Pooled Igs purified with protein A/G beads were adjusted to the final concentration of 10 mg/mL. (B) Platelet aggregation induced by Hp 49503 was inhibited in PRP and in washed platelets with pooled Igs where anti-CD62P antibodies applied.
No aggregation occurred in PRP with an inhibition of P-selectin expression during H pylori infection. Stored PRP (24 hours) was preserved in a sealed, air-permeable bag and oscillated in a flat-bottomed shaking device at room temperature. (A) During pro-aggregatory Hp 49503 infection (platelet/bacteria ratio = 25:1), no aggregation was observed in stored Hp IgG+ PRP, of which standard agonists (AA, ADP, and collagen) were capable of aggregation. (B) P-selectin expressions were elevated in stored PRP, but there was no significant increase during Hp 49503 infection (platelet/bacteria ratio = 100:1).
No aggregation occurred in PRP with an inhibition of P-selectin expression during H pylori infection. Stored PRP (24 hours) was preserved in a sealed, air-permeable bag and oscillated in a flat-bottomed shaking device at room temperature. (A) During pro-aggregatory Hp 49503 infection (platelet/bacteria ratio = 25:1), no aggregation was observed in stored Hp IgG+ PRP, of which standard agonists (AA, ADP, and collagen) were capable of aggregation. (B) P-selectin expressions were elevated in stored PRP, but there was no significant increase during Hp 49503 infection (platelet/bacteria ratio = 100:1).
VWF depletion occurred in the pro-aggregatory strain of H pylori. VWF depletion assays were carried out in PRP with or without H pylori infection. VWF depletion occurred only in Hp 49503–induced platelet aggregation mixtures.
VWF depletion occurred in the pro-aggregatory strain of H pylori. VWF depletion assays were carried out in PRP with or without H pylori infection. VWF depletion occurred only in Hp 49503–induced platelet aggregation mixtures.
PS exposure and membrane blebbing were observed during H pylori infection
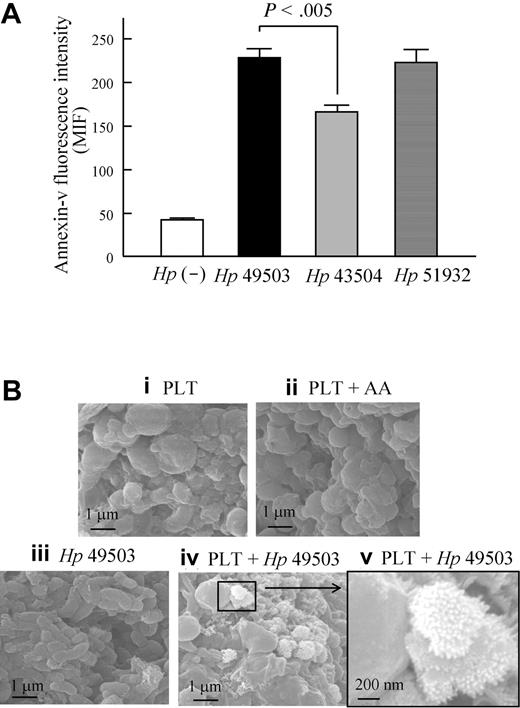
PS exposure in platelets (Figure 7A) and the apoptotic sign of membrane blebbing on platelet aggregates (Figure 7B) were observed during H pylori infection. Significantly high levels of PS exposure were observed in infections with both the pro-aggregatory strain (Hp 49503) and nonaggregatory strains (Hp 43504 and Hp 51932) of H pylori.
PS exposure and membrane blebbing were observed during H pylori infection. Using flow cytometry, platelet surface exposure of PS was examined in response to H pylori stimulation. PS expression was observed by binding with annexin V-FITC, and platelet was labeled with PE-conjugated mouse anti-CD41 IgG. (A) All strains of H pylori can induce PS expression on platelets; however, a higher level of PS expression was found in pro-aggregatory Hp 49503 compared with that of nonaggregatory Hp 43504. (B) Using a scanning electron microscope (Quanta 400F; FEI), the apoptotic sign of membrane blebbing was also observed on platelet aggregates induced by Hp 49503.
PS exposure and membrane blebbing were observed during H pylori infection. Using flow cytometry, platelet surface exposure of PS was examined in response to H pylori stimulation. PS expression was observed by binding with annexin V-FITC, and platelet was labeled with PE-conjugated mouse anti-CD41 IgG. (A) All strains of H pylori can induce PS expression on platelets; however, a higher level of PS expression was found in pro-aggregatory Hp 49503 compared with that of nonaggregatory Hp 43504. (B) Using a scanning electron microscope (Quanta 400F; FEI), the apoptotic sign of membrane blebbing was also observed on platelet aggregates induced by Hp 49503.
Discussion
It has previously been described that virulence factors of H pylori, such as CagA and VagA, have no influence in H pylori–induced platelet aggregation19 and that H pylori–induced platelet aggregation may be strain-dependent.39 After assessing different strains of H pylori for their ability to induce platelet aggregation, the H pylori strain ATCC 49503 and H pylori strain ATCC 43504 were chosen as the pro-aggregatory strain and nonaggregatory strain, respectively, for this study. By analyzing Hp IgG+ PRP, Hp IgG− PRP (Figure 1C), and washed platelets of Hp IgG+ samples (Figure 4B), we further strengthened evidence previously described19 that H pylori–induced platelet aggregation requires the presence of Hp IgGs.
Human platelet aggregation has also been associated with bacterial LPS.40 LPS from Escherichia coli is known to bind to platelets,41 and the LPSs of some specific H pylori strains carry the sialyl Lewis x antigen.42 The sialyl Lewis x structure can be recognized by 3 members of the selectin receptor family.43 However, the LPSs extracted from the specific H pylori strains used in this study were unable to induce aggregation or activate P-selectin; we therefore used these as negative controls for P-selectin expression assays. Further investigation is needed into whether the specific H pylori LPS has a role in adhesion between living bacteria and P-selectin of activated platelets.
In this study, we showed the limitation of pro-aggregatory strains of Hp 49503 in platelet aggregation induction. Aggregation induction appears to be concentration- and storage time-dependent (Figure 1), and certain amounts of living bacteria are necessary to interact with platelets for the induction of platelet aggregation. Once the amount of living bacteria reaches the threshold, aggregation can be initiated. This theory is supported by our adhesion assays, where strong adhesions between platelets and the pro-aggregatory strain Hp 49503 (Figure 2A) were observed. The appearance of pro-aggregatory strain Hp 49503 DNA in platelet aggregates (Figure 2B) also demonstrates a high level of adhesion between platelets and the pro-aggregatory strain Hp 49503. These results indicate that a threshold of bacterial bound platelets exists and is necessary to initiate platelet aggregation. Despite the absence of H pylori from routine blood and serum sample cultures, H pylori has been detected in atherosclerotic plaques by the polymerase chain reaction method.4 This indicates that bacteria from a chronic localized H pylori infection in the gastric mucosa may have, at some point, had the chance to leak into the blood, inducing platelet aggregation within the blood, thus forming multiple emboli. This may have eventually led to the development of subtle cardiovascular diseases.
H pylori has been reported to stimulate the expression of P-selectin44 ; however, the functions of redistributed P-selectins remain unclear. Our data show that Hp 49503 can induce aggregation (Figure 1C) and stimulate high levels of P-selectin expression in Hp IgG+ PRP (Figure 3D). The inability of Hp 43504 to induce platelet aggregations may be a result of its inability to induce a significant release of P-selectins (Figure 3B-C). Again, platelet aggregation did not occur (Figure 5A) when stored platelets were applied, in which no significant release of P-selectin was observed (Figure 5B). There was a 2.5- to 3.0-fold increase (Figure 5B) in P-selectin expression in Hp IgG− stored PRP compare with that of fresh PRP, with or without Hp 49503 infection; we therefore suspect that the high levels of P-selectin expression observed in the stored platelets were not induced by the bacteria infection. The necessity of P-selectin in H pylori–induced platelet aggregation was demonstrated in Figure 4 with the addition of anti–P-selectin antibodies (anti-CD62). This further confirms the indispensable role of P-selectin in H pylori–induced platelet aggregation. In this study, we have found that P-selectin is not only involved in H pylori–induced platelet aggregation, but also has as an essential role in the induction of platelet aggregation.
It has also been suggested that H pylori triggers the formation of TTP by inducing platelet aggregation through an interaction with the VWF.7 A previous study demonstrated that direct binding between VWF and Hp 49503 exists and that H pylori–induced platelet aggregation is inhibited by the anti-VWF antibody, aspirin, and by a GPIIb/GPIIIa antagonist.19 The D′-D3 domains of VWF in Weibel-Palade bodies have also been found to interact with membrane P-selectin.21 In this study, a significant consumption of VWF was observed when Hp 49503 was applied to PRP. Without further addition of VWF, platelet aggregation was also induced in washed platelets by adding pooled Igs. This indicates that newly released VWFs from activated platelets are enough to initiate aggregation. Based on these findings, we propose that H pylori–induced platelet aggregation is initiated with the binding of bacteria/antibodies to the platelet receptor FcγRIIA. Strong adhesions are necessary for the platelets to become active, after which there is a significant release of P-selectin and VWF. Adhesions between the bacteria/anti-Hp IgG/platelet complex, platelet-released VWF, and P-selectin finally induce aggregation.
H pylori infections have been associated with some adult patients with ITP8,,,,,,–15 ; however, a few studies have found conflicting results in the pediatric field.45,46 Although the discrepancy in the clinical response to H pylori eradication therapy may be the result of differences in the bacterial strains, there has also been a suggestion that chronic infection with H pylori is a cause of secondary ITP, which differs from primary ITP (seen mostly in children).47 It is highly plausible that adult ITP patients, who respond to H pylori eradication therapy and show subsequent improvement in their posteradication platelet counts, have secondary ITP induced by long-term H pylori infection, although in cases, mostly childhood ITP, where patients do not respond to bacterial eradication,45,46 the ITP may not be secondary to H pylori infection. Accurate diagnosis of ITP is therefore essential.
In a study carried out by Ahn et al,48 eradication of H pylori from patients with ITP was found to reduce P-selectin expression but seldom to improve the subsequent posteradication platelet counts. This may partly be the result of the complex underlying etiology of ITP.47 However, it may also be because, after H pylori eradication therapy, Hp IgG+ patients will no longer carry P-selectin inducing H pylori, resulting in the reduction of P-selectin expression seen in the patients in this study. Other etiologies of ITP, excluding ITP secondary to H pylori infection, should be considered where posteradication platelet counts do not improve.
Not every strain of H pylori can induce platelet aggregation, but most H pylori strains can induce apoptosis in human gastric epithelial cells and its cell line, AGS cells. Our results demonstrate that both the pro-aggregatory strain Hp 49503 and the nonaggregatory strain Hp 43504 can induce PS exposure on the surface of platelets. Although PS expression does not necessarily imply certain apoptosis in platelets,49,50 the apoptotic membrane blebbing on the surface of aggregates induced by the pro-aggregatory Hp 49503 further supports our hypothesis. The detailed mechanisms of H pylori–induced platelet apoptosis need further investigation.
In conclusion, this study provides an insight into the association between increased platelet P-selectin expression and H pylori–induced platelet aggregation, as well as how a decrease in platelet count can be triggered both by platelet aggregation and platelet surface exposure of PS during H pylori infection.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the National Science Council (Taiwan; NSC96-2320-B-110-008) and Center for Nanoscience and Nanotechnology, the Aim for the Top University Plan, National Sun Yat-Sen University, Kaohsiung, Taiwan.
Authorship
Contribution: J.-J.Y., S.T., and J.-Y.W. designed and performed research; D.-C.W. prepared H pylori and analyzed data; and T.-C.L. and A.C. designed research, supervised the work, analyzed data, and prepared the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Angela Chen, Institute of Biomedical Sciences, National Sun Yat-Sen University, PO Box 59-69, Kaohsiung 80424, Taiwan; e-mail: achen@mail.nsysu.edu.tw.