Histone hypoacetylation occurs in many cancers and inhibition of histone deacetylation is a promising approach to modulate these epigenetic changes. Our laboratory previously demonstrated that the histone deacetylase inhibitors (HDACis) vorinostat and AR-42 reduced the viability of a canine malignant mast cell line. The purpose of this study was to further investigate the mechanisms of pan-HDAC inhibition in normal and malignant mast cells. Mouse and canine malignant mast cell lines expressing various Kit mutations, normal canine mast cells, and primary canine malignant mast cells were treated with AR-42 (a novel HDACi) and effects on cell viability, cycling, and signaling were evaluated. Treatment with AR-42 induced growth inhibition, cell- cycle arrest, apoptosis, and activation of caspases-3/7. AR-42 promoted hyperacetylation of H3, H4, and alpha-tubulin, and up-regulation of p21. Down-regulation of Kit occurred after AR-42 treatment via inhibition of Kit transcription. Disassociation between Kit and heat shock protein 90 (HSP90) and up-regulation of HSP70 were observed after AR-42 treatment, suggesting potential loss of HSP90 chaperone function. Lastly, AR-42 down-regulated the expression of p-Akt, total Akt, phosphorylated STAT3/5 (pSTAT3/5), and total STAT3/5. In summary, AR-42 exhibits in vitro and ex vivo biologic activity against malignant mast cells, representing a promising therapeutic approach for malignant mast cell disease.
Introduction
Global DNA hypermethylation and histone hypoacetylation are hallmarks of many cancers.1 These epigenetic modifications alter gene expression in the absence of changes to the DNA sequence and play important roles in tumorigenesis by modulating the expression of tumor suppressor, cell-cycle regulatory, and DNA repair genes. The potential reversibility of these epigenetic changes has made the pathways involved attractive targets for therapeutic intervention.1 Histone deacetylase inhibitors (HDACis) are a promising class of antitumor agents that can induce growth arrest, differentiation, and apoptosis of cancer cells through the accumulation of acetylated histones leading to chromatin remodeling and restored transcription of genes regulating proliferation, cell-cycle progression, and cell survival.2
The major mechanism of HDACis is believed to be through alteration in transcription of several genes such as p21 via histone modification.2 However, a growing number of nonhistone substrates have been identified and implicated in the antitumor activities of HDACis, including molecular chaperones, such as heat shock protein 90 (HSP90), and transcription factors, including signal transducer and activator of transcription 3 (STAT3) and nuclear factor κB.3,4 Specifically, HSP90 is a substrate of HDAC6 and is hyperacetylated after HDACi treatment, resulting in the loss of chaperone function.5 This HSP90-dependent pathway has been recognized as an important histone acetylation–independent anticancer mechanism for the HDACi-induced down-regulation of Kit in human gastrointestinal stromal tumor cell lines,6 Bcr-Abl in human chronic myeloid leukemia lines,7 estrogen receptor and DNA methyltransferase 1.8,9
Mast cell–associated malignancies are important diseases in both humans and dogs,10,11 and are characterized by activating mutations in Kit in a significant portion of patients. More than 90% of human patients with systemic mastocytosis carry the D816V mutation in Kit and exhibit resistance to imatinib (Gleevec) therapy.12 Similarly, up to 30% of dogs with high-grade mast cell tumors (MCTs) possess internal tandem duplications (ITDs) in the Kit juxtamembrane (JM) domain.13,14 Targeted inhibitors of Kit such as imatinib mesylate and toceranib phosphate (Palladia) have demonstrated clinical efficacy against malignant mast cell disease.15,16 However, different Kit mutations exhibit variable resistance toward Kit inhibitors, and the potential development of secondary resistance mutations is a concern. Previous studies performed by our laboratory and others demonstrated that inhibition of HSP90 activity using 17-(Allylamino)-17-demethoxygeldanamycin (17-AAG) or STA-9090 down-regulated the expression of both wild-type and mutant Kit, resulting in apoptosis of malignant mast cells in vitro, ex vivo, and in a mouse xenograft model.17,18 A subsequent phase 1 study of STA-1474 (prodrug of STA-9090) demonstrated activity against malignant mast cell tumors in dogs (C.A.L. et al, unpublished data, July 2008).
Recent studies have demonstrated that HDAC inhibitors exhibit activity against human gastrointestinal stromal tumor cell lines possessing activating mutations in Kit.6 The proposed mechanism of action was down-regulation of mutated Kit due to HSP90-dependent degradation and alteration of Kit gene transcription. Given the demonstrated role of Kit in malignant mast cell disease, we hypothesized that HDACis may have activity against these tumors via similar pathways. In a previous study investigating the biologic activity of HDACis against canine tumor cell lines, the pan-HDACi, AR-42 (Arno Therapeutics), previously shown to be effective against mouse models of prostatic and hepatocellular carcinoma,19,–21 demonstrated superior growth inhibition of the C2 canine malignant mast cell line compared with the HDACi vorinostat (Zolinza; Merck).22 The purpose of this study was to expand upon these initial findings and evaluate the biologic effects and mechanism of action of AR-42 against both canine and mouse malignant mast cells.
Methods
Reagents, cell lines, and fresh tumor samples
The novel pan-HDACi, AR-42 ((S)-(+)-N-hydroxy-4-(3-methyl-2-phenyl-butyrylamino) benzamide; Arno Therapeutics), was synthesized as previously described23 with purities exceeding 99% as determined by nuclear magnetic resonance spectroscopy (300 MHz). 17-AAG was kindly provided by Synta Pharmaceuticals. The following antibodies were obtained from Cell Signaling Technologies: p-Kit (Tyr719), Kit, p-Akt (Ser473 and Tyr308), p-STAT3 (Tyr705), p-STAT5 (Tyr694), STAT3, STAT5, acetylated lysine, HSP70, and HSP90. A monoclonal antibody against acetylated tubulin (clone 6-11B-1) and Stemline serum-free medium were obtained from Sigma-Aldrich. Antibodies against Akt, Bcl-2, and poly(adenosine diphosphate ribose) polymerase (PARP) were obtained from BD Biosciences. Bcl-XL/S, HSP90α/β, and β-actin antibodies were from Santa Cruz Biotechnology. Kit antibody was obtained from Calbiochem. Monoclonal antibodies against HSP organizer protein (HOP) and p23 were purchased from Stressgen and Abcam, respectively. Monoclonal anticonstitutive HSP70 antibody was kindly provided by Dr Michael Oglesbee (Ohio State University). Antibodies against acetylated histones H3 (Lys9 and Lys14) and H4 (Lys5, Lys8, Lys12, and Lys16) were obtained from Millipore.
Mouse P815 (activating D814V Kit mutation; homologous to the human KIT D816V mutation found in the HMC-1 cell line) and canine C2 (activating internal tandem duplication [ITD] mutation in the JM domain of Kit) and BR (activating point mutation L575P in the JM domain of Kit) cells were kindly provided by Dr Stephen Galli (Stanford University) and Dr Warren Gold (University of California) and were maintained in RPMI 1640 with 10% fetal bovine serum and antibiotics. Canine bone marrow–derived cultured mast cells (canine BMCMCs) were generated from 2 different dogs and maintained in Stemline medium supplemented with recombinant canine stem cell factor (rcSCF) as previously described.24 Fine needle aspirates were performed on spontaneously occurring canine MCTs to obtain small numbers of primary malignant mast cells and processed as previously detailed,18 resulting in 60% to 95% purity. Aspirates were obtained from 17 different affected dogs presented to the Veterinary Teaching Hospital at The Ohio State University in accordance with a protocol approved by the hospital Clinical Trials Advisory Committee.
Assessment of proliferation and cell viability
Changes in cell proliferation were assessed using a commercially available bromodeoxyuridine (BrdU) incorporation assay (Roche). Briefly, 15 × 104 P815, C2, and BR cells were treated with dimethyl sulfoxide (DMSO), AR-42, or 17-AAG as indicated for 24 hours in 96-well plates. The BrdU reagent was added, cells were incubated for another 2 to 3 hours and then harvested, fixed, and digested by nuclease for 30 minutes at 37°C. Cells were then incubated with conjugate for 1 hour and washed, and the plates were developed by adding 100 μL of substrate for 30 minutes. The absorbance was measured using an enzyme-linked immunosorbent assay (ELISA) plate reader (Spectra Max; Molecular Devices). To measure changes in the viability of primary canine malignant mast cells after drug treatments, the WST-1 assay (Clontech) was used. Briefly, 2 to 4 × 105 fresh malignant canine mast cells were treated with 0.1% DMSO, AR-42, or 17-AAG for 48 hours in 96-well plates. The WST-1 working solution was then added, plates were incubated for 4.5 hours, and absorbances were measured by ELISA plate reader.
Cell-cycle analysis and evaluation of apoptosis
The effects of drug treatment on cell cycle and apoptosis of canine and mouse mast cell lines were measured by annexin V/propidium iodide (PI) and PI staining as previously described.18 Briefly, 1.0 × 106 P815, C2, and BR cells were treated with 0.1% DMSO, AR-42, or 17-AAG for 24 hours at 37°C. Cells were collected, washed, and stained with annexin V–fluorescein isothiocyanate and PI for 15 minutes before evaluation by flow cytometry. Alternatively, cells were treated as previously described, collected, washed 3 times in 0.1% glucose/PBS, and then fixed with cold 70% EtOH at 4°C overnight. After 3 0.1% glucose/PBS washes, 200 μL of PI working solution (50 μg/mL in PBS) was added before analysis by flow cytometry.
Induction of caspases-3/7 activity was measured using the SensoLyte homogeneous AMC caspases-3/7 assay kit (Anaspec). Briefly, 5.0 × 104 C2 and BR cells, 5.0 × 103 P815 cells, and 2 to 4 × 105 fresh malignant mast cells were treated with DMSO, AR-42, or 17-AAG for 24 hours in 96-well plates, after which the substrate (Ac-DEVD-AMC) was added to each well and plates were incubated for 40 minutes at room temperature. Wells containing equal amounts of medium with 0.1% DMSO and substrate were used as blanks. Fluorescence was measured by ELISA plate reader, and the data are presented as relative fluorescence units.
Western blotting and coimmunoprecipitation
P815, C2, and BR cells (1.0 × 107 cells) and fresh malignant canine mast cells (1 × 106 cells) were treated with DMSO, AR-42, or 17-AAG for 24 hours. Cells were collected, washed, and lysed in protein lysis buffer containing protease and phosphatase inhibitors as previously described.18 Equal amounts of protein lysate were used for sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE). After transfer, the membranes were incubated with primary antibodies overnight at 4°C and corresponding horseradish peroxidase–conjugated secondary antibodies for 1 hour at room temperature, and then developed with Super Signal West Pico-chemiluminescence substrate (Pierce).
To assess the effects of drug treatment on the association of HSP90 with Kit, 3.0 × 107 P815, C2, and BR cells were treated with DMSO, AR-42, or 17-AAG for 8 hours. Cells were collected and lysates generated as previously described. The lysates were then precleared with 50 μL of True-blot anti–rabbit agarose beads (eBioscience) for 30 minutes on ice. Rabbit anti-HSP90 antibody (5 μL) was added to samples, which were then incubated for another 60 minutes on ice, followed by the addition of 50 μL of True-blot anti–rabbit agarose beads. The samples were incubated at 4°C overnight and then analyzed by Western blotting to detect acetyl-lysine, HSP90, or Kit.
Kit cell-surface expression
P815, C2, and BR cells (1.0 × 106) were treated for 6 hours, and 0.5 × 106 fresh malignant mast cells were treated for 24 hours with 1μM AR-42 or 17-AAG. Cells were harvested, washed, and analyzed for Kit cell-surface expression by flow cytometry as previously described.18 Analysis was performed using gates set on the forward/side scatter untreated cell population to minimize the inclusion of dead/dying cells. As the analysis was performed after only 6 hours of drug treatment, cell death was minimal at this time point.
Quantitative reverse-transcription–PCR analysis of Kit expression
P815, C2, and BR cells were treated with AR-42 or 17-AAG for 4 and 8 hours, and RNA was extracted using TRIzol (Invitrogen). cDNA was made from 1 μg of total RNA using Superscript III (Invitrogen). Real-time quantitative polymerase chain reaction (PCR) was performed using the Applied Biosystems 7900HT Sequence Detection System. Kit and 18S were detected using Fast SYBR green PCR master mix (Applied Biosystems) according to the manufacturer's protocol, and primer sets are detailed in Table 1. All reactions were performed in triplicate and included no-template controls for each gene. Relative expression was calculated using the comparative threshold cycle method.25 Experiments were repeated 3 times using samples in triplicate.
Primers for quantitative reverse-transcriptase polymerase chain reaction
| Gene/primers . | Primer sequences . |
|---|---|
| Canine cKit | |
| 232F | 5′-GAG AAC ACA CAC AAC GAA TG-3′ |
| 414R | 5′-GCA GCG GAC CAG CGT ATC ATT G-3′ |
| Mouse cKit | |
| 152F | 5′-GCG ACA CCC TCA GCC TGA CGT G-3′ |
| 406R | 5′-GTG GGT CTG TCA GAG GGC AGC G-3′ |
| 18S | |
| V2F | 5′-AAA TCC TTT AAC GAG GAT CCA TT-3′ |
| V2R | 5′-AAT ATA CGC TAT TGG AGC TGG A-3′ |
| Gene/primers . | Primer sequences . |
|---|---|
| Canine cKit | |
| 232F | 5′-GAG AAC ACA CAC AAC GAA TG-3′ |
| 414R | 5′-GCA GCG GAC CAG CGT ATC ATT G-3′ |
| Mouse cKit | |
| 152F | 5′-GCG ACA CCC TCA GCC TGA CGT G-3′ |
| 406R | 5′-GTG GGT CTG TCA GAG GGC AGC G-3′ |
| 18S | |
| V2F | 5′-AAA TCC TTT AAC GAG GAT CCA TT-3′ |
| V2R | 5′-AAT ATA CGC TAT TGG AGC TGG A-3′ |
Matrigel invasion assay
To assess the ability of AR-42 to inhibit invasion, a Matrigel invasion assay using cell culture inserts (8-μm pore size; Falcon) coated with 100 μL of BD Matrigel (BD Bioscience) was used. Briefly, C2 cells (5 × 105/mL) were pretreated with DMSO, AR-42, or 17-AAG for 8 hours. Cells were then transferred into the upper chamber of inserts. After 20 hours at 37°C, the cells in the lower chamber were collected and stored at −80°C until cell numbers were determined using the CyQUANT assay as previously described (Invitrogen). To account for cell death after drug treatment, an equivalent number of the C2 cells, treated for the same duration with the same concentration of drugs, served as the baseline comparison for each individual assay.
Statistics
All experiments with the exception of those involving canine BMCMCs and primary mast cells were performed in triplicate and repeated 3 times. Experiments using canine BMCMCs were performed in triplicate, but repeated only twice because of limited cell numbers. Experiments using primary malignant mast cells cultured ex vivo were performed in triplicate but undertaken only once because of the limited cell numbers and lack of long-term viability in culture. Data were presented as mean plus or minus standard deviation. The difference between 2 group means was analyzed using the Student t test and a P value less than .05 was considered significant.
Results
AR-42 induces apoptosis of canine and mouse malignant mast cells
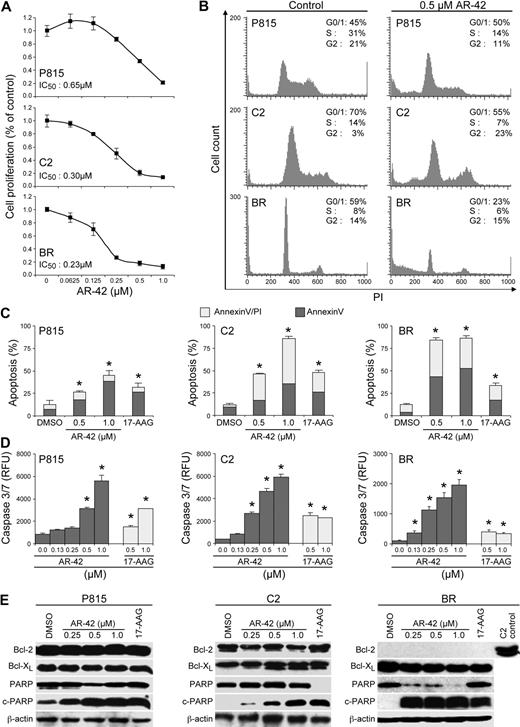
The biologic effects of AR-42 on both mouse (P815) and canine (C2 and BR) malignant mast cell lines were investigated. Cells were treated with AR-42 for 24 hours, after which the cell proliferation rate was assessed using a BrdU incorporation assay. As shown in Figure 1A, AR-42 inhibited cell proliferation in a dose-dependent manner in all 3 lines. The median IC50 concentrations for P815, C2, and BR cells were 0.65, 0.30, and 0.23μM, respectively. To determine whether AR-42 treatment induced cell death, effects of drug treatment on cell cycle and apoptosis were analyzed. AR-42 induced cell-cycle arrest at G1 in the P815 cells and at G1/G2 in the C2 cells at a concentration of 0.5μM (Figure 1B). BR cells failed to show significant cell-cycle arrest, but a large number of dead cells (sub G1) were noted. In addition, AR-42 caused a dose-dependent induction of apoptosis as determined by enhanced labeling with annexin/PI (Figure 1C), a significant increase in caspases-3/7 activity (Figure 1D), and increased levels of PARP cleavage (Figure 1E) in all 3 cell lines. In contrast to the previous findings of AR-42 effects on hepatocellular carcinoma and prostate cancer cell lines,20,21 expression levels of Bcl-xL and Bcl-2 were unaffected by AR-42 treatment (Figure 1E). Bcl-2 levels in BR cells were undetectable. These findings suggest that in P815, C2, and BR malignant mast cells, AR-42 does not alter the expression of Bcl family member genes and that apoptosis induced by treatment with the drug may be independent of this pathway. As expected, 17-AAG, which was used as the positive control, induced apoptosis in all cell lines.
AR-42 inhibits the proliferation of malignant mast cells via cell-cycle arrest and apoptosis. (A) P815 (top panel), C2 (middle panel), and BR (bottom panel) cells were treated with increasing concentrations of AR-42 for 24 hours. The cell proliferation rate was assessed by BrdU incorporation and the median inhibitory concentration was then calculated for each cell line. Three independent experiments were performed, and 1 representative result is presented. (B) P815, C2, and BR cells were treated with 0.5μM AR-42 for 24 hours. Cells were then evaluated for effects on cell cycle using propidium iodide (PI) staining and flow cytometry. Three independent experiments were performed, and 1 representative result is presented. (C) P815, C2, and BR cells (10 × 106) were treated with increasing concentrations of AR-42 and 1μM 17-AAG for 24 hours. Apoptosis was assessed by annexin V/PI staining and flow cytometry. Three independent experiments were performed and their data were calculated (*P < .05). (D) P815, C2, and BR cells were incubated with various concentrations of AR-42 and 17-AAG for 24 hours and caspases-3/7 activation was assessed (*P < .05). Experiments were performed in triplicate and repeated 3 times. (E) P815, C2, and BR cells were treated with various concentrations of AR-42 or 1μM 17-AAG for 24 hours. Effects on the expression of Bcl-2, Bcl-xL, and PARP were determined by Western blot analysis.
AR-42 inhibits the proliferation of malignant mast cells via cell-cycle arrest and apoptosis. (A) P815 (top panel), C2 (middle panel), and BR (bottom panel) cells were treated with increasing concentrations of AR-42 for 24 hours. The cell proliferation rate was assessed by BrdU incorporation and the median inhibitory concentration was then calculated for each cell line. Three independent experiments were performed, and 1 representative result is presented. (B) P815, C2, and BR cells were treated with 0.5μM AR-42 for 24 hours. Cells were then evaluated for effects on cell cycle using propidium iodide (PI) staining and flow cytometry. Three independent experiments were performed, and 1 representative result is presented. (C) P815, C2, and BR cells (10 × 106) were treated with increasing concentrations of AR-42 and 1μM 17-AAG for 24 hours. Apoptosis was assessed by annexin V/PI staining and flow cytometry. Three independent experiments were performed and their data were calculated (*P < .05). (D) P815, C2, and BR cells were incubated with various concentrations of AR-42 and 17-AAG for 24 hours and caspases-3/7 activation was assessed (*P < .05). Experiments were performed in triplicate and repeated 3 times. (E) P815, C2, and BR cells were treated with various concentrations of AR-42 or 1μM 17-AAG for 24 hours. Effects on the expression of Bcl-2, Bcl-xL, and PARP were determined by Western blot analysis.
AR-42 induces hyperacetylation of histones H3 and H4 and α-tubulin
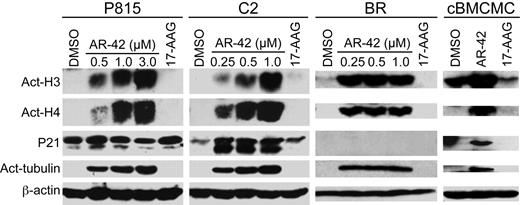
Biomarkers of HDAC inhibition include hyperacetylation of histones and α-tubulin, and the up-regulation of p21 expression. The effects of AR-42 on these biomarkers were assessed in P815, C2, and BR cell lines and canine BMCMCs. As shown in Figure 2, after 24 hours of AR-42 treatment, a dose-dependent hyperacetylation of histone H3, histone H4, and α-tubulin was observed in all cell lines as well as the normal canine mast cells. Although up-regulation of p21 was noted in the C2 line and canine BMCMCs, this did not occur in the P815 and BR cells after exposure to AR-42. However, P815 cells exhibited a higher basal level of p21 expression relative to C2 cells, whereas the p21 level in BR cells was undetectable regardless of treatment. As expected, the HSP90 inhibitor 17-AAG did not induce hyperacetylation of histones or α-tubulin in treated cells.
AR-42 treatment induces acetylation of histones and α-tubulin in malignant mast cells. P815, C2, and BR cell lines were treated with the indicated concentrations of AR-42 or 1μM 17-AAG and canine BMCMCs were treated with 1μM AR-42 or 17-AAG for 24 hours. Effects on the acetylation status of histones H3 and H4 and α-tubulin were determined by Western blotting.
AR-42 treatment induces acetylation of histones and α-tubulin in malignant mast cells. P815, C2, and BR cell lines were treated with the indicated concentrations of AR-42 or 1μM 17-AAG and canine BMCMCs were treated with 1μM AR-42 or 17-AAG for 24 hours. Effects on the acetylation status of histones H3 and H4 and α-tubulin were determined by Western blotting.
AR-42 modulates the expression of mutant Kit via down-regulation of Kit mRNA transcript levels
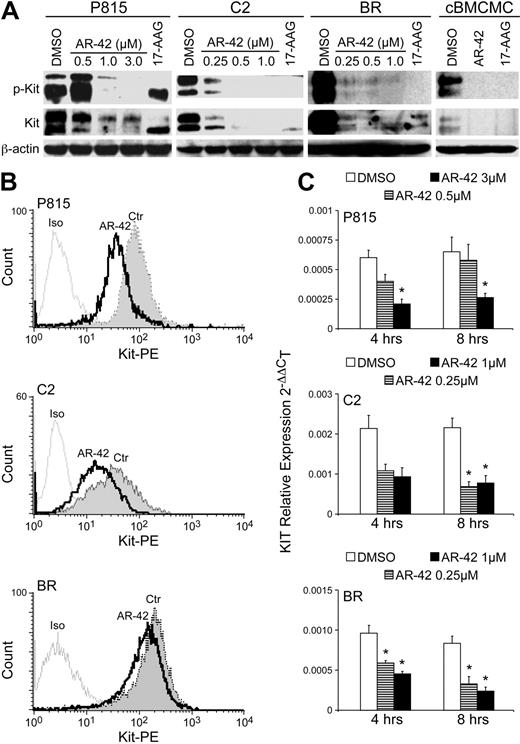
Canine BMCMCs express wild-type Kit and are highly SCF dependent for their survival and proliferation.24 In contrast, malignant mast cell lines and spontaneous primary malignant mast cell tumors often possess activating mutations in Kit including catalytic domain point mutations (D814V/D816V mouse/human cell lines and human systemic mastocytosis) and juxtamembrane domain ITDs and point mutations (dog mast cell tumors and dog mast cell lines).10,13,26,27 These gain-of-function mutations in Kit are important mediators of proliferation, migration, and survival and represent a relevant target for therapeutic intervention.15,16,18,26 To determine how HDAC inhibition may affect Kit in malignant mast cells, we evaluated Kit expression after AR-42 treatment. Western blotting analysis demonstrated reduced levels of phosphorylated and total Kit in all cell lines and canine BMCMCs (wild type) after 24 hours of treatment with AR-42 (Figure 3A). 17-AAG, used as the control for these experiments, was previously shown to down-regulate various forms of constitutively activated Kit in malignant mast cell lines.17 The canine BMCMCs were maintained in the presence of 50 ng/mL rcSCF, resulting in the observed phosphorylation of wild-type Kit. In P815, C2, and BR cells, Kit cell-surface expression was down-regulated after only 6 hours of AR-42 treatment (Figure 3B). To determine whether modulation of Kit protein expression was secondary to loss of Kit mRNA, we performed quantitative reverse-transcription–PCR for Kit after treatment of cell lines AR-42 for 4 or 8 hours. We found that AR-42 down-regulated Kit mRNA transcription at both time points (Figure 3C), indicating that alteration of gene transcription is at least partially responsible for the observed effects of AR-42 on Kit protein levels.
AR-42 down-regulates the expression of Kit protein and mRNA. (A) P815, C2, and BR cells and canine BMCMCs were treated with either AR-42 or 17-AAG (1μM AR-42 and 17-AAG for BMCMCs) at the indicated concentrations for 24 hours. Effects on the expression of phosphorylated Kit and total Kit were determined by Western blot analysis. The top band represents the mature form and the bottom band the immature form of Kit. (B) P815, C2, and BR cells (1.0 × 106) were treated with 1μM AR-42. Cells were collected at 6 hours after drug treatment and evaluated for cell-surface Kit expression by flow cytometry. PE-conjugated rat-IgG antibody was used as isotype control. Three independent experiments were performed, and 1 representative result is shown. (C) P815, C2, and BR cells were treated with AR-42 at the indicated concentrations and were collected at 4 and 8 hours after treatment, and real-time PCR for Kit was performed. Experiments were performed in triplicate and repeated 3 times. The difference between treatment groups and DMSO control group was analyzed using the Student t test. *P < .05.
AR-42 down-regulates the expression of Kit protein and mRNA. (A) P815, C2, and BR cells and canine BMCMCs were treated with either AR-42 or 17-AAG (1μM AR-42 and 17-AAG for BMCMCs) at the indicated concentrations for 24 hours. Effects on the expression of phosphorylated Kit and total Kit were determined by Western blot analysis. The top band represents the mature form and the bottom band the immature form of Kit. (B) P815, C2, and BR cells (1.0 × 106) were treated with 1μM AR-42. Cells were collected at 6 hours after drug treatment and evaluated for cell-surface Kit expression by flow cytometry. PE-conjugated rat-IgG antibody was used as isotype control. Three independent experiments were performed, and 1 representative result is shown. (C) P815, C2, and BR cells were treated with AR-42 at the indicated concentrations and were collected at 4 and 8 hours after treatment, and real-time PCR for Kit was performed. Experiments were performed in triplicate and repeated 3 times. The difference between treatment groups and DMSO control group was analyzed using the Student t test. *P < .05.
AR-42 induces dissociation between Kit and HSP90
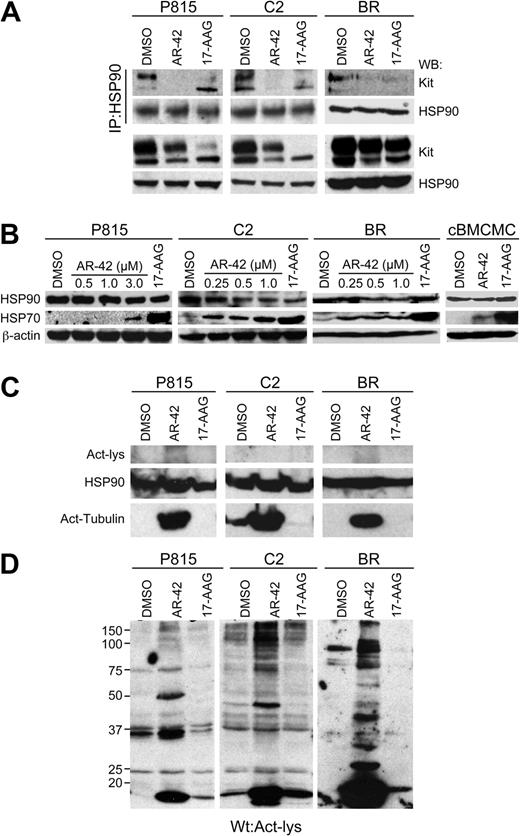
Previous studies have shown that HDAC inhibition can induce HSP90 hyperacetylation, resulting in loss of its chaperone function and consequent degradation of client proteins.5 As Kit is a client protein of HSP9017,18 and HSP90 function is lost after HDACi treatment of human gastrointestinal stromal tumor lines,6 we investigated the potential role of HSP90 function in AR-42–induced repression of Kit in the malignant mast cell lines. First, we examined the effects of AR-42 on the interaction between HSP90 and Kit. Lysates of P815, C2, and BR cells treated for 8 hours with AR-42 or 17-AAG were generated and HSP90 was immunoprecipitated, followed by Western blotting for Kit. Blots were then stripped and reprobed for HSP90. Both AR-42 and 17-AAG treatments reduced the amount of Kit associated with HSP90 in all cell lines (Figure 4A top panels). As a control, the P815, C2, and BR cell lines were treated identically and straight Western blotting was performed for Kit and HSP90 (Figure 4A bottom panels), demonstrating the presence of these proteins in the lysates. Next, we evaluated the P815, C2, and BR cells and normal canine BMCMCs for up-regulation of HSP70, a marker of HSP90 inhibition, after AR-42 treatment. As shown in Figure 4B, after exposure to AR-42, HSP70 expression was up-regulated, indicative of loss of HSP90 activity, whereas total HSP90 expression remained relatively unchanged. However, the magnitude of the HSP70 up-regulation was substantially less than that induced by 17-AAG treatment.
AR-42 promotes disassociation of Kit from HSP90 without evidence of HSP90 hyperacetylation. (A) Mast cell lines were treated with 3μM (P815 cells) or 1μM (C2 and BR cells) AR-42 or 1μM 17-AAG for 8 hours. HSP90 was immunoprecipitated from the cell lysates, and the levels of Kit and HSP90 in the immunoprecipitates were determined by Western blot analysis (top panel). Western blotting for Kit and HSP90 was also performed on total cell lysates (50 μg) before immunoprecipitation as a control (bottom panel). (B) P815, C2, and BR cells were treated with increasing concentrations of AR-42 or 1μM 17-AAG for 24 hours. Equal amounts of cell lysates were analyzed by Western blotting to detect induced HSP70, HSP90, and β-actin. (C) Mast cell lines were treated with 3μM (P815 cells) or 1μM (C2 and BR cells) AR-42 or 1μM 17-AAG for 24 hours. Protein lysates were generated and, after 7% SDS-PAGE of 200 μg of total protein, Western blotting was performed for acetyl-lysine. Blots were then stripped and reprobed first for total HSP90 and then for acetyl-tubulin. The top panel demonstrates the lack of any 90-kDa acetylated protein, although the 90-kDa HSP90 protein is evident after Western blotting for this protein (middle panel). (D) Mast cell lines were treated with 3μM (P815 cells) or 1μM (C2 and BR cells) AR-42 or 1μM 17-AAG for 24 hours. Protein lysates were generated and, after 10% SDS-PAGE of 200 μg of total protein, Western blotting was performed for acetyl-lysine. Multiple acetylated proteins are evident on the Western blot, although none corresponds directly to HSP90. All experiments shown in this figure were performed 3 times.
AR-42 promotes disassociation of Kit from HSP90 without evidence of HSP90 hyperacetylation. (A) Mast cell lines were treated with 3μM (P815 cells) or 1μM (C2 and BR cells) AR-42 or 1μM 17-AAG for 8 hours. HSP90 was immunoprecipitated from the cell lysates, and the levels of Kit and HSP90 in the immunoprecipitates were determined by Western blot analysis (top panel). Western blotting for Kit and HSP90 was also performed on total cell lysates (50 μg) before immunoprecipitation as a control (bottom panel). (B) P815, C2, and BR cells were treated with increasing concentrations of AR-42 or 1μM 17-AAG for 24 hours. Equal amounts of cell lysates were analyzed by Western blotting to detect induced HSP70, HSP90, and β-actin. (C) Mast cell lines were treated with 3μM (P815 cells) or 1μM (C2 and BR cells) AR-42 or 1μM 17-AAG for 24 hours. Protein lysates were generated and, after 7% SDS-PAGE of 200 μg of total protein, Western blotting was performed for acetyl-lysine. Blots were then stripped and reprobed first for total HSP90 and then for acetyl-tubulin. The top panel demonstrates the lack of any 90-kDa acetylated protein, although the 90-kDa HSP90 protein is evident after Western blotting for this protein (middle panel). (D) Mast cell lines were treated with 3μM (P815 cells) or 1μM (C2 and BR cells) AR-42 or 1μM 17-AAG for 24 hours. Protein lysates were generated and, after 10% SDS-PAGE of 200 μg of total protein, Western blotting was performed for acetyl-lysine. Multiple acetylated proteins are evident on the Western blot, although none corresponds directly to HSP90. All experiments shown in this figure were performed 3 times.
To explore acetylation as a potential mechanism for loss of HSP90 chaperone activity, we treated the P815, C2, and BR cells with DMSO (control), AR-42, or 17-AAG for 24 hours, then immunoprecipitated HSP90 from protein lysates and performed Western blotting for acetyl-lysine, then stripped and reprobed the blots for HSP90. We detected no evidence of acetyl-lysine, despite demonstrating the presence of HSP90 on the reprobed blots (data not shown). We then repeated the treatment of P815, C2, and BR cells with DMSO, AR-42, or 17-AAG for 24 hours, generated protein lysates, and used 7% SDS-PAGE to obtain good separation of proteins in the 90-kDa size range. Western blotting was performed for acetyl-lysine and the blots were then stripped and reprobed for HSP90 (Figure 4C). Once again, no acetyl-lysine was evident at 90 kDa despite the presence of HSP90 at this location upon reprobe. In contrast, acetylated α-tubulin was shown to be present in treated cells when the blots were again stripped, then reprobed for acetylated tubulin (Figure 4C). Lastly, multiple cellular proteins were acetylated after AR-42, but not 17-AAG, treatment (Figure 4D). These data indicate that AR-42 is capable of inducing the acetylation of nonhistone targets, although HSP90 does not appear to be one of these targets in either the mouse or canine malignant mast cell lines. We also found that the HSP90 cochaperones HSP70, HOP, and P23 were not acetylated after AR-42 treatment (data not shown), indicating that direct acetylation of the super-chaperone complex is likely not responsible for the observed loss of HSP90 function.
AR-42 modulates several key cellular proteins
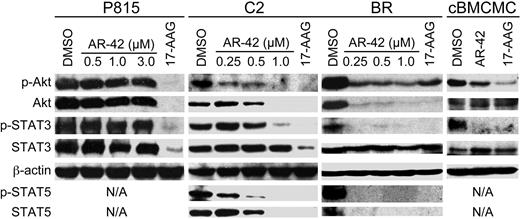
HDACis are known to modulate the activity of multiple molecular targets, including Akt and transcription factors such as STAT3. As these signaling mediators are involved in the regulation of mast cell viability and proliferation, we next investigated the effect of AR-42 on these targets. P815, C2, and BR cell lines and canine BMCMCs were treated with AR-42 or 17-AAG for 24 hours and Western blotting for various proteins was performed. As shown in Figure 5A, AR-42 reduced levels of p-Akt/total Akt and p-STAT3 in C2 and BR cell lines as well as canine BMCMCs, although it also down-regulated both p-STAT5 and total STAT5 in C2 and BR cells; total STAT3 levels remained unchanged in these cell lines. Interestingly, AR-42 failed to modulate Akt and STAT3 in the P815 cells. As expected, the HSP90 inhibitor 17-AAG induced degradation of its client proteins Akt, STAT3, and STAT5.
AR-42 modulates Akt and STAT3/5 pathways in malignant mast cells. P815, C2, and BR cells and canine BMCMCs (1μM AR-42 and 17-AAG) were treated with AR-42 (0.5-3μM) or 17-AAG (1μM) for 24 hours. Effects on the expression of phosphorylated and total levels of Akt, STAT3, and STAT5 were determined by Western blotting. Three independent experiments were performed.
AR-42 modulates Akt and STAT3/5 pathways in malignant mast cells. P815, C2, and BR cells and canine BMCMCs (1μM AR-42 and 17-AAG) were treated with AR-42 (0.5-3μM) or 17-AAG (1μM) for 24 hours. Effects on the expression of phosphorylated and total levels of Akt, STAT3, and STAT5 were determined by Western blotting. Three independent experiments were performed.
AR-42 inhibits invasion of C2 cells
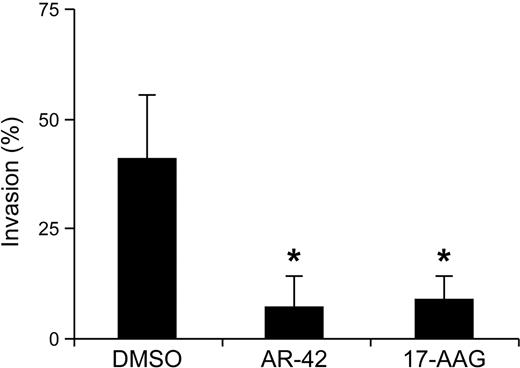
High-grade canine mast cell tumors arising from skin often metastasize to regional lymph nodes, or systemically to the liver and spleen, resulting in a grave prognosis.11 Previous work from our laboratory and others has shown that Kit signaling plays an important role in regulating mast cell motility and invasion.24,28,–30 To assess the effects of AR-42 treatment on malignant mast cell invasion, C2 cells were pretreated with AR-42 or 17-AAG for 8 hours before transfer onto cell culture inserts coated with Matrigel. After 20 hours of incubation, cells that had invaded and migrated into the lower chambers were collected and measured by CyQUANT fluorescence. To adjust for treatment-associated cell death, C2 cells were treated in parallel under the same conditions, but without transfer onto cell-culture inserts. Cell invasion was significantly suppressed by AR-42 and 17-AAG treatment compared with the DMSO-treated controls (Figure 6). Similar experiments were performed with the P815 cells, although no cell invasion was observed in the absence of any drug treatment (data not shown), and thus an effect of AR-42 on migration of these cells could not be determined using this assay.
AR-42 blocks the invasion of C2 cells. C2 cells were pretreated with 1μM AR-42 or 17-AAG for 8 hours and then transferred onto cell-culture inserts coated with Matrigel for another 20 hours. Cells that had invaded and migrated into the lower chambers were collected and enumerated using the CyQUANT assay. To account for drug-induced loss of cell viability, equivalent numbers of C2 cells were treated identically in standard culture wells and the proportion of cells surviving at the end of treatment served as the control for each group (*P < .05). These experiments were performed in triplicate and repeated 3 times.
AR-42 blocks the invasion of C2 cells. C2 cells were pretreated with 1μM AR-42 or 17-AAG for 8 hours and then transferred onto cell-culture inserts coated with Matrigel for another 20 hours. Cells that had invaded and migrated into the lower chambers were collected and enumerated using the CyQUANT assay. To account for drug-induced loss of cell viability, equivalent numbers of C2 cells were treated identically in standard culture wells and the proportion of cells surviving at the end of treatment served as the control for each group (*P < .05). These experiments were performed in triplicate and repeated 3 times.
AR-42 exhibits biologic activity against fresh canine malignant mast cells ex vivo
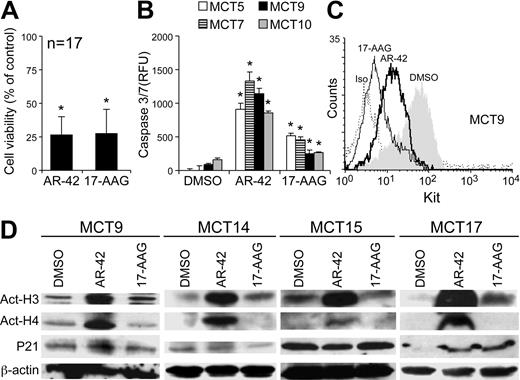
Canine mast cells share many functional similarities with their human counterparts30 and can serve as a good biologic model for human mast cell–associated diseases. Although different Kit mutations have been identified in human and canine malignant mast cells, therapies targeting Kit have been proposed, validated, and intensively studied in both species. The prevalence of spontaneous canine malignant mast cell disease is high compared with human malignant mast cell disease, and as such, it is relatively easy to obtain fresh tumor specimens for biologic analysis. We therefore chose to validate the activity and target modulation of AR-42 using primary canine malignant mast cells derived directly from affected dogs. Malignant mast cells were purified from fine needle aspiration samples of tumors from 17 dogs diagnosed with this disease at the Veterinary Teaching Hospital at The Ohio State University. Cells were treated with AR-42, 17-AAG, or DMSO for 24 or 48 hours then evaluated for cell viability, caspases-3/7 activity, acetylated histones H3 and H4, and changes in expression of p21, Kit, and HSP70. AR-42 and 17-AAG treatment significantly reduced the viability of fresh malignant mast cells cultured ex vivo after 48 hours (Figure 7A). This loss of cell viability was associated with significant up-regulation of caspases-3/7 activity, suggesting involvement of caspase-dependent cell death (Figure 7B). Consistent with findings in the cell lines, AR-42 treatment induced greater caspases-3/7 activity than 17-AAG treatment in the fresh malignant mast cells compared with cells treated with DMSO alone. Furthermore, both AR-42 and 17-AAG down-regulated cell-surface Kit expression 24 hours after treatment (Figure 7C). Hyperacetylation of histones H3 and H4 was observed after AR-42 treatment in the fresh malignant mast cells, as was observed previously in normal canine BMCMCs, but p21 expression was largely unchanged in most of the ex vivo samples (Figure 7D). As with the mast cell lines, 17-AAG treatment did not affect acetylation of histones in the fresh malignant mast cells.
AR-42 induces Kit down-regulation and caspase-3/7 activation in malignant canine mast cells cultured ex vivo. Primary malignant canine mast cells were obtained from clinical patients by fine needle aspiration. Red blood cells were removed by hypotonic shock, and the purity of the cells was determinate by cytologic evaluation. (A) Fresh malignant canine mast cells from 17 individual tumor samples were treated with DMSO, 1μM AR-42, or 1μM 17-AAG in triplicate, and the WST-1 assay was used to assess cell viability after 48 hours of culture. Data from all 17 tumor samples was pooled, and the percentage of cell viability after AR-42 or 17-AAG treatment was calculated using the DMSO group as the 100% control (*P < .05). (B) Fresh malignant canine mast cells (from 4 different patients: MCT nos. 4, 6, 9, and 12 as indicated) were treated with or without 1μM AR-42 or 17-AAG in triplicate for 48 hours, and activation of caspases-3/7 was measured. The difference was analyzed between treatment groups (AR-42 and 17-AAG) and the DMSO control group for each individual tumor sample (*P < .05). (C) Fresh malignant canine mast cells were left untreated or treated with 1μM AR-42 or 17-AAG for 24 hours. Cells were collected and evaluated for Kit cell-surface expression by flow cytometry. The data presented here are from MCT patient no. 9; a total of 3 experiments from 3 different patients were performed once with similar results (data not shown). (D) Fresh malignant canine mast cells (from 4 different patients: MCT nos. 9, 14, 15, and 17 as indicated) were left untreated or treated with 1μM AR-42 or 17-AAG for 24 hours. Effects on the acetylation of histones H3 and H4 and p21 were determined by Western blotting.
AR-42 induces Kit down-regulation and caspase-3/7 activation in malignant canine mast cells cultured ex vivo. Primary malignant canine mast cells were obtained from clinical patients by fine needle aspiration. Red blood cells were removed by hypotonic shock, and the purity of the cells was determinate by cytologic evaluation. (A) Fresh malignant canine mast cells from 17 individual tumor samples were treated with DMSO, 1μM AR-42, or 1μM 17-AAG in triplicate, and the WST-1 assay was used to assess cell viability after 48 hours of culture. Data from all 17 tumor samples was pooled, and the percentage of cell viability after AR-42 or 17-AAG treatment was calculated using the DMSO group as the 100% control (*P < .05). (B) Fresh malignant canine mast cells (from 4 different patients: MCT nos. 4, 6, 9, and 12 as indicated) were treated with or without 1μM AR-42 or 17-AAG in triplicate for 48 hours, and activation of caspases-3/7 was measured. The difference was analyzed between treatment groups (AR-42 and 17-AAG) and the DMSO control group for each individual tumor sample (*P < .05). (C) Fresh malignant canine mast cells were left untreated or treated with 1μM AR-42 or 17-AAG for 24 hours. Cells were collected and evaluated for Kit cell-surface expression by flow cytometry. The data presented here are from MCT patient no. 9; a total of 3 experiments from 3 different patients were performed once with similar results (data not shown). (D) Fresh malignant canine mast cells (from 4 different patients: MCT nos. 9, 14, 15, and 17 as indicated) were left untreated or treated with 1μM AR-42 or 17-AAG for 24 hours. Effects on the acetylation of histones H3 and H4 and p21 were determined by Western blotting.
Discussion
Epigenetic changes are common in many cancers. Unlike genetic changes, epigenetic changes are reversible and evidence suggests that strategies to alter epigenetic changes have therapeutic potential. Inhibition of histone deacetylation is one approach to modify the expression of various genes that control cell proliferation and survival. Several HDACis are currently undergoing clinical evaluation including vorinostat (Merck), which has been approved for the treatment of cutaneous lymphoma in people. We previously evaluated both vorinostat and a novel pan-HDAC inhibitor, AR-42, against a canine malignant mast cell line (C2) and found that both compounds inhibited the viability of these cells, although AR-42 was more potent.22 Another HDACi, trichostatin A, was shown to induce apoptosis in the P815 cell line via histone-dependent and intrinsic apoptosis pathways.31 The purpose of this study was to further characterize the biologic effects of HDAC inhibition with AR-42 on malignant mast cells and demonstrate that AR-42 induces down-regulation of Kit expression in all cell lines through both transcriptional down-regulation and loss of chaperone (HSP90) activity.
Hyperacetylation of histones H3 and H4 is an important biomarker for HDAC inhibition, as this is necessary for restoration of gene expression. Although hyperacetylation of H3 and H4 occurred in all cell lines and tumor samples tested after AR-42 treatment, up-regulation of the cyclin-dependent kinase inhibitor p21 was observed only in the C2 line, canine BMCMCs, and MCT patient no. 17, presumably resulting in cell-cycle arrest at the G1 phase. The G1 cycle arrest was not observed using 1 to 10μM AR-42 in C2 cells in the previous study.22 This may be secondary to the higher concentrations of drug used, potentially resulting in earlier cell-cycle arrest that was missed at the time cell-cycle analysis was performed. Although the data concerning p21 expression may appear inconsistent between the cell lines and primary cells and somewhat incongruous with prior investigations, we have observed differing effects on p21 gene expression after treatment of several different tumor cell lines with AR-42 (W.C.K., unpublished data, September 2009). As the hyperacetylation of H3/H4 serves only as a biomarker, additional studies are currently under way to determine whether the expression of additional cell-cycle regulators are altered after histone modification by AR-42 in malignant mast cells.
Both wild-type Kit and various forms of constitutively activated Kit found in canine and mouse malignant mast cells were down-regulated after AR-42 treatment. In contrast, Kit small molecule inhibitors often exhibit variable potencies against specific Kit mutants. For example, imatinib mesylate exhibits minimal activity against human malignant mast cells possessing the D816V Kit mutation.32 Similarly, the concentration of toceranib phosphate necessary to inhibit the D814V Kit mutant in P815 is 2.5 to 5 times higher than that sufficient to inhibit Kit possessing a juxtamembrane ITD.26 Thus, our data suggest that HDACi, particularly AR-42, may exhibit broader efficacy against tumor cells expressing diverse forms of mutant Kit, thereby potentially circumventing issues with drug resistance recognized with typical Kit small molecular inhibitors.
We found that the down-regulation of Kit was at least partly due to inhibition of Kit gene transcription. Furthermore, our data indicate that a reduction in HSP90 chaperone activity may contribute to and possibly enhance the observed loss of Kit expression. Up-regulation of inducible HSP70, a biomarker of HSP90 inhibition, occurred after AR-42 treatment, indirectly supporting the notion that HSP90 activity was repressed in the malignant mast cells. It is important to point out, however, that loss of Kit protein through down-regulation of gene transcription after AR-42 treatment may have contributed to the observed absence of Kit after immunoprecipitation of HSP90. Although we cannot quantitate the relative contribution of loss of Kit gene expression and loss of HSP90 chaperone activity to down-regulation of Kit protein, it is likely that both contribute to the efficiency of cell death induced by AR-42. Previous studies have demonstrated that HSP90 is a potential target of HDACi, resulting in acetylation of HSP90, loss of chaperone function, and subsequent degradation of client proteins.5,8 Potential HSP90 clients that could be affected by acetylation of this chaperone include several oncogenes such as Kit, Bcr-Abl, Her2/Neu, B-Raf, and Akt.33,34 Our data suggest that AR-42 treatment disrupted the interaction between HSP90 and Kit, presumably contributing to its degradation. The mechanistic paradigm for the HSP90-inhibitory activity of HDACi is that inhibition of HDAC6, an HSP90 deacetylase, leads to the hyperacetylation of HSP90, with subsequent dissociation from cochaperones and loss of chaperone activity. In the present study, however, increased acetylation of HSP90, HSP70, or its cochaperones, HOP and p23, was not detected in AR-42–treated P815, BR, and C2 cells, although tubulin acetylation was evident. Interestingly, FK228 (a class I HDACi), but not vorinostat, disrupts HSP90 chaperone function, resulting in Bcr-Abl degradation via hyperacetylation of HSP70 but not HSP90 in leukemia cells.35 Thus, whether the lack of HSP90 acetylation is unique to AR-42 relative to other HDACis or is characteristic of malignant mast cells is unclear and requires further investigation. Although the effect of AR-42 against wild-type Kit may be a concern regarding the potential for bone marrow toxicity, no substantial clinical effects other than infertility were noted in previous studies exploring the activity of AR-42 in mouse models.19
AR-42 modulated the activation status and/or expression of several cellular proteins including Akt, STAT3, and STAT5. With respect to the loss of pAkt, this is likely secondary to loss of total Akt, possibly due to inhibition of HSP90 chaperone activity. We have previously shown that direct inhibition of HSP90, Kit, or Akt significantly reduces the viability of canine malignant mast cells.18 Furthermore, the novel STAT3 inhibitor, LLL3,36 decreases the viability of C2 canine malignant mast cells in a dose-dependent manner (data not shown). Thus, simultaneous inhibition of multiple cellular pathways may contribute to the observed effects of AR-42 on normal and malignant mast cells. Down-regulation of both p-STAT5 and STAT5 was observed after AR-42 treatment of canine malignant mast cell lines. Previous studies with different HDACis demonstrated a decrease in p-STAT5 in human leukemia cells after treatment, but total STAT5 remained unchanged.37,–39 In the current study, both AR-42 and 17-AAG reduced p-STAT5/STAT5 in the malignant mast cells, suggesting that this transcription factor may be an HSP90 client protein in mast cells. Lastly, HDACi is known to modulate Akt via several mechanisms. For example, down-regulation of total Akt by HDACi can be achieved through loss of HSP90 chaperone activity as it is a known client protein. Both pAkt/Akt were reduced in canine mast cell lines. In addition, AR-42 can bind HDAC6, causing its dissociation from protein phosphatase 1, which is then free to dephosphorylate Akt.20
In the current study, AR-42 inhibited the migration of C2 cells through Matrigel. Although Kit is likely important in tumor cell migration, hyperacetylation of α-tubulin has been reported to perturb microtubule dynamics, resulting in the inhibition of migration.40 Thus, both down-regulation of Kit and hyperacetylation of α-tubulin may play a role in the effects of AR-42 on C2 cell motility.
In summary, the work presented here is the first to demonstrate that AR-42 down-regulates wild-type and mutant Kit in normal and malignant mast cells, resulting in cell death. Our data suggest that both alteration of Kit gene expression and loss of HSP90 chaperone activity contribute to the observed loss of Kit expression. Furthermore, the effects of AR-42 on multiple cell signaling proteins such as Akt and STAT3 likely enhance the loss of Kit-driven survival signals present in the normal and malignant mast cells. As such, HDAC inhibition may represent a promising therapeutic approach for the treatment of malignant mast cell disease.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This study was supported by a grant from the AKC Canine Health Foundation.
Authorship
Contribution: T.-Y.L. designed and performed research, analyzed data, and wrote the paper; J.F. performed the real-time PCR and sequencing of Kit; S.M. generated preliminary data that led to work with AR-42 and mast cells; M.D.B. provided technical support and performed some of the experiments in this study; S.K.K. assisted with experimental design and editing of the paper; D.W. contributed vital new reagents (AR-42) for this study; C.-S.C. contributed vital new reagents and assisted with analysis of data; W.C.K. performed initial studies with AR-42 that supported work with compound in malignant mast cells, edited the paper, and assisted in analysis; and C.A.L. assisted in the design of research, oversaw analysis of data, assisted in writing of the paper, and edited the paper.
Conflict-of-interest disclosure: C.-S.C. received royalty payments from Arno Therapeutics per the licensing agreement of AR-42 between The Ohio State University Research Foundation and the company. The remaining authors declare no competing financial interests.
Correspondence: Cheryl A. London, 454 VMAB, 1925 Coffey Rd, Department of Veterinary Biosciences, The Ohio State University, Columbus, OH 43210; e-mail: london.20@osu.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal