Abstract
Hematopoietic stem (HSC) and progenitor (HPC) cell fate is governed by intrinsic and extrinsic parameters. We examined the impact of hematopoietic niche elements on HSC and HPC function by analyzing the combined effect of osteoblasts (OBs) and stromal cells (SCs) on Lineage−Sca-1+CD117+ (LSK) cells. CFU expansion and marrow repopulating potential of cultured Lineage−Sca-1+CD117+ cells were significantly higher in OB compared with SC cultures, thus corroborating the importance of OBs in the competence of the hematopoietic niche. OB-mediated enhancement of HSC and HPC function was reduced in cocultures of OBs and SCs, suggesting that SCs suppressed the OB-mediated hematopoiesis-enhancing activity. Although the suppressive effect of SC was mediated by adipocytes, probably through up-regulation of neuropilin-1, the OB-mediated enhanced hematopoiesis function was elaborated through Notch signaling. Expression of Notch 2, Jagged 1 and 2, Delta 1 and 4, Hes 1 and 5, and Deltex was increased in OB cultures and suppressed in SC and OB/SC cultures. Phenotypic fractionation of OBs did not segregate the hematopoiesis-enhancing activity but demonstrated that this function is common to OBs from different anatomic sites. These data illustrate that OBs promote in vitro maintenance of hematopoietic functions, including repopulating potential by up-regulating Notch-mediated signaling between HSCs and OBs.
Introduction
Hematopoietic stem cells (HSCs) are multipotent progenitor cells that give rise to all types of mature blood cells. HSCs reside in a complex cellular microenvironment containing osteoblasts (OBs), osteoclasts, endothelial cells, stromal cells (SCs), mesenchymal progenitor cells, and adipocytes as well as multiple components of the extracellular matrix. Collectively, these cellular elements and the extracellular matrix constitute the hematopoietic niche, which most probably regulates the size of the stem cell pool and controls HSC fate.1
OBs play a critical role in HSC function and self-renewal. Primitive HSCs that are in association with the endosteal region have high proliferative and repopulating capacities.2 OBs can deliver proliferative signals to HSCs during mobilization.3 Human OBs secrete cytokines, such as granulocyte colony-stimulating factor, granulocyte-macrophage colony-stimulating factor, and leukemia inhibitory factor, thereby supporting hematopoietic progenitor cell (HPC) function in vitro.4-6 Furthermore, OBs secrete angiopoietin, thrombopoietin, and stromal cell–derived factor-1, all of which regulate HSC maintenance.7-9 Physical and molecular interactions between HPCs and OBs supported in vitro hematopoiesis5 and survival,10 whereas cotransplantation of OBs with HSCs improved engraftment.11 However, others questioned whether OBs contribute to the formation of niches where vascular and perivascular cells play a major role in maintaining HSC function.12
In addition to stem cell–enhancing activity, microenvironment cells in multiple systems can down-regulate stem cell function. Endothelial cells in the perivascular niche reduce the adipogenic potential of adipose stromal cells by up-regulating inhibitors of adipogenesis.13 In the hematopoietic system, adipocytes inhibit lineage-specific differentiation14 and engraftment of transplanted cells.15 These observations suggest that different cells of the hematopoietic niche mediate both positive and negative effects on stem and progenitor cells.
Notch signaling is crucial for HSC formation during embryonic development16 and is critical for HSC maintenance.17 Notch signaling regulates differentiation and maintenance of HSCs, and Notch1 activation promotes stem cell self-renewal.18 Calvi19 and Weber et al20 demonstrated the role of the endosteal niche in maintaining HSC self-renewal through the activation of Notch receptors on HSCs by Jagged1 expressed by OBs. However, the role of Notch signaling in HSC homeostasis has been questioned21,22 because impeding key signaling molecules was ineffective in immediately decreasing HSC numbers or suppressing hematopoiesis.
At present, we do not know precisely how different cellular elements of the hematopoietic niche collaborate to promote HSC self-renewal and to maintain the stem cell pool. Similarly, the interplay between different cell types of the hematopoietic niche that promotes or impedes self-renewing signaling pathways is also not well understood. Herein, we investigated the potential role of OBs and SCs singularly or together on the in vitro and in vivo HSC and HPC function. Collectively, our data provide strong evidence that OBs and SCs play opposing roles in maintaining and expanding hematopoietic function and illustrate that these activities are mediated by the regulation of Notch signaling between OBs and hematopoietic cells.
Methods
Animals
Adult B6.SJL-PtγcqPep3b/BoyJ (BoyJ) mice (6- to 8-week-old), C57BL/6 mice (2-day pups and 6- to 8-week-old), C57BL/6 × BoyJ F1 mice (6- to 8-week-old) were used. Mice were bred and housed in the animal facility at Indiana University. For transplantation, recipient mice received 1100 cGy ionizing radiation from a cesium source (700 and 400 cGy split dose). Cells were infused via the tail vein. All procedures were approved by the Laboratory Animal Research Facility of the Indiana University School of Medicine and followed National Institutes of Health guidelines.
Preparation of OBs
Two-day calvariae OBs.
Calvarial OBs were prepared after a modification of published methods.23,24 Calvariae from C57BL/6 mice less than 48 hours old were dissected, pretreated with ethylenediaminetetraacetic acid in phosphate-buffered saline (PBS) for 30 minutes, and then subjected to sequential collagenase digestions (200 U/mL). Fractions 3 to 5 (collected between 45-60, 60-75, and 75-90 minutes through the digestion) were collected and used as OBs. These cells are more than 95% OBs or OB precursors as previously demonstrated.24 Freshly prepared OBs were used for all studies.
Two-day long bone OBs.
Neonatal long bones (tibiae and femurs) were dissected from C57BL/6 mice less than 48 hours old. Bones were cut into less than 1-mm segments, washed twice with PBS, and then subjected to 2 consecutive collagenase digestions (30 minutes and 1 hour). Cells were collected from both cycles, pooled, and used as described.
Six- to 8-week long bone and calvariae OBs.
For the long bones, the epiphyses were removed and saved. After flushing bone marrow (BM) cells in PBS, diaphyses and epiphyses were combined, cut into less than 1-mm segments, and washed twice with PBS. Calvariae were washed twice with PBS, and less than 1-mm segments were prepared. Bone segments were subjected to 2 consecutive collagenase digestions (30 minutes and 1 hour). Cells were collected after each cycle, pooled, and used as described.
Preparation of SCs
SCs were prepared in “Dexter” cultures as described.25,26 BM cells were flushed from femurs and tibias of C57BL/6 mice, and low-density cells were obtained by Ficoll centrifugation (GE Healthcare). Cells were cultured in Iscove modified Dulbecco medium supplemented with 10% fetal calf serum, 1% penicillin/streptomycin, and 1% L-glutamine, 0.2mM β-mercaptoethanol, and 0.2μM methylprednisolone for 4 to 5 weeks until a typical SC monolayer was formed.
Cell staining and flow cytometry
Cells were washed once with stain wash (PBS, 1% bovine calf serum, and 1% penicillin-streptomycin) followed by antibody staining for 15 minutes on ice. Cells were washed with cold stain wash after each step.
LSK cell sorting and phenotyping.
Low-density BM cells from C57BL/6 (CD45.2) or BoyJ (CD45.1) mice were stained with phycoerythrin (PE)–conjugated CD3, CD4, CD45R, Ter119, and Gr1; allophycocyanin-conjugated c-Kit (CD117); fluorescein isothiocyanate-conjugated Sca1 (BD Biosciences). Lineage−Sca-1+CD117+ (LSK) cells were sorted on BD FACSAria (BD Biosciences). Cells harvested from cocultures were stained with the aforementioned antibody combinations along with Pacific blue-conjugated CD45.1 and PE-Cy7–conjugated CD45.2. CD45.1+ cells were gated and analyzed for the presence of Lin−Sca1+ cells on a BD LSRII (BD Biosciences). Because cultured cells quickly lose the expression of c-Kit, they were not analyzed for CD117. This is why persistence of primitive cells in culture was assessed via the presence of Lin−Sca1+ cells only.
OB phenotyping and sorting.
OBs from 4 different sources, calvariae, and long bones of 2-day and 6- to 8-week-old mice were stained with allophycocyanin-conjugated CD45, CD31, and Ter119; PE-Cy7–conjugated Sca1; PE–conjugated ALCAM (eBiosciences); and purified osteopontin (OPN; Rockland) followed by AlexaFluor 488–conjugated species and subclass specific secondary antibody (Invitrogen).
Progenitor cell assay
Cells were plated in duplicate in 3-cm Petri dishes containing 1 mL of methylcellulose with cytokines (MethoCult GF M3434; StemCell Technologies). Cultures were maintained at 37°C in humidified incubator at 5% CO2, and colonies were counted on an inverted microscope after 7 days.
Quantitative real-time RT-PCR
cDNA was made on μMACS columns (Miltenyi Biotec) using the μMACS one-step cDNA kit (Miltenyi Biotec, catalog no. 130-091-902) following the manufacturer's instructions. Quantitative PCR was performed in an MX3000 detection system using SYBR Green PCR reagents following the manufacturer's instructions (Stratagene). PCR amplification was performed in a 25-μL final volume containing 12.5 μL of 2× SYBR Mastermix, 2.5 μL of ROX (300nM, reference dye), 2.5 μL of each primer (at desired concentration), and 5 μL of template (cDNA diluted) using 95°C for 10 minutes, followed by 40 cycles at 95°C for 30 seconds and 55°C for 1 minute and 72°C for 30 seconds. For each gene analyzed, a calibration curve was performed and all the oligonucleotides were tested to ensure specificity and to determine the optimum concentration. For each sample, arbitrary units obtained using the standard curve and the expression of glyceraldehyde-3-phosphate dehydrogenase was used to normalize the amount of the investigated transcript.
ALP activity
Alkaline phosphatase (ALP) activity was determined by the colorimetric conversion of p-nitrophenol phosphate to p-nitrophenol (Sigma-Aldrich) and normalized to total protein (bicinchoninic acid, Pierce Chemical).23 Cells were washed twice with PBS, lysed with 0.1% (vol/vol) Triton X-100 supplemented with a cocktail of broad-range protease inhibitors (Pierce Chemical), frozen and thawed twice, and cleared via centrifugation. Lysates were incubated with 3 mg/mL p-nitrophenol phosphate in an alkaline buffer (pH 8.0) for 30 minutes at 37°C. The reaction was stopped by the addition of 20mM NaOH and read at 405nM (GENios Plus; Tecan). ALP activity was determined by comparison with known p-nitrophenol standards.
Quantitative analysis of Ca deposition
Ca deposition was assessed by eluting Alizarin Red S from cell monolayers.23 Monolayers were washed twice with PBS, fixed in ice cold 70% (vol/vol) ethanol for 1 hour, and then washed 2 times with water. Monolayers were stained with 40mM Alizarin Red S (pH 4.2) for 10 minutes. Unbound dye was removed by washing with water (5 times) and PBS (1 time for 15 minutes). Bound Alizarin Red was eluted by incubating monolayers with 1% (vol/vol) cetylpyridinium chloride in 10mM sodium phosphate (pH 7.0) for 15 minutes. Absorbance from aliquots was measured at 562 nm (GENios Plus; Tecan), and Alizarin Red concentrations were calculated from measured standards (Ca/mol of dye in solution).
Statistical analysis
At least 3 individual experiments were performed unless stated otherwise. Where applicable, data are presented as mean plus or minus SD and were analyzed using a 2-tailed Student t test. Differences were considered statistically significant with a P value of less than .05. All statistical analyses were performed with the Excel 2003 program (Microsoft). Where applicable (Figure 7C-D), Pearson correlation coefficients (bivariate correlation) were used to determine R2 values. Linear regressions using analysis of variance model were performed to compare groups. All analyses involving these methods were performed with the Statistical Package for Social Sciences (SPSS 17; Norusis/SPSS Inc) software and were 2-tailed with a level of significance set at .05.
Results
Kinetics of cell growth in cocultures of OBs and SCs
OBs were freshly isolated from calvariae (C) or long bones (LB) of 2-day-old pups or 6- to 8-week-old C57BL/6 mice.23,24 All OB preparations were used either directly or sorted then used in cocultures within 6 to 8 hours of their isolation. SCs were prepared from murine low-density BM cells in “Dexter” cultures25 and maintained for 4 to 5 weeks until a typical SC monolayer was formed. SCs were then harvested and used in cocultures as described. Phenotypic characterization of SCs maintained in culture for 4 to 5 weeks is shown in supplemental Figure 1 (available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Invariably, SC cultures contained a substantial number of adipocytes as evidenced by Oil Red O staining (supplemental Figure 2). In general, adipocytes constituted between 12% and 17% of all cells present in SC cultures at week 4 (supplemental Figure 2). Different proliferation kinetics were observed for fresh OBs cocultured with SCs that had been in culture for 4 to 5 weeks. However, neither cell type was eliminated from the coculture after 7 or 10 days (supplemental Figure 3), illustrating that neither cell type was totally purged by the other.
Hematopoietic properties of LSK cells cultured with OBs and SCs
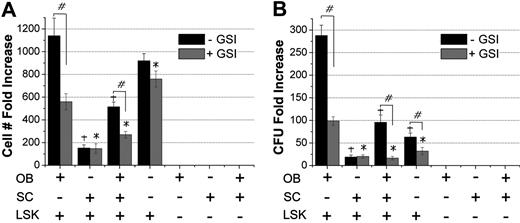
Cocultures of OBs and SCs from C57BL/6 mice were initiated 24 hours before seeding at time 0 (day 0) with 1000 freshly sorted B6.SJL-PtγcqPep3b/BoyJ (BoyJ)–derived LSK cells per well. Cultures were supplemented with exogenous cytokines as detailed in Figure 1. After 7 days, fold increase in total cell number generated from LSK cells was significantly higher in OB + LSK cocultures (1353.4 ± 236.5-fold) compared with SC + LSK cocultures (474.8 ± 129.8-fold; Figure 1A). When all 3 cell types were cocultured, the presence of SCs significantly suppressed the OB-mediated LSK cell expansion (463.8 ± 168.3-fold; equivalent to 75.6% ± 7.3% suppression). A significant suppressive effect was still evident when the total cell number of OBs and SCs was reduced by 50% to minimize crowding (data not shown).
Impact of OB, SC, and OB + SC cocultures on stem and progenitor cell function. (A) Cells were cultured in different combinations as indicated, and all wells received recombinant murine stem cell factor and interleukin-3 (10 ng/mL), insulin-like growth factor 1 and thrombopoietin (20 ng/mL), interleukin-6 and Fms-like tyrosine kinase 3 (25 ng/mL), and OPN (50 ng/mL). Cells were harvested on day 7 and counted. Fold increase in total cell number from the original 1000 LSK cells was calculated relative to day 0; n = 6 to 8 independent experiments. (B) LSK progeny cells harvested on day 7 were plated in methylcellulose-based clonogenic assays, and colony formation was assessed 7 days later. CFU fold increase was calculated relative to that obtained from 250 freshly isolated LSK cells assayed on day 0; n = 4 or 5 independent experiments. (C) LSK progeny harvested on day 7 were stained and analyzed for the Lin−Sca1+ content; n = 3 independent experiments. (D) Lin−Sca1+ cells were sorted from each group and analyzed for cell-cycle status with propidium iodide; n = 5 independent experiments. (E) BM repopulating potential of freshly isolated and in vitro expanded LSK cells for 10 days in cocultures of OBs, SCs, or OBs + SCs or on plastic. LSK cells from C57Bl/6 (CD45.2) mice were cotransplanted with 100 000 BoyJ (CD45.1) competitor cells in lethally irradiated (1100 cGy, split dose) CD45.2 × CD45.1 F1 recipients. Control mice (Fresh) received 1000 freshly isolated LSK cells and 100 000 competitor cells. At monthly intervals, chimerism was assessed as [CD45.2/(CD45.2 + CD45.1)] × 100, thus eliminating the contribution of residual host-derived HSCs. Data are from 1 experiment, 4 or 5 mice per group, except for the LSK cells cultured on plastic where only 1 mouse survived. (F) Secondary transplantations from primary recipients. At 4 months after primary transplantation, the BM content of 1 femur from each primary recipient was transplanted into a lethally irradiated secondary recipient without competitor cells, and engraftment was assessed at monthly intervals. Each group contained 4 mice, except the LSK cells group, which had 2 mice transplanted with cells from a single primary recipient. *Significant at P < .01 compared with OB + LSK group for panels A, B, C, and D and at P < .05 for panels E and F. Differences between fresh and OB + LSK groups for primary and secondary transplantations were not significant.
Impact of OB, SC, and OB + SC cocultures on stem and progenitor cell function. (A) Cells were cultured in different combinations as indicated, and all wells received recombinant murine stem cell factor and interleukin-3 (10 ng/mL), insulin-like growth factor 1 and thrombopoietin (20 ng/mL), interleukin-6 and Fms-like tyrosine kinase 3 (25 ng/mL), and OPN (50 ng/mL). Cells were harvested on day 7 and counted. Fold increase in total cell number from the original 1000 LSK cells was calculated relative to day 0; n = 6 to 8 independent experiments. (B) LSK progeny cells harvested on day 7 were plated in methylcellulose-based clonogenic assays, and colony formation was assessed 7 days later. CFU fold increase was calculated relative to that obtained from 250 freshly isolated LSK cells assayed on day 0; n = 4 or 5 independent experiments. (C) LSK progeny harvested on day 7 were stained and analyzed for the Lin−Sca1+ content; n = 3 independent experiments. (D) Lin−Sca1+ cells were sorted from each group and analyzed for cell-cycle status with propidium iodide; n = 5 independent experiments. (E) BM repopulating potential of freshly isolated and in vitro expanded LSK cells for 10 days in cocultures of OBs, SCs, or OBs + SCs or on plastic. LSK cells from C57Bl/6 (CD45.2) mice were cotransplanted with 100 000 BoyJ (CD45.1) competitor cells in lethally irradiated (1100 cGy, split dose) CD45.2 × CD45.1 F1 recipients. Control mice (Fresh) received 1000 freshly isolated LSK cells and 100 000 competitor cells. At monthly intervals, chimerism was assessed as [CD45.2/(CD45.2 + CD45.1)] × 100, thus eliminating the contribution of residual host-derived HSCs. Data are from 1 experiment, 4 or 5 mice per group, except for the LSK cells cultured on plastic where only 1 mouse survived. (F) Secondary transplantations from primary recipients. At 4 months after primary transplantation, the BM content of 1 femur from each primary recipient was transplanted into a lethally irradiated secondary recipient without competitor cells, and engraftment was assessed at monthly intervals. Each group contained 4 mice, except the LSK cells group, which had 2 mice transplanted with cells from a single primary recipient. *Significant at P < .01 compared with OB + LSK group for panels A, B, C, and D and at P < .05 for panels E and F. Differences between fresh and OB + LSK groups for primary and secondary transplantations were not significant.
Progeny of LSK cells were assayed on day 7 for their colony-forming unit (CFU) content and corrected for the total number contained in each culture (Figure 1B). Although the number of CFU increased in OB cultures by more than 80.7 plus or minus 13.0-fold, CFU expansion was significantly lower in cultures supported by SCs (30.1 ± 7.3-fold) and in cultures of LSK only (24.5 ± 5.6-fold). More importantly, the OB-mediated enhanced HPC function was significantly suppressed in the presence of SCs (Figure 1B). SC conditioned medium did not suppress OB-mediated enhancement of hematopoietic properties (supplemental Figure 4), illustrating the need for cell-cell contact for the transmission of the suppressive effect of SCs on OB-mediated enhancement of hematopoiesis.
Cells harvested on day 7 were analyzed for the expression of Sca1 and lineage markers (Figure 1C) and for cell-cycle status (Figure 1D). Because the expression of CD117 is quickly down-regulated via the internalization of the receptor in cultures supplemented with exogenous stem cell factor,27,28 we did not use CD117 to track the phenotypic makeup of cultured cells on day 7. A significantly higher percentage of Lin−Sca1+ cells (34.5% ± 8.3%) was present in OB + LSK cultures relative to SC + LSK and OB + SC + LSK cultures, suggesting that maintenance of Lin−Sca1+ cells may be responsible for the fold increase in CFU in these cultures (Figure 1B). The significant increase in LSK progeny in OB + LSK cultures on day 7 (Figure 1A) probably resulted from the low percentage (75%) of Lin−Sca1+ cells in the G0/G1 phase of cell cycle (Figure 1D). It should be noted that analysis of apoptosis (with annexin V staining) of cells in cultures shown in Figure 1C and D (and in other culture conditions; Figures 2,4,5,7) showed a very small percentage of apoptotic cells and no significant differences between cells in any culture condition (data not shown). Taken together, a higher number of LSK progeny (in the absence of changes in apoptosis), increased number of clonogenic cells, higher percentage of Lin−Sca1+ cells, and decreased percentage of cells in G0/G1 in OB + LSK cultures suggest that OBs promote cell proliferation and expansion while maintaining primitive hematopoietic cell properties.
These results suggested that LSK progeny may retain a high level of BM repopulating potential. To examine this, the expansion equivalent of 1000 LSK cells (CD45.2) maintained under different culture conditions for 10 days was used in a competitive repopulation assay. Figure 1E illustrates that LSK cells maintained in OB cultures retained a high level of repopulating activity relative to freshly isolated cells (P > .05 at all time points). In contrast, LSK cells maintained with SCs only failed to repopulate lethally irradiated recipients. Furthermore, SCs significantly suppressed the OB-mediated maintenance of LSK function when all 3 cell types were cocultured together (Figure 1E). Only 1 mouse survived in the group of LSK cells maintained on plastic, probably resulting from the small number of competitor cells used in these studies. As expected, cultures not seeded with LSK cells did not sustain chimerism (Figure 1E). BM repopulating ability of LSK cells maintained in OB cultures was also evident in secondary recipients that received BM cells from primary recipients 4 months after transplantation (Figure 1F). Levels of chimerism in secondary recipients of cells maintained with OBs were significantly higher than those in mice receiving cells cocultured with SCs under any condition (each secondary recipient received the cell content of 1 femur from a primary recipient). Results from secondary transplantations illustrate the ability of OBs to maintain HSC function and demonstrate the suppressive effect of SCs on the OB-mediated hematopoiesis-enhancing activity.
Mechanism of hematopoiesis-enhancing activity in OB cocultures
We investigated whether observations made in cocultures of OBs and HPCs were mediated by the HSC receptor Notch and its OB-expressed ligand Jagged. To that effect, cocultures of OB, SC, and LSK cells were treated with γ-secretase inhibitor (GSI). In 3 independent experiments, the addition of GSI suppressed both proliferation of LSK cells and their progeny (Figure 2A) and generation of CFU (Figure 2B), especially in cocultures of OB + LSK and OB + SC + LSK, suggesting that the hematopoiesis-enhancing activity of OBs is mediated in part through Notch signaling. Cocultures of SCs and LSK, where Notch signaling is not expected to play a major role in sustaining hematopoietic cells, were not impacted by GSI. Interestingly, autonomous Notch signaling in LSK cells29 was also inhibited by GSI.
Impact of Notch signaling inhibition on OB-mediated enhancement of HPC function. Increase in total cell number from the original 1000 LSK cells (A) and production of CFU (B) in cocultures of OB, SC, and LSK cells with and without the Notch inhibitor, GSI. Cell numbers and CFU content were assayed, and fold increase was calculated to day 0 values. Data shown are from 1 of 3 independent experiments with similar results. GSI was added at 10nM on day 0 and replenished twice during the next 7-day culture period. #P < .01, comparisons within each group between treatments with and without GSI. +P < .01 compared with OB + LSK group without GSI. *P < .05 compared with OB + LSK with GSI.
Impact of Notch signaling inhibition on OB-mediated enhancement of HPC function. Increase in total cell number from the original 1000 LSK cells (A) and production of CFU (B) in cocultures of OB, SC, and LSK cells with and without the Notch inhibitor, GSI. Cell numbers and CFU content were assayed, and fold increase was calculated to day 0 values. Data shown are from 1 of 3 independent experiments with similar results. GSI was added at 10nM on day 0 and replenished twice during the next 7-day culture period. #P < .01, comparisons within each group between treatments with and without GSI. +P < .01 compared with OB + LSK group without GSI. *P < .05 compared with OB + LSK with GSI.
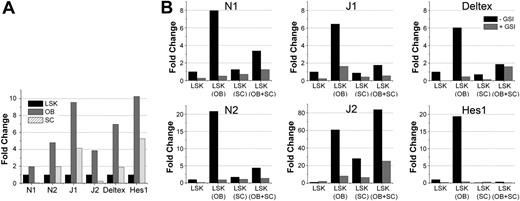
To verify the involvement of Notch in mediating the effects seen in Figure 2, we monitored Notch activation in LSK cells cocultured with OBs or SCs and the potential role of intrinsic Notch signaling in regulating OBs. First, we examined the expression of Notch receptors, ligands, and downstream Notch targets in LSK, OBs, and SCs. OBs and LSK expressed high levels of N1, whereas SCs expressed N1 at relatively low levels (Figure 3A). Both Notch ligands J1 and J2 were highly expressed on OBs compared with LSK and SCs. Coexpression of Notch receptors and their ligands, in various combinations, is found in all cell types in the BM microenvironment30 and accounts for basal Notch signaling through homotypic cell-cell interactions. Analysis of Notch signaling activation by expression of downstream transcriptional targets Hes1 and Deltex revealed a basal activation of Notch signaling in all cell types, with the strongest signaling in OBs (Figure 3A).
Notch activation in OB, SC, and LSK cocultures. (A) Endogenous expression of components of the Notch pathway. Quantitative RT-PCR was performed on cDNA generated from mRNA derived from sorted LSK cells, cultured SCs, and freshly isolated 2-day C OBs (performed in triplicate for each sample). Bar graphs represent ratio of each specific transcript to glyceraldehyde-3-phosphate dehydrogenase. Expression of each transcript in OBs and SCs was expressed as fold change relative to the expression of that transcript in LSK cells, which was normalized to 1. (B) Quantitative RT-PCR data from cells isolated from cocultures. Cocultures were established with SCs from C57Bl/6 GFP mice (CD45.2), OBs (2-day C) from C57Bl/6 mice (CD45.2), and LSK cells from BoyJ mice (CD45.1). On day 7, cells were harvested and stained with PE-CD45.1. mRNA was prepared from GFP-CD45.1+ cells (LSK progeny only) and analyzed. Bar graphs show fold increase in the expression of the indicated genes in LSK cultured alone (normalized to 1 in cultures without GSI) or with other cell types shown for each condition in parentheses. Quantitative RT-PCR was performed in triplicates for each sample and each condition. Data are representative of 2 independent experiments with similar results. GSI was added at 10nM on day 0 and replenished twice during the next 7-day culture period. The legend shown in the plot of Deltex in panel B applies to all other plots in the figure (N1, N2, J1, J2, and Hes1).
Notch activation in OB, SC, and LSK cocultures. (A) Endogenous expression of components of the Notch pathway. Quantitative RT-PCR was performed on cDNA generated from mRNA derived from sorted LSK cells, cultured SCs, and freshly isolated 2-day C OBs (performed in triplicate for each sample). Bar graphs represent ratio of each specific transcript to glyceraldehyde-3-phosphate dehydrogenase. Expression of each transcript in OBs and SCs was expressed as fold change relative to the expression of that transcript in LSK cells, which was normalized to 1. (B) Quantitative RT-PCR data from cells isolated from cocultures. Cocultures were established with SCs from C57Bl/6 GFP mice (CD45.2), OBs (2-day C) from C57Bl/6 mice (CD45.2), and LSK cells from BoyJ mice (CD45.1). On day 7, cells were harvested and stained with PE-CD45.1. mRNA was prepared from GFP-CD45.1+ cells (LSK progeny only) and analyzed. Bar graphs show fold increase in the expression of the indicated genes in LSK cultured alone (normalized to 1 in cultures without GSI) or with other cell types shown for each condition in parentheses. Quantitative RT-PCR was performed in triplicates for each sample and each condition. Data are representative of 2 independent experiments with similar results. GSI was added at 10nM on day 0 and replenished twice during the next 7-day culture period. The legend shown in the plot of Deltex in panel B applies to all other plots in the figure (N1, N2, J1, J2, and Hes1).
Next, we determined whether basal activation of Notch on LSK cells was modulated by coculture with OBs or SCs. As is evident by the expression of Notch target genes Hes1 and Deltex, Notch signaling was up-regulated in LSK cells when cocultured with OBs and was significantly inhibited in the presence of SCs (Figure 3B). The presence of SCs in LSK + OB cocultures resulted in a dominant inhibitory effect on Notch, suggesting that SCs suppress the OB-mediated regulation and maintenance of HSC function. Expression of Notch receptors and ligands mirrored the pattern of Notch activation on LSK cells: they were up-regulated by OBs and dominantly inhibited by SCs. Addition of GSI abrogated the stimulatory effect of OBs on hematopoietic cells but did not rescue the suppressive effect of SCs, thus excluding the possibility of a negative feedback loop triggered by Notch signaling itself. We also investigated whether LSK cells had a reciprocal effect on OBs, thus altering their intrinsic Notch activity. PCR analysis of sorted OBs cocultured with LSK cells showed a decrease in the expression of N1, N2, J1, and Hes1 and a significant up-regulation of J2 (data not shown). Overall, activation of Notch signaling in LSK cells highly correlated with their functional activity in colony assays and BM transplantation (Figure 1), suggesting a major role of Notch signaling in HSC maintenance.
Impact of different cell types within SCs on in vitro hematopoietic function
Recently, it was shown that BM adipocytes suppress granulopoiesis14 and may prevent hematopoietic expansion in homeostasis and after transplantation.15 Because SCs contain various numbers of adipocytes, we examined the impact of an established mesenchymal stromal cell line, GZL,31 with various numbers of adipocytes on the in vitro maintenance of hematopoietic cells. GZL cells with a higher content of adipocytes (GZL/Adi) were less efficient than their counterparts with normal adipocyte content (GZL) in supporting both LSK proliferation and CFU production (Figure 4A). That GZL/Adi contained a higher proportion of adipocytes compared with GZL was confirmed by the expression of adiponectin and FABP4 (Figure 4B). Belaid-Choucair et al14 recently demonstrated that BM adipocytes block granulopoiesis through neuropilin-1, a coreceptor to a tyrosine kinase receptor. We assessed the expression of this receptor on both variants of GZL cells used in this assay relative to primary SCs. Neuropilin-1 was up-regulated on GZL/Adi relative to GZL cells and SCs (Figure 4C). These data suggest that the suppressive effect of SCs on the hematopoiesis-enhancing activity of OBs is partially mediated by adipocytes within SCs, possibly via the production of neuropilin-1. Given that adipocytes are a critical component of the hematopoietic microenvironment and that the BM content of adipocytes increases with age (concomitant with a decline in hematopoietic activity), these data suggest that adipocytes may play a significant role in down modulating HSC function.
Impact of adipocytes on the maintenance of HPCs in vitro. Without frequent media changes and at high cell density, GZL, an established MSC cell line, differentiates preferentially into adipocytes (GZL/Adi). The number of adipocytes is greatly reduced when the cells are propagated under more favorable conditions (GZL). Both GZL and GZL/Adi were used along with primary SCs to sustain LSK cells for 7 days. (A) Cells were harvested on day 7, counted, and used in clonogenic assays. (B) SC, GZL, and GZL/Adi were assayed by quantitative RT-PCR for the expression of adiponectin and FABP4 as indicators of adipogenic differentiation. Data were normalized to primary SCs. (C) SCs, GZL, and GZL/Adi were separated from LSK progeny on day 7 by cell sorting and assayed by quantitative RT-PCR for the expression of neuropilin-1. Expression of Np1 was normalized to primary SCs. P < .05 between SC and GZL/Adi groups in panel A.
Impact of adipocytes on the maintenance of HPCs in vitro. Without frequent media changes and at high cell density, GZL, an established MSC cell line, differentiates preferentially into adipocytes (GZL/Adi). The number of adipocytes is greatly reduced when the cells are propagated under more favorable conditions (GZL). Both GZL and GZL/Adi were used along with primary SCs to sustain LSK cells for 7 days. (A) Cells were harvested on day 7, counted, and used in clonogenic assays. (B) SC, GZL, and GZL/Adi were assayed by quantitative RT-PCR for the expression of adiponectin and FABP4 as indicators of adipogenic differentiation. Data were normalized to primary SCs. (C) SCs, GZL, and GZL/Adi were separated from LSK progeny on day 7 by cell sorting and assayed by quantitative RT-PCR for the expression of neuropilin-1. Expression of Np1 was normalized to primary SCs. P < .05 between SC and GZL/Adi groups in panel A.
Phenotypic makeup of OBs with hematopoiesis-enhancing activity
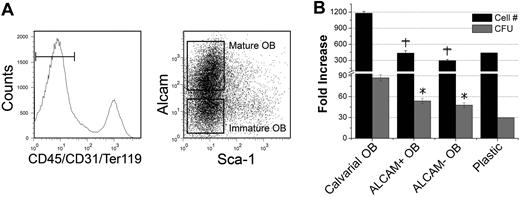
OBs used in our studies were not phenotypically defined. We therefore attempted to prospectively identify OB flow cytometrically, arguing that this will not only identify OBs capable of enhancing HSC function but will also begin to establish a model for the osteoblastic developmental hierarchy. We first examined the expression of ALCAM (CD166) on 2-day calvarial OBs, which was recently identified by Arai et al32 as a marker capable of distinguishing mature and immature OBs from long bones of adult mice when combined with Sca1 and a CD45, CD31, and Ter119 “lineage” cocktail. These markers defined Lin−Sca1−ALCAM− cells as immature OBs and Lin−Sca1−ALCAM+ cells as mature OBs.32 Using this approach, we isolated 2 distinct phenotypes of OBs from 2-day calvariae (Figure 5A) and examined their hematopoiesis-enhancing activities (Figure 5B). Interestingly, the hematopoiesis-enhancing activity could not be segregated into either Lin−Sca1−ALCAM+ or Lin−Sca1−ALCAM− cells, suggesting that these markers are not sufficient to fully define OBs or to segregate the observed hematopoiesis-enhancing activity.
Effect of phenotypically defined groups of 2-day C OBs on HPC function. (A) Two-day C OBs were stained as described in “Methods.” Gated CD45−CD31−Ter119− cells were separated into Sca1−ALCAM+ and Sca1−ALCAM− cells, which were used in assays shown in panel B. (B) Increase in cell number from the original LSK cells and production of clonogenic progenitors in the presence of phenotypically defined groups of OBs. +P < .01 compared with fold increase in cell number in calvarial OB cultures. *P < .01 compared with fold increase in CFU in calvarial OB cultures.
Effect of phenotypically defined groups of 2-day C OBs on HPC function. (A) Two-day C OBs were stained as described in “Methods.” Gated CD45−CD31−Ter119− cells were separated into Sca1−ALCAM+ and Sca1−ALCAM− cells, which were used in assays shown in panel B. (B) Increase in cell number from the original LSK cells and production of clonogenic progenitors in the presence of phenotypically defined groups of OBs. +P < .01 compared with fold increase in cell number in calvarial OB cultures. *P < .01 compared with fold increase in CFU in calvarial OB cultures.
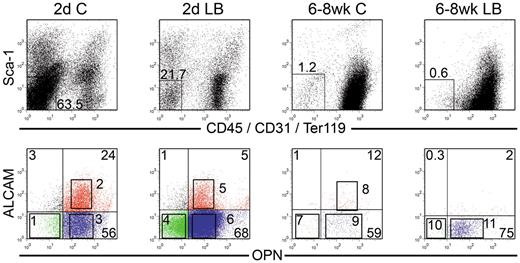
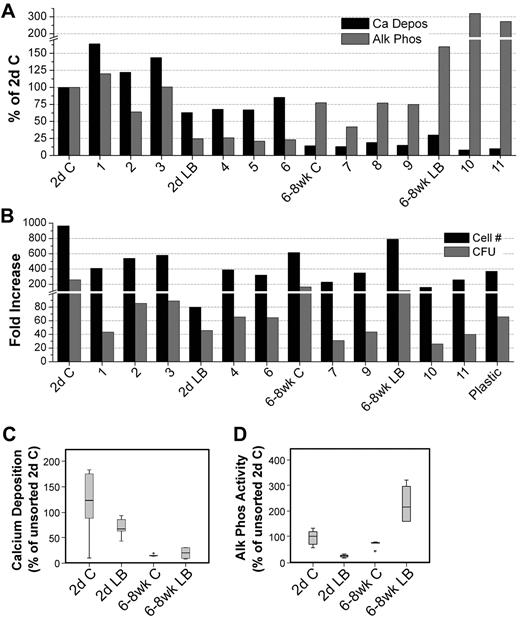
We next examined OPN, which has been previously used to identify OBs and characterize their function.3 Because OB function varies considerably with age and anatomic location,33 we examined OBs from 2 distinct anatomic sites, namely, the calvariae (C) and LB and from 2 different ages; either newborn mice (2-day-old) or adult (6- to 8-week-old mice). All 4 sources of OBs (2-day C, 2-day LB, 6- to 8-week C, 6- to 8-week LB) contained cells that lacked the expression of the lineage markers CD45/CD31/Ter119 and Sca1 (Lin−Sca1−; Figure 6). Arai et al32 defined Lin−Sca1+ cells in bone preparations as mesenchymal progenitors. We therefore focused on Lin−Sca1− cells (Figure 6) as candidate OBs. Lin−Sca1− cells were analyzed for OPN and ALCAM expression to identify 11 distinct groups of cells from the 4 sources of OBs as shown in sort windows 1 through 11 in the lower row of dot plots in Figure 6. Lin−Sca1− cells decreased from 2-day C to 6- to 8-week LB, suggesting that the percentage of total OBs decreased with age and anatomic site. All 11 groups of OBs and the 4 parent populations were examined for 2 fundamental functional osteoblastic properties34 : Ca deposition (mineralization) and ALP activity (Figure 7A). ALP is the major OB enzymatic activity and Ca deposition is a surrogate marker for mineralization.34 The highest amount of Ca deposition was among 2-day C OB and the lowest was in 6- to 8-week C, although these cells displayed similar activities as those observed with 6- to 8-week LB, suggesting that Ca deposition diminished with location and age. ALP activity was lowest in 2-day LB with very high activity in 6- to 8-week LB. The importance of ALP+ SCs in supporting ex vivo and in vivo hematopoiesis was recognized many years ago.35
Phenotypic analysis of OBs from calvariae (C) or long bones (LB) of newborn (2-day) and 6- to 8-week-old mice. OBs were stained as described in “Cell staining and flow cytometry.” Cells were analyzed for Sca1 versus CD45/CD31/Ter119 (top row), and double-negative cells were then analyzed for OPN versus ALCAM (bottom row). Different numbers of events were collected and are displayed for each file. Sort gates defining groups 1 through 11 were established based on fluorescence levels of control samples. Cells in these gates were sorted and used in different assays depending on the number of cells recovered for each fraction. Dot plots are from 1 representative experiment of 3.
Phenotypic analysis of OBs from calvariae (C) or long bones (LB) of newborn (2-day) and 6- to 8-week-old mice. OBs were stained as described in “Cell staining and flow cytometry.” Cells were analyzed for Sca1 versus CD45/CD31/Ter119 (top row), and double-negative cells were then analyzed for OPN versus ALCAM (bottom row). Different numbers of events were collected and are displayed for each file. Sort gates defining groups 1 through 11 were established based on fluorescence levels of control samples. Cells in these gates were sorted and used in different assays depending on the number of cells recovered for each fraction. Dot plots are from 1 representative experiment of 3.
Osteogenic and hematopoietic functional studies of isolated fractions of OBs. (A) Ca deposition and enzymatic ALP activity of parental populations and groups 1 through 11 shown in Figure 6. Cells were cultured in osteogenic media (αminimal essential medium supplemented with 10% fetal calf serum, 50 μg/mL ascorbic acid, 2 times per week). Starting on day 7, cultures were supplemented with 5mM β-glycerophosphate to induce mineralization and were assayed on day 14. ALP activity and Ca deposition were assessed as described in “ALP activity” and “Quantitative analysis of Ca deposition.” With the exception of the unsorted 2-day C and 2-day LB data (n = 3 in duplicate), results are from 2 experiments each performed in duplicate. (B) Parental populations and cell fractions collected in sufficient numbers were used in cocultures with LSK cells. Cultured cells were harvested on day 7, counted, and assayed for HPC content performed in triplicate. Data shown were collected from 2 sorting experiments. (C-D) Results from Ca deposition (C) and enzymatic ALP activity (D) from 2-day C, 2-day LB, 6- to 8-week C, and 6- to 8-week LB (n = 6-8 for all groups) are reported as box plots depicting the range of data points and the mean (line). Error bars represent SD associated with the mean. Data points more than 2 SD from the mean are identified on the plot and were still used in statistical determinations. Statistical analysis methods used to analyze data shown in panels C and D are described in “Statistical analysis.”
Osteogenic and hematopoietic functional studies of isolated fractions of OBs. (A) Ca deposition and enzymatic ALP activity of parental populations and groups 1 through 11 shown in Figure 6. Cells were cultured in osteogenic media (αminimal essential medium supplemented with 10% fetal calf serum, 50 μg/mL ascorbic acid, 2 times per week). Starting on day 7, cultures were supplemented with 5mM β-glycerophosphate to induce mineralization and were assayed on day 14. ALP activity and Ca deposition were assessed as described in “ALP activity” and “Quantitative analysis of Ca deposition.” With the exception of the unsorted 2-day C and 2-day LB data (n = 3 in duplicate), results are from 2 experiments each performed in duplicate. (B) Parental populations and cell fractions collected in sufficient numbers were used in cocultures with LSK cells. Cultured cells were harvested on day 7, counted, and assayed for HPC content performed in triplicate. Data shown were collected from 2 sorting experiments. (C-D) Results from Ca deposition (C) and enzymatic ALP activity (D) from 2-day C, 2-day LB, 6- to 8-week C, and 6- to 8-week LB (n = 6-8 for all groups) are reported as box plots depicting the range of data points and the mean (line). Error bars represent SD associated with the mean. Data points more than 2 SD from the mean are identified on the plot and were still used in statistical determinations. Statistical analysis methods used to analyze data shown in panels C and D are described in “Statistical analysis.”
Most of these OB fractions and their parent populations were also examined for their hematopoiesis-enhancing activities (Figure 7B). All sources of OBs, except the 2-day LB, supported hematopoietic properties beyond those observed for LSK cells cultured on plastic. Data in Figure 7B illustrate that (1) OPN, as previously observed for ALCAM (Figure 5), does not fractionate the OB-enhancing activity of HSC function; hematopoiesis-enhancing activities were detected for example in OPN− (groups 1 and 4) and OPN+ (groups 3 and 6) cells; (2) even together, OPN and ALCAM fail to completely segregate the hematopoiesis-enhancing activity into 1 group of cells; activity was detected in OPN+ALCAM+ (group 2) and OPN+ALCAM− (group 3) cells; and (3) Ca and ALP activities were significantly correlated with anatomic location and age. As illustrated in Figure 7C and D, a significant association was observed between the source of OB and both Ca deposition (P < .001, R2 = 0.746) and ALP activity (P = .016, R2 = 0.486). Surprisingly, however, CFU content was not significantly correlated with either one of these primary OB functional properties regardless of whether the analysis focused on anatomic location and age or on the subfractions of these sources of OBs. This observation illustrates that the OB-mediated enhancement of hematopoiesis, which was investigated here predominantly with 2-day C OBs, is not a unique characteristic of these OBs, but instead, is a functional property of OBs from other anatomic sites. Furthermore, although our data in Figure 7B examined the impact of OBs on progenitor and not stem cell function, these data suggest that the OB-mediated hematopoiesis supportive activity is not age dependent. Consequently, these data most probably describe an OB-mediated activity that is representative of the impact of OBs from different anatomic sites and different ages on HSC function.
Discussion
Self-renewal and differentiation divisions of HSCs are tightly regulated to achieve a balance between homeostasis and maintenance of the stem cell pool. This balance is regulated through a complex signaling network involving a large number of soluble factors and multiple cell-cell interactions.36 Interactions between HSCs and OBs37 and between HSCs and SCs38 are very prominent in determining HSC fate. Based on these interactions, the hematopoietic microenvironment is considered to be composed of 2 specialized niches, the endosteal and the vascular niche.38 At present, it is thought that primitive long-term repopulating HSCs associate with cells of the OB lineage that line bone surfaces, whereas more mature hematopoietic cells (such as short-term HSCs and HPCs) associate more with endothelial (and other) cells in the vascular niche.7,39-42 We therefore sought to examine the impact of single or multiple interactions between HSCs, OBs, and SCs on hematopoietic function. Our results demonstrate that, although OBs mediate a positive regulatory effect on stem cell function, most probably through the up-regulation of Notch signaling, SCs suppress the OB-mediated hematopoiesis-enhancing activity via the down-regulation of Notch signaling, possibly through mechanisms involving adipocytes.
In the presence of OBs, LSK cells had a higher rate of proliferation, produced more CFU, and maintained a higher percentage of Lin−Sca1+ cells than when cocultured with SCs alone or a mixture of OBs and SCs. Although the OB-enhancing activity was not significantly higher for some datasets than what was observed with LSK cells cultured alone (Figure 1), it is critical to point out that the novel observation of our studies is the significant suppressive effect of SCs on the OB-mediated hematopoiesis-enhancing activity, which was demonstrated in all of our studies presented here. Furthermore, it should be noted that interexperimental variability probably contributed to lack of significance and that, when normalized, significant differences were observed between datasets, including, for example, the fold increase in cell number between OB + LSK and LSK cultured on plastic (data not shown). In particular, a significantly higher repopulating potential was noted in primary and secondary recipients of LSK cells expanded with OBs only compared with cells cultured with SCs or in an OB + SC coculture. At 1 month after transplantation, chimerism in primary recipients of LSK cells cultured with OBs only was higher than that observed for fresh LSK cells, suggesting that OBs may have preferentially expanded the number of short-term repopulating cells. These results may have a profound impact on the design of culture conditions intended for the expansion of short- and long-term repopulating cells. These data suggest that, although SCs support hematopoiesis as previously reported by several laboratories,43-46 the hematopoiesis-enhancing activity mediated by OBs is superior. Furthermore, our data demonstrate that the hematopoiesis-enhancing activity of OBs is suppressed by SCs, even when these cells are present in small numbers relative to the number of cocultured OBs.
The Notch pathway is a highly conserved signaling cascade present in all vertebrates studied so far.30 It plays a crucial role in determining cell fate decisions and HSC self-renewal. The Notch-specific inhibitor GSI significantly suppressed the OB-mediated hematopoiesis-enhancing activity, illustrating that Notch signaling is critical for the support of HSC function via their direct interaction with OBs. Because GSI did not affect the behavior of LSK cultured with SCs, Notch signaling is probably not involved in the interaction of these 2 cell types. That Notch signaling is central to the OB-induced support of HSCs was further demonstrated by the expression of different ligands, receptors, and intermediate molecules involved in this pathway. Endogenous expression of these moieties was highest in OBs. When cocultured for 7 days and then separated by flow cytometric cell sorting, LSK cells cultured in the presence of OBs demonstrated significant up-regulation of Notch signaling components compared with their counterparts cultured with SCs. The relative expression of these molecules was substantially lower in LSK cells cocultured with OBs and SCs, simultaneously demonstrating the presence of a correlative relationship between Notch signaling and the hematopoiesis-enhancing versus hematopoiesis-suppressing activities of OBs and SCs, respectively. The specificity of these observations vis-à-vis the involvement of Notch signaling in our results was corroborated by the fact that GSI significantly interfered with the expression of all these intermediate molecules.
Further investigation revealed that adipocytes are most probably responsible for the suppressive effect of SCs on HPC function. This speculation is in agreement with the recent data from Naveiras et al15 demonstrating the negative regulatory role of adipocytes in the BM microenvironment. It has been previously demonstrated that BM adipocytes can block granulopoiesis via a coreceptor to a tyrosine kinase receptor, neuropilin-1.14 In our studies, the expression of neuropilin-1 in GZL/Adi was much higher than in the parental cell line, GZL. Furthermore, expression of neuropilin-1 in primary SCs used throughout these studies was almost equal to that in GZL/Adi. Together, these data illustrate that the negative impact of SCs on hematopoiesis is at least partially the result of adipocytes and that neuropilin-1 may be responsible for this suppressive activity. Whether adipocytes also impact Notch signaling to suppress hematopoiesis remains to be determined.
The developmental hierarchy of OBs is not well defined nor is it clear whether OBs from different anatomic sites and chronologic stages share the same phenotypic makeup. We therefore phenotypically examined OBs from different aged mice and from different sites to better define the developmental hierarchy of OBs and to establish which stage of OB maturation supports this observed hematopoiesis-enhancing activity. Contrary to the classification of Arai et al32 in which Lin−Sca1−ALCAM− cells were defined as immature OBs whereas Lin−Sca1−ALCAM+ cells were identified as mature OBs, our data suggest that ALCAM− cells may represent mature OBs compared with ALCAM+ cells. We base this argument on observations made between groups 1 and 3 versus group 2 in Figure 7A, for example, in which both Ca deposition and ALP production were higher in ALCAM− cells (groups 1 and 3) than in ALCAM+ cells (group 2), suggesting that the former is more mature than the latter. On the other hand, Mayack and Wagers3 identified Lin−OPN+ cells as OBs. Because ALCAM can be used to classify OBs based on their maturational stage, it may be argued that OPN+ALCAM− cells are mature OBs, whereas OPN+ALCAM+ cells are immature OBs. Hence, data shown in the bottom row in Figure 6 suggest that, although the percentage of OPN+ cells remains relatively constant within the Lin−Sca1− group of cells, the percentage of ALCAM+ cells (immature OBs) decreases with age and varies with anatomic location (left to right in Figure 6).
Proliferation and expansion of HPC in cocultures with fractionated OB groups indicated that the markers we used so far are not sufficient to fully segregate the hematopoiesis-enhancing capacity of OBs into 1 phenotypically defined group (Figure 7B). Similarly, all sorted fractions displayed different levels of Ca deposition and ALP activity (Figure 7A). However, Ca and ALP activities were significantly correlated with anatomic location and age, but neither one was associated with LSK proliferation and CFU expansion. Obviously, additional markers are still required to compartmentalize the hematopoiesis-enhancing activity of OBs into a phenotypically defined group of cells. Such studies will also help defining why, for example, OBs from 2-day long bones have a reduced hematopoiesis-enhancing activity and possibly identify more precisely the maturational stage of OBs responsible for this activity. Many studies that examined the impact of OBs on hematopoiesis or made direct observations between HSCs and the endosteal region, used or focused on calvariae OBs.47 As such, the validity of using calvariae OBs or interpreting microscopic observations made in the calvariae as true representatives of HSC-OB interactions have been questioned.48 Our present studies do not show any significant correlation between the hematopoiesis-enhancing activity of OBs and their anatomic location. In addition, these data do not support an age-dependent impact on the ability of OBs to support progenitor cell function. Therefore, collectively, these results strongly suggest that the calvariae are a valid choice for the collection of OBs suitable for the type of investigations presented here.
In these studies, measurement of Ca deposition is a marker for the mineralization occurring in newly formed osteoid bone matrix. Ca and phosphate form hydroxyapatite crystals, which is the mineral content of bone. Therefore, our data are not inconsistent with those of Adams et al49 in which the authors suggest that preferential localization of HSCs in the endosteal niche is probably the result of high Ca concentration given that HSCs express a Ca-sensing receptor.49 One of the functions of the skeleton is to provide a reservoir of minerals, including Ca and phosphate,50 and through bone remodeling, osteoclasts release these minerals under the control of various stimuli to maintain homeostasis. Ca that is freed by bone resorption through osteoclastic activity is bioavailable; however, Ca measured in these studies is not free Ca as described by Adams et al49 and, as such, is not bioavailable but rather is a component of the mineral matrix of bone.
Taken together, our data demonstrate that hematopoiesis is most probably maintained in the hematopoietic niche through opposing functions of OBs and SCs. Furthermore, the activities of these cell types on the fate of HSCs appear to be mediated via Notch signaling and its powerful impact on self-renewal and the maintenance of the HSC pool. Our data also suggest that the observed negative activity of SCs on hematopoiesis most probably involves multiple cellular and soluble mediators, including adipocytes and neuropilin-1, the role of which requires further investigations.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the operators of the Indiana University Melvin and Bren Simon Cancer Center Flow Cytometry Resource Facility for their outstanding technical help and support.
This work was supported in part by grant NHLBI HL55716 (E.F.S.). The Flow Cytometry Research Facility is partially funded by NCI P30 CA082709.
National Institutes of Health
Authorship
Contribution: B.R.C. performed the majority of the experimental work and assisted in writing the manuscript; Y.-H.C. performed all the experimental work involving preparation and culturing of osteoblasts; B.P. contributed to the culture and animal work; S.R.-R. performed all the PCR work involving Notch signaling; W.S.G. helped in the design of experiments involving adipocytes; N.C. designed and interpreted the work focusing on Notch signaling; M.A.K. designed all the work related to osteoblast preparation and culturing, helped in the design of other experiments, and assisted in writing the manuscript; and E.F.S. designed the research, interpreted data, and wrote the manuscript.
Conflict-of-interest disclosure: W.S.G. is a medical director of General BioTechnology LLC, Indianapolis, IN. The remaining authors declare no competing financial interests.
Correspondence: Edward F. Srour, Department of Medicine, Indiana University School of Medicine, 980 W Walnut St, R3-C312, Indianapolis, IN 46202; e-mail: esrour@iupui.edu.

![Figure 1. Impact of OB, SC, and OB + SC cocultures on stem and progenitor cell function. (A) Cells were cultured in different combinations as indicated, and all wells received recombinant murine stem cell factor and interleukin-3 (10 ng/mL), insulin-like growth factor 1 and thrombopoietin (20 ng/mL), interleukin-6 and Fms-like tyrosine kinase 3 (25 ng/mL), and OPN (50 ng/mL). Cells were harvested on day 7 and counted. Fold increase in total cell number from the original 1000 LSK cells was calculated relative to day 0; n = 6 to 8 independent experiments. (B) LSK progeny cells harvested on day 7 were plated in methylcellulose-based clonogenic assays, and colony formation was assessed 7 days later. CFU fold increase was calculated relative to that obtained from 250 freshly isolated LSK cells assayed on day 0; n = 4 or 5 independent experiments. (C) LSK progeny harvested on day 7 were stained and analyzed for the Lin−Sca1+ content; n = 3 independent experiments. (D) Lin−Sca1+ cells were sorted from each group and analyzed for cell-cycle status with propidium iodide; n = 5 independent experiments. (E) BM repopulating potential of freshly isolated and in vitro expanded LSK cells for 10 days in cocultures of OBs, SCs, or OBs + SCs or on plastic. LSK cells from C57Bl/6 (CD45.2) mice were cotransplanted with 100 000 BoyJ (CD45.1) competitor cells in lethally irradiated (1100 cGy, split dose) CD45.2 × CD45.1 F1 recipients. Control mice (Fresh) received 1000 freshly isolated LSK cells and 100 000 competitor cells. At monthly intervals, chimerism was assessed as [CD45.2/(CD45.2 + CD45.1)] × 100, thus eliminating the contribution of residual host-derived HSCs. Data are from 1 experiment, 4 or 5 mice per group, except for the LSK cells cultured on plastic where only 1 mouse survived. (F) Secondary transplantations from primary recipients. At 4 months after primary transplantation, the BM content of 1 femur from each primary recipient was transplanted into a lethally irradiated secondary recipient without competitor cells, and engraftment was assessed at monthly intervals. Each group contained 4 mice, except the LSK cells group, which had 2 mice transplanted with cells from a single primary recipient. *Significant at P < .01 compared with OB + LSK group for panels A, B, C, and D and at P < .05 for panels E and F. Differences between fresh and OB + LSK groups for primary and secondary transplantations were not significant.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/115/16/10.1182_blood-2009-09-246173/4/m_zh89991051170001.jpeg?Expires=1767757088&Signature=SVBymDkOlduVctUFNSvNcQ1nN2fHKf~Ii~b3s-zOG10MIDF5lvn1E7IgSYPVDH9D77F4z2q89OOUV3ytCS69uFe3JyrWt-RCcK~IhLo6XBgaZLzqXmgMMWcGxGrrml3jtT4uYE-iApYhl3QK5mGR9rCi7H7N4~6JNm3V~Vc0AKJGMysrenfxRk7bAeF-MQy2--UY2euAD3EE7dZOBXJF--FQ3Pei62fBpzGZs4pNviJyGB~0hB9ZC1FlXZaY785o9UligZTVyUoOeNoN~3jwi52pf3LWav5l3JQo8nojNUuJ7vk~SoMq5PPtebA5PL-pl9jWI7euSGgGq0rDc58Rmw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal