To assess whether treatment with enoxaparin and low-dose aspirin, along with intensive pregnancy surveillance, reduces rate of pregnancy loss compared with intensive pregnancy surveillance alone in women with history of 2 or more consecutive previous pregnancy losses, a parallel group, multicenter, randomized controlled trial was performed in the United Kingdom and New Zealand. Participants (n = 294) presenting for initial antenatal care at fewer than 7 weeks' gestation with history of 2 or more consecutive previous pregnancy losses at 24 or fewer weeks' gestation and no evidence of anatomic, endocrine, chromosomal, or immunologic abnormality were randomly assigned to receive either enoxaparin 40 mg subcutaneously and 75 mg of aspirin orally once daily along with intense pregnancy surveillance or intense pregnancy surveillance alone from random assignment until 36 weeks' gestation. The primary outcome measure was pregnancy loss rate. Of the 147 participants receiving pharmacologic intervention, 32 (22%) pregnancy losses occurred, compared with 29 losses (20%) in the 147 subjects receiving intensive surveillance alone, giving an odds ratio of 0.91 (95% confidence interval, 0.52-1.59) of having a successful pregnancy with pharmacologic intervention. Thus, we observed no reduction in pregnancy loss rate with antithrombotic intervention in pregnant women with 2 or more consecutive previous pregnancy losses. The trial was registered at http://www.controlled-trials.com as ISRCTN06774126.
Continuing Medical Education online
This activity has been planned and implemented in accordance with the Essential Areas and policies of the Accreditation Council for Continuing Medical Education (ACCME) through the joint sponsorship of Medscape, LLC and the American Society of Hematology. Medscape, LLC is accredited by the ACCME to provide continuing medical education for physicians. Medscape, LLC designates this educational activity for a maximum of 0.5 AMA PRA Category 1 credits™. Physicians should only claim credit commensurate with the extent of their participation in the activity. All other clinicians completing this activity will be issued a certificate of participation. To participate in this journal CME activity: (1) review the learning objectives and author disclosures; (2) study the education content; (3) take the post-test and/or complete the evaluation at http://cme.medscape.com/journal/blood; and (4) view/print certificate. For CME questions, see page 4319.
Disclosures
Andrew Thomson has received honoraria from Sanofi Aventis and Leo for lectures. Ian A. Greer has received honoraria from Sanofi Aventis and Leo for lectures and advisory boards. The remaining authors; Associate Editor David P. Lillicrap; and CME questions author Charles P. Vega, University of California, Irvine, CA, declare no competing financial interests.
Learning objectives
Upon completion of this activity, participants will be able to:
Describe hematologic factors associated with recurrent pregnancy loss and potential treatment to prevent pregnancy loss
Identify the efficacy of enoxaparin plus aspirin in improving rates of pregnancy success
Specify the prevalence of identified causes of thrombophilia in the study cohort of women with recurrent pregnancy loss
Compare the rate of adverse events in pregnant women treated with enoxaparin plus aspirin versus intensive surveillance only
Introduction
Recurrent pregnancy loss is a main issue for women's health, with 3 or more successive losses affecting 1% to 2% of women of reproductive age and 2 or more successive losses affecting approximately 5%.1 Although a small proportion is associated with identifiable abnormalities in the mother or the fetus, the cause of most cases of recurrent loss remains unknown. It is recognized, however, that successful pregnancy outcome depends on the development and maintenance of an adequate utero-placental circulation, with evidence that prothrombotic factors underlie some pregnancy losses.2 In particular, antithrombotic therapy may prevent pregnancy loss3,–5 in the antiphospholipid syndrome,6,7 although this may not be due to an exclusively anticoagulant effect. Recent data also implicate heritable thrombophilia in pregnancy loss.8 This association may be important as a substantial proportion of whites carry an identifiable thrombophilic predisposition. Despite this, preliminary data examining pregnancy success with the use of antithrombotic therapy in women with heritable thrombophilia are inconclusive.9,10 However, it has also been suggested that heparin could be of benefit in preventing pregnancy loss in women without thrombophilia.11 Consequently, given that 2 successive pregnancy losses are relatively common and distressing and that there is a lack of effective treatments, antithrombotic therapy is often prescribed in these women. However, although low-molecular-weight heparins (LMWHs) and low-dose aspirin are generally seen as being safe,12,13 there is no direct evidence of efficacy. As a result, there have been repeated calls for randomized trials in this area, particularly a comparison of anticoagulant treatment with no pharmacologic intervention.9,14,–16
In clinical practice, the investigation of recurrent pregnancy loss varies widely; clinical histories will not usually permit an accurate classification of miscarriage, and an identifiable cause is usually absent. In addition, there is no clear consensus on what constitutes appropriate thrombophilia testing or, indeed, a standardized laboratory method for thrombophilia assessment.17,18 Moreover, whether antithrombotic therapy is beneficial in antiphospholipid syndrome only or has an effect relating to a nonspecific thrombogenic phenotype is unclear. Thus, such women can often be managed only pragmatically on the basis of the number of previous losses.
The Scottish Pregnancy Intervention (SPIN) study was designed as a pragmatic, multicenter, randomized controlled trial to assess whether treatment with enoxaparin and low-dose aspirin, along with intensive pregnancy surveillance, in those with a history of 2 or more consecutive pregnancy losses at 24 or fewer weeks' gestation and with no evidence of anatomic, endocrine, chromosomal, or immunologic abnormality, results in a reduction in the rate of loss in the current pregnancy compared with intensive pregnancy surveillance alone. Data were also collected on the tolerance and safety of enoxaparin therapy in pregnancy. Although not intended to be analyzed in relation to the primary outcome, information on the carriage of common thrombophilias was also collected to inform future studies in this area.
Methods
Study design and patients
SPIN is a multicenter international randomized hospital-based clinical trial. Between the June 30, 2004, and May 12, 2008, a total of 294 women were randomly assigned in 11 centers in Scotland, 2 centers in England, and 1 center in New Zealand. All women were to be followed up until completion of the index pregnancy. Subjects were eligible for enrollment if they had a history of a minimum of 2 consecutive early pregnancy losses (defined as at or before 24 weeks' gestation) and presented for initial antenatal care at fewer than 7 weeks' gestation with a positive pregnancy test. In all cases, on confirmation of a positive pregnancy test, subjects attended their hospital for the routine assessment of full blood count, coagulation screen, red cell grouping, red cell antibody screening, thyroid function testing, and ultrasound scanning confirmation of pregnancy. Those participants with satisfactory results of these tests were provided with information and asked to give consent to the study. Women were excluded if (1) previous fetal loss investigations (as outlined by United Kingdom national guidelines19,20 ) had determined that a previous loss was associated with anatomic, chromosomal, endocrine, or immunologic causes; (2) they had a history of venous or arterial thrombosis; (3) they were already known to have antiphospholipid syndrome (as defined locally by the presence of a persistent antiphospholipid antibody and 3 consecutive early pregnancy failures); (4) at enrollment they were not previously known to have antiphospholipid antibodies but had a history of 3 or more pregnancy losses and were screened at enrollment and found to have a positive lupus inhibitor screen or immunoglobulin G/immunoglobulin M (IgG/IgM) anticardiolipin antibodies (ACAs) above the local reference range; (5) at enrollment they were already known to have a thrombophilic disorder, or (6) they were found to have an excluding condition on booking for the current pregnancy (such as anemia requiring therapy, platelet count < 150 × 1012/L, abnormal thyroid function, multiple or rare red cell alloantibodies or autoantibodies). Furthermore, in women with a previous successful pregnancy, only those women in whom the 2 most recent pregnancies had resulted in consecutive losses were eligible for inclusion.
Participants were enrolled by appropriate staff at the participating centers. Randomization was administered by telephone centrally by the Department of Transfusion Medicine, Ninewells Hospital, using consecutively numbered randomization envelopes supplied by the Medical Statistics Unit, Public Health Sciences, University of Edinburgh. Once eligibility was confirmed and the necessary baseline details were logged, patients were randomly assigned by telephone to either (1) enoxaparin 40 mg subcutaneously, once per day by self-injection and 75 mg of aspirin orally once daily until 36 weeks' gestation along with intense pregnancy surveillance or (2) intensive pregnancy surveillance with no specific pharmacologic intervention. The fetal surveillance to be offered consisted of scans every 2 weeks from the diagnosis of pregnancy until 12 weeks' gestation, then monthly ultrasound scans to assess fetal growth until 28 weeks' gestation. In the heparin/aspirin treatment study arm, full blood counts were performed 7 to 10 days after commencement of treatment and thereafter at 28 and 36 weeks' gestation.
The protocol was approved by the multicenter research ethics committee (REF, 03/8/041) and by the local ethics committees of all contributing centers. Informed, written, consent for entry into the study was obtained before randomization in accordance with the Declaration of Helsinki. The study is registered as ISRCTN06774126.
Thrombophilia investigations
Once women were randomly assigned, a further blood aliquot was sent to a central laboratory (Department of Hematology, Royal Infirmary) to test for thrombophilia. The results of these tests were not to be disclosed to the women or their attending clinicians until 6 weeks after delivery or pregnancy loss. At 12 weeks' gestation, a further blood sample was also sent to the central coagulation laboratory for confirmatory thrombophilia testing. Factor V Leiden and prothrombin G20210A status were determined by standard methods.21,22 IgG and IgM ACA assays were performed by enzyme-linked immunoabsorbent assay (Cambridge Life Sciences). The presence of a lupus inhibitor was determined by a screening test and mixing studies (an activated partial thromboplastin time and an activated partial thromboplastin time on a 50:50 mixture of test and normal plasma), as well as a second screening test (using a dilute Russell viper venom time) with a confirmatory platelet correction procedure.
Statistical analysis
Initial estimates of probable loss rates in the SPIN study were derived from data on pregnancy outcome and past obstetric history in a Scottish cohort of 2500 pregnancies managed conventionally.23 Therefore, we anticipated that 25% to 30% of women with at least 2 pregnancy losses would be expected to experience another loss in the next pregnancy. Initial plans were to continue the study until 300 recruits were achieved in each group. As based on the initial estimate of loss, such a study would have 90% power (at 5% significance) to detect a reduction in the pregnancy loss rate from 25% to 15%. However, because the overall pregnancy loss rate was lower than anticipated, along with slower recruitment and funding limitations, the decision was made in late 2007 to continue recruitment until May 2008. This was the last date that would allow complete follow-up of recruits within the remaining period of funding. A post hoc power calculation, based on the observed pregnancy loss rate of the study participants, indicates that the study had greater than 80% power (P = .05) to detect an improvement in outcome of approximately 60% with anticoagulants, an effect size not dissimilar to that observed when aspirin/heparin is used in women with recurrent miscarriage and antiphospholipid syndrome.24
The prespecified analysis of the primary end point was to be by intention to treat, using a χ2 analysis to compare the pregnancy failure rates in the treatment and surveillance arms. No formal statistical analysis was planned to be performed on the secondary outcomes of tolerance and safety of enoxaparin and aspirin therapy in pregnancy or on the thrombophilia information. Twin pregnancies were classified as successful if they resulted in any live birth.
Results
The profile of trial enrollment and outcomes is shown in Figure 1. The demographic information for women randomly assigned to treatment is shown in Table 1. The groups were well matched. For all subjects randomly assigned, 42.9% had experienced more than 2 previous losses. In the index pregnancy an overall pregnancy loss rate of 20.8% was observed. Of those women randomly assigned to enoxaparin and low-dose aspirin plus intensive surveillance, 4 subjects were lost to follow-up and 32 pregnancy losses were observed in 143 subjects. In the 147 subjects randomly assigned to intensive surveillance alone, 7 subjects were lost to follow-up and 29 losses were observed in 140 subjects. The χ2 (Yates correction) value was 0.04 (df = 1; P = .85), and the odds ratio of having a successful pregnancy was 0.91 (95% confidence interval, 0.52-1.59) in women randomly assigned to pharmacologic intervention compared with intensive surveillance alone. In addition to 2 pregnancy terminations, 5 losses occurred after 16 weeks' gestation in the group randomly assigned to pharmacologic intervention, whereas none occurred after 16 weeks in subjects undergoing surveillance only. These later losses in the intervention arm comprised one case of premature rupture of membranes at 22 weeks, resulting in death associated with prematurity, one stillbirth at 36 weeks associated with cord prolapse, one intrauterine death accompanied by a cystic hygroma and talipes at 21 weeks, one miscarriage at 17 weeks' gestation despite insertion of a cervical stitch, and one stillbirth at 36 weeks' gestation accompanied by preeclampsia and growth restriction.
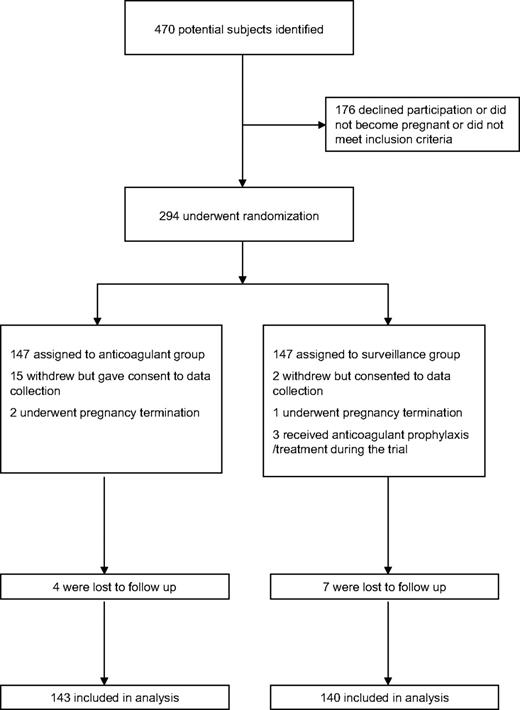
Enrollment and outcomes. The enrollment and outcomes of participants in the trial are shown. In the anticoagulant group, 4 of the 15 withdrawals occurred after an adverse event (1 with a new diagnosis of von Willebrand disease, 2 with unrelated antepartum hemorrhage, and 1 with gastric symptoms). Of the 15 who withdrew, 10 received therapy until at least 12 weeks of gestation. In the surveillance-only group the 3 who received anticoagulant prophylaxis/treatment did so from 6, 16, and 26 weeks, respectively. The 2 who withdrew from surveillance did so at 10 and 24 weeks.

Enrollment and outcomes. The enrollment and outcomes of participants in the trial are shown. In the anticoagulant group, 4 of the 15 withdrawals occurred after an adverse event (1 with a new diagnosis of von Willebrand disease, 2 with unrelated antepartum hemorrhage, and 1 with gastric symptoms). Of the 15 who withdrew, 10 received therapy until at least 12 weeks of gestation. In the surveillance-only group the 3 who received anticoagulant prophylaxis/treatment did so from 6, 16, and 26 weeks, respectively. The 2 who withdrew from surveillance did so at 10 and 24 weeks.
Baseline characteristics of randomly assigned women
| Characteristic . | Anticoagulant group, n = 147 . | Intensive surveillance group, n = 147 . |
|---|---|---|
| Age, y, median (interquartile range) | 31 (26-36) | 32 (27-36) |
| No. with at least 1 previous live birth (%) | 67 (45.6) | 66 (44.9) |
| No. of previous losses, median (interquartile range) | 2 (2-3) | 2 (2-3) |
| No. with more than 2 previous losses (%) | 66 (44.9) | 60 (40.8) |
| BMI, kg/m2, median (interquartile range) | 25.4 (21.8-29.0) | 26.4 (23.1-32.4) |
| Current smoker, n (%) | 29 (19.7) | 25 (17.0) |
| Gestation at random assignment, wk, median (interquartile range) | 6 (6-7) | 6 (6-6) |
| Presence of multiple pregnancy, n (%) | 2 (1.4) | 3 (2.0) |
| Characteristic . | Anticoagulant group, n = 147 . | Intensive surveillance group, n = 147 . |
|---|---|---|
| Age, y, median (interquartile range) | 31 (26-36) | 32 (27-36) |
| No. with at least 1 previous live birth (%) | 67 (45.6) | 66 (44.9) |
| No. of previous losses, median (interquartile range) | 2 (2-3) | 2 (2-3) |
| No. with more than 2 previous losses (%) | 66 (44.9) | 60 (40.8) |
| BMI, kg/m2, median (interquartile range) | 25.4 (21.8-29.0) | 26.4 (23.1-32.4) |
| Current smoker, n (%) | 29 (19.7) | 25 (17.0) |
| Gestation at random assignment, wk, median (interquartile range) | 6 (6-7) | 6 (6-6) |
| Presence of multiple pregnancy, n (%) | 2 (1.4) | 3 (2.0) |
BMI indicates body mass index.
Of all women randomly assigned, a lupus inhibitor assessment that was positive at both random assignment and at 12 weeks' gestation and/or an IgG or IgM ACA assessment that was above the local reference range on both occasions was detected in 7 of the 292 subjects (2.4%), when appropriate samples were received for analysis. Of these women, 5 were observed in those randomly assigned to intensive surveillance, with one of these subjects identified as having both a persistent positive lupus inhibitor and positive IgG ACA assessment. Eight of 289 subjects (2.8%) were identified as heterozygous carriers of factor V Leiden, with 5 carriers identified in the group randomly assigned to pharmacologic intervention. Two of 288 subjects (0.7%) were identified as heterozygous carriers of the prothrombin G20210A mutation, with one identified in each arm of the trial.
No suspected serious adverse reactions were recorded, but the adverse events that could conceivably relate to anticoagulant/aspirin medication are shown in Table 2. Sixteen events, classified as “nonserious” (as defined by European Union Directive 2001/20/EC, Article 2), were coded by the local investigators as “probably” related to the trial medication. All but 6 of these nonserious events were coded as being of “mild”' severity with only one (a rash at the injection site) coded as being of “severe” intensity. In the anticoagulant group, one report of fetal thrombocytopenia was recorded. However, no maternal thrombocytopenia was reported in this case, making any potential link with the study drugs unlikely. One maternal persistent thrombocytopenia was reported as a nonserious adverse event in the surveillance arm. No complications attributable to osteopenia and no allergic or skin reactions were reported as serious adverse events in either group.
Adverse events in the SPIN trial
| Event category/episode . | Pharmacologic intervention group, n . | Intensive surveillance group, n . |
|---|---|---|
| Serious adverse event | ||
| Antepartum hemorrhage/vaginal bleed | 10 | 10 |
| Postpartum hemorrhage | 14 | 13 |
| Low hemoglobin | 0 | 1 |
| Nonserious adverse event | ||
| Antepartum hemorrhage/vaginal bleed | 45 | 31 |
| Injection site/abdominal bruising | 16 | 0 |
| Nosebleed | 13 | 0 |
| Bleeding (other) | 6 | 0 |
| Postpartum hemorrhage | 3 | 5 |
| Anemia | 3 | 1 |
| Injection site pain | 1 | 0 |
| Injection site itch | 2 | 0 |
| Injection site or other rash | 2 | 0 |
| Gastric upset | 2 | 0 |
| Low platelet count | 0 | 1 |
| Event category/episode . | Pharmacologic intervention group, n . | Intensive surveillance group, n . |
|---|---|---|
| Serious adverse event | ||
| Antepartum hemorrhage/vaginal bleed | 10 | 10 |
| Postpartum hemorrhage | 14 | 13 |
| Low hemoglobin | 0 | 1 |
| Nonserious adverse event | ||
| Antepartum hemorrhage/vaginal bleed | 45 | 31 |
| Injection site/abdominal bruising | 16 | 0 |
| Nosebleed | 13 | 0 |
| Bleeding (other) | 6 | 0 |
| Postpartum hemorrhage | 3 | 5 |
| Anemia | 3 | 1 |
| Injection site pain | 1 | 0 |
| Injection site itch | 2 | 0 |
| Injection site or other rash | 2 | 0 |
| Gastric upset | 2 | 0 |
| Low platelet count | 0 | 1 |
No serious adverse reactions were reported.
Discussion
We believe that this is the first reported randomized controlled trial in women with 2 or more miscarriages, which represents an important area of women's health. We have found that the pragmatic use of an LMWH and low-dose aspirin for women with 2 or more recurrent pregnancy losses has no measurable benefit in preventing further loss compared with intensive fetal surveillance. This contrasts to antiphospholipid syndrome in which such therapy is known to reduce the rate of pregnancy loss.5,–7 These data should inform current practice where, in the absence of any effective intervention, obstetricians have been prescribing antithrombotic therapy for recurrent miscarriage due to the reported association with heritable thrombophilia, the effectiveness in antiphospholipid syndrome, and the safety of LMWH in pregnancy. Our findings do not exclude such an effect in women with a particular heritable thrombophilia. However, the association between heritable thrombophilia and recurrent pregnancy loss is uncertain9 because only a modest association was shown in largely retrospective case-control studies and little association was seen in prospective studies.8,9,25,26 Interestingly, the rate of the common thrombophilias found in the women in this trial was similar to that in the general population.
Heparin has been shown to have potentially beneficial effects on trophoblast that may facilitate implantation,27,–29 including an influence on trophoblast apoptosis. It is possible that to be beneficial heparin may require to be given at the time of the initial implantation, a hypothesis not tested by this trial. In the antiphospholipid syndrome it has been assumed, perhaps, that the beneficial effect of heparin is mediated by an antithrombotic mechanism. However, this may not be the case, and heparin may have its effect by reducing excessive complement activation.30,31 Correspondingly, a beneficial effect of antithrombotic therapy may not be seen in other cases of pregnancy loss. It is also possible that a beneficial effect of pharmacologic intervention may be more apparent in women with 3 or more recurrent miscarriages,32,33 or in women with primary recurrent miscarriage who may represent more homogeneous groups. In this trial, 57.1% of subjects randomly assigned had experienced only 2 previous miscarriages, and so the current study may not be directly applicable to women with 3 or more losses. In this regard, although the study was not designed to robustly examine subgroups, it is of interest that in those with 3 or more previous losses, 17 losses (26.6%) were observed in those 64 subjects randomly assigned to pharmacologic intervention and 14 loses (24.2%) were observed in those 58 subjects randomly assigned to intensive surveillance (with 2 randomly assigned subjects in each category among those lost to follow-up). Similarly, the current study also included some women who had had a previous successful pregnancy. In women randomly assigned to surveillance, comparable failure rates were observed in subjects with such a previous success (20.5%) compared with subjects without (20.9%). By comparison, intervention resulted in a failure rate of 29.2% in subjects with a previous success and 16.7% in subjects without. A further trial would, of course, be required to formally address the effect of intervention in these separate groups. The overall success rate observed in the current study was 79.2%, which is similar to that seen in observational and noncontrolled studies that included various definitions of pregnancy loss, including women with 3 or more losses,9,10,34,35 in which success rates of up to 80% are reported. Finally, there has been debate surrounding the effects of aspirin on miscarriage,36,37 and it is possible that heparin and aspirin had counteracting effects in the current study. Our study identified no significant safety issues, a finding that is consistent with a previous systematic review.12
Our trial does not support the use of this intervention in women with recurrent pregnancy loss not associated with antiphospholipid syndrome. However, a substantially larger trial would be required to exclude a beneficial effect of antithrombotic intervention, either in women with specific thrombotic risk factors, or in women with specific subgroups of pregnancy loss, which may carry differing natural prognoses. Importantly, however, our study does indicate that women are willing to be randomly assigned to a pharmacologic intervention or control group after 2 pregnancy losses. The study also identifies a number of potential areas for future research. These include a trial of women with recurring loss and evidence of a specific heritable thrombophilia and a pragmatic trial of heparin around the time of implantation, perhaps linked to assisted reproduction. More particularly, such studies should consider a comparison of heparin and aspirin. However, it is evident that such studies will be increasingly expensive and difficult to perform, particularly with the lack of interest from the pharmaceutical industry in pregnancy-related trials.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank all the women who agreed to participate in this trial and to all the midwifery staff who contributed their time and effort. We also thank Dr Catherine Hewitt, Department of Health Sciences, University of York, for the statistical analysis of the trial results.
This work was supported by The Chief Scientist Office of the Scottish Government Health Department (reference CVB/4/110). At the behest of the funding source, Sanofi-Aventis UK supplied enoxaparin to a small number of participating centers. The trial was cosponsored by the University of Glasgow/National Health Service Greater Glasgow and Clyde.
Authorship
Contribution: All authors contributed to the trial conception and design and development of the trial protocol. SW conducted local GCP training and site monitoring; P.C. and S.W. collected and collated the data; P.C. and I.A.G. drafted the first version of the manuscript; P.C., I.D.W., P.L., L.C., A.T., M.G., S.W., and I.A.G. critically reviewed, revised, and supplemented the manuscript. All authors approved the final version. P.C. is the guarantor.
Conflict-of-interest disclosure: A.T. has received honoraria from Sanofi Aventis and Leo for lectures. I.A.G. has received honoraria from Sanofi Aventis and Leo for lectures and advisory boards. P.L. has had no competing financial interests in the past 5 years. The remaining authors declare no competing financial interests.
The study sponsors and funding sources had no role in data collection, data storage, data analysis, preparation of this report, or the decision to publish. All authors had full access to the primary data in the study, and the trial steering committee had final responsibility for the decision to submit for publication.
Correspondence: Peter Clark, Department of Transfusion Medicine, Ninewells Hospital and Medical School, Dundee, United Kingdom DD1 9SY; e-mail: peterclark@nhs.net.
Appendix
The SPIN trial collaborators are Dr P. Agustsson, Ninewells Hospital and Medical School, Dundee, United Kingdom; Mr I. Aird, Gateshead Maternity Hospital, Gateshead, United Kingdom; Dr G. Cumming, Dr N. Smith, Royal Infirmary, Aberdeen, United Kingdom; Dr L. Crichton, Dr J. Brennand, Dr A. Cameron, Queen Mother's Maternity Hospital, Glasgow United Kingdom; Dr M. Gaudoin, Southern General Hospital, Glasgow, United Kingdom; Dr J. Ramsay, Ayrshire Central Hospital, Irvine, United Kingdom; Dr S. Maharaj, Dr M. Maclean, Dr D. McLellan, Wishaw General Hospital, Wishaw, United Kingdom; Dr A. Mathers, Royal Infirmary, Glasgow, United Kingdom; Dr C. McLintock, University of Auckland, New Zealand; Prof J. Thornton, Nottingham City Hospital, Nottingham, United Kingdom; Dr A. Thomson, Royal Alexandra Hospital, Paisley, United Kingdom; Dr S. Wisdom, Dumfries and Galloway Royal Infirmary, Dumfries, United Kingdom.
The Trial Monitoring Committee consists of Dr D. Meiklejohn (chair), Department of Haematology, Ninewells Hospital and Medical School, Dundee, United Kingdom; Prof G. D. O. Lowe, Division of Cardiovascular and Medical Sciences, University of Glasgow, United Kingdom; and Prof S. Senn, Department of Statistics, University of Glasgow, United Kingdom.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal