Two decades ago, Eschbach reported an instructive case of a patient with chronic kidney disease (CKD) treated with first-time available recombinant human Epo (rhEpo).3 The 40-year-old man had undergone hemodialysis for 7 years, unsuccessful kidney transplantation, and androgen therapy, received 313 units of red blood cells (RBCs), and was HIV-positive. Within 8 weeks, his hematocrit (Hct) increased from 15% to 38%, RBC transfusions were no longer required, and the patient was again able to work and participate in sports.3
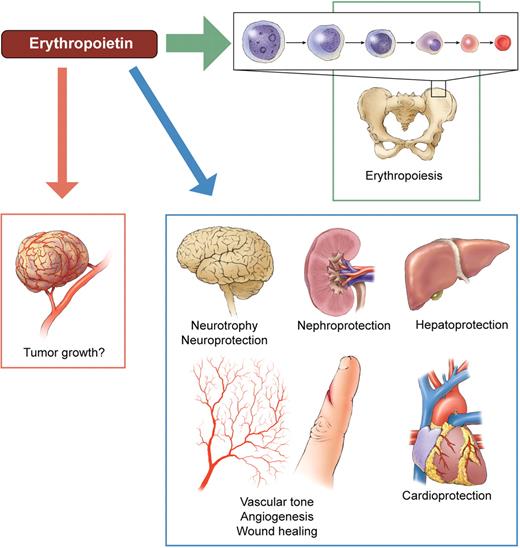
Alleged targets of erythropoietin. The primary task of the hormone (and its therapeutic analogs) is to maintain myeloid erythropoiesis, by preventing erythrocytic progenitors from undergoing apoptosis and by stimulating their proliferation and differentiation. Erythropoietin receptors have been claimed to be present throughout the body, suggesting that erythropoietin is a pleiotropic survival and growth factor. As a result, the alleged erythropoietin's tissue protective potential has already been investigated in clinical trials, for example, in brain and heart diseases. Whether erythropoietin can stimulate cancer growth by promoting tumor cell survival and angiogenesis is an even more controversial issue. Professional illustration by Kenneth X. Probst.
Alleged targets of erythropoietin. The primary task of the hormone (and its therapeutic analogs) is to maintain myeloid erythropoiesis, by preventing erythrocytic progenitors from undergoing apoptosis and by stimulating their proliferation and differentiation. Erythropoietin receptors have been claimed to be present throughout the body, suggesting that erythropoietin is a pleiotropic survival and growth factor. As a result, the alleged erythropoietin's tissue protective potential has already been investigated in clinical trials, for example, in brain and heart diseases. Whether erythropoietin can stimulate cancer growth by promoting tumor cell survival and angiogenesis is an even more controversial issue. Professional illustration by Kenneth X. Probst.
About 25% of CKD patients on dialysis required regular RBC transfusions before rhEpo (“epoetin”) was approved by the FDA in 1989, “to elevate or maintain the red blood cell level and to decrease the need for transfusions.”4 p189 RhEpo and its analog darbepoetin (erythropoiesis stimulating agents [ESAs]) have been liberating for millions of CKD patients and, more recently, cancer patients receiving chemotherapy. Apart from being free from RBC transfusions, the benefits have included improvements in life quality, cognitive function, stamina, and the prevention of left ventricular hypertrophy.5
These triumphs stimulated further clinical trials and off-label uses of ESAs that have, over time, proven how complex and potentially detrimental use of biologic agents may be. First, investigators and primary care physicians extrapolated the data to ask why or in many cases simply assume that raising hemoglobin levels (Hb) into the normal range would further improve clinical outcomes. Unexpectedly, the results were the opposite, as the higher (Hbs) increased thromboembolism. Second, possible cytoprotective actions of ESAs outside the bone marrow were explored (see figure). Pleiotropism is a double-edged sword: would ESAs prevent normal and also malignant cells from undergoing apoptosis? Hence, it is of major importance to identify tissues that possess functional Epo receptors (EpoR) and have physiologically relevant responses to Epo concentrations reached in vivo in patients receiving ESAs.
Sinclair et al report that EpoR mRNA was consistently expressed in human neuronal, cardiac, endothelial and renal cell lines, albeit at low levels compared with cells in the erythroid lineage.1 Yet the presence of EpoR mRNA does not necessarily equate with functional cell-surface EpoR protein. With use of what the authors stress is the first specific anti-EpoR antibody,6 no EpoR protein was detectable on the surface of these cell lines. In case receptor was present and functional, but not at levels detectable by flow cytometry, the authors also investigated downstream signaling. Human EpoR is a homodimer of two 59 kDa transmembrane glycoproteins. On Epo binding, the receptor undergoes a conformational change resulting in the phosphorylation of intracellular signaling proteins. Even on addition of very high doses of rhEpo, no evidence for downstream EpoR signaling occurred in the non-erythrocytic cells tested.1 RhEpo failed to inhibit cytotoxic drug effects in primary cardiac and renal tubular cell cultures, and it did not promote angiogenesis in vivo. These findings prompt reevaluation of the concept that Epo stimulates angiogenesis and careful consideration of the rationale in clinical trials using Epo to treat stroke, acute coronary syndrome and infarction, kidney or liver hypoxic damage, or other ischemic injuries.7
In a study using the same EpoR antibody carried out by some of the same authors, Swift et al measured low levels of EpoR mRNA in 209 human tumor cell lines representing 16 tumor types.2 EpoR protein was barely detectable in the 66 cell lines investigated. EpoR signaling in response to rhEpo was not detected in the nonerythrocytic cells. These results corroborate the findings of several earlier smaller studies.8 If confirmed, these results suggest that Epo's effects on most types of non-hematopoietic tumor cells are minimal, and only likely with doses greatly exceeding the plasma level of Epo even with pharmacologic therapy with this agent. A crucial issue remains regarding the earlier reports of immunochemical positivity for EpoR protein in human tumor biopsies. However, the staining in these studies may have resulted from the use of nonspecific anti-EpoR antibodies that cross-react with heat-shock proteins, for example.8
With respect to safety concerns, the most recent and comprehensive meta-analysis of controlled ESA oncology trials (> 15 000 patients) has shown no significant effect on survival or disease progression.9 However, there was an increased risk for venous-thromboembolic events. Earlier detrimental outcomes were reported only in trials not following current guidelines on the use of ESAs in cancer patients. Both the baseline and the achieved Hb often exceeded the recommended values. The higher blood viscosity in combination with elevated platelet counts likely increased the incidence of thrombus formation.
In CKD patients, Hb less than 100 g/L is unfavorable for health and survival; however, Hb more than 120 g/L is linked with an increased risk for thromboembolic events. The use of rhEpo in a stroke trial for neuroprotection resulted in a higher death rate in patients receiving rhEpo compared with patients receiving placebo, particularly in those requiring thrombolytic therapy.10 Even in healthy persons, the likelihood of a cerebral infarction increases with Hct. Humans, particularly men, have luxuriously high Hb values, more likely an evolutionary relict than a requirement for good health.11 The FDA has recently voiced concerns regarding the use of ESAs to increase Hb in CKD patients above a level intended solely to avert the need for RBC transfusions.4 This may be overcautious, as the target Hb in CKD and in cancer patients receiving chemotherapy is with reason set to 100 to 120 g/L.
The ongoing saga of the use of ESAs should serve as a cautionary tale. The enthusiastic use of these agents got ahead of the science on their beneficial effects. This cautionary tale needs to be heeded by investigators (who need to include long-term outcomes, not just short-term results in clinical trials), pharmaceutical companies (who need to support such trials, conduct long-term follow-up, and refocus their drug promotion more toward hard data shared with physicians) and practitioners (who should stick to hard data, not unproven possibilities, and stay within safety recommendations). It is also a sad tale—these agents, with their promise of benefit, may have harmed some patients. The current negative stream of data has created an environment that makes it difficult to conduct clinical trials to determine their safe use and has scared both patients who could benefit and practitioners away from their use. Let us hope that everyone has learned from this experience.
Conflict-of-interest disclosure: W.J. has received honoraria for consultations and educational lectures from several pharmaceutical companies marketing ESAs. ■