Abstract
The prognostic impact of minimal residual disease (MRD) was analyzed in 259 patients with mantle cell lymphoma (MCL) treated within 2 randomized trials of the European MCL Network (MCL Younger and MCL Elderly trial). After rituximab-based induction treatment, 106 of 190 evaluable patients (56%) achieved a molecular remission (MR) based on blood and/or bone marrow (BM) analysis. MR resulted in a significantly improved response duration (RD; 87% vs 61% patients in remission at 2 years, P = .004) and emerged to be an independent prognostic factor for RD (hazard ratio = 0.4, 95% confidence interval, 0.1-0.9, P = .028). MR was highly predictive for prolonged RD independent of clinical response (complete response [CR], complete response unconfirmed [CRu], partial response [PR]; RD at 2 years: 94% in BM MRD-negative CR/CRu and 100% in BM MRD-negative PR, compared with 71% in BM MRD-positive CR/CRu and 51% in BM MRD-positive PR, P = .002). Sustained MR during the postinduction period was predictive for outcome in MCL Younger after autologous stem cell transplantation (ASCT; RD at 2 years 100% vs 65%, P = .001) and during maintenance in MCL Elderly (RD at 2 years: 76% vs 36%, P = .015). ASCT increased the proportion of patients in MR from 55% before high-dose therapy to 72% thereafter. Sequential MRD monitoring is a powerful predictor for treatment outcome in MCL. These trials are registered at www.clinicaltrials.gov as #NCT00209222 and #NCT00209209.
Introduction
Mantle cell lymphoma (MCL) is characterized by a mostly advanced stage of disease at diagnosis and an aggressive clinical course with a short median overall survival (OS) of 3 to 4 years after standard treatment. However, recent studies have reported an improved outcome with an almost doubled median survival of 5 to 6 years.1,2 The biologic hallmark of MCL is the chromosomal translocation t(11;14)(q13;q32) leading to cyclin D1 protein overexpression. The translocation is detectable by molecular cytogenetics in more than 95% of MCL.3,4
Current treatment strategies include combinations of the monoclonal anti-CD20 antibody rituximab with different chemotherapy regimens as well as more intensive treatment protocols, including high-dose cytosine arabinoside (Ara-C).5-7 Furthermore, there is increasing evidence that autologous stem cell transplantation (ASCT) as part of the first-line treatment leads to a substantial prolongation of disease-free survival (DFS) and overall survival (OS) in younger patients with MCL.8-14 Although in particular combinations of rituximab, Ara-C-based consolidation and ASCT can achieve long-lasting remissions in significant proportions of patients,7,10,15-18 individual patients may still have an early relapse. Recent evidence suggests that clinical relapses might be prevented by experimental consolidation treatments, such as interferon-α or antibody maintenance or even allogeneic SCT. Therefore, prediction of quality and duration of response becomes increasingly important for early individual risk estimation.
The MCL International Prognostic Index (MIPI) based on the 4 independent factors age, ECOG performance status, lactate dehydrogenase (LDH), and white blood cell count is of proven value for pretreatment risk assessment in patients with advanced-stage MCL.19 However, parameters for early response assessment and individual risk assignment during treatment are currently lacking.
Molecular monitoring of minimal residual disease (MRD) by real-time quantitative polymerase chain reaction (RQ-PCR) is a broadly applicable tool for the assessment of circulating residual lymphoma cells with a great impact on prognosis in different B-cell lymphoma entities.20-27 We have shown that quantitative MRD assessment during treatment allows comparison of the relative impact of different treatment modalities (ie conventional chemotherapy, ASCT, with and without monoclonal antibodies) on the tumor load, and to study the kinetics of tumor depletion and regrowth after cytotoxic treatment in MCL.26 Clonal IGH VH-JH rearrangements as well as the t(11;14) translocation are suitable targets for molecular MRD assessment in MCL. Achievement of a molecular remission (MR) defined as achievement of MRD negativity after ASCT demonstrated a high prognostic significance for progression-free survival and OS in MCL.26 However, to date, only sparse data on the prognostic impact of MRD in the setting of modern combined immunochemotherapy approaches are available.10,11,26,28
In the present study, we therefore addressed the prognostic potential of quantitative MRD monitoring after combined immunochemotherapy followed by ASCT or maintenance treatment in MCL patients. Taking advantage of 2 large and homogeneously treated patient cohorts from the current Intergroup European MCL Network trials (MCL net), we evaluated the prognostic impact of MRD kinetics on disease control and compared the specific effect of different treatment modalities (combined immunochemotherapy, myeloblative radio-chemotherapy, and maintenance treatment with interferon-α or rituximab) on quantitative MRD load.
Methods
Patients and sample collection for MRD
Patients with histologically confirmed MCL were randomized within the clinical trials of the MCL net according to age and eligibility to receive a high-dose therapy. The trials were investigating the role of different induction protocols followed by either 2 different high-dose regimens with ASCT (MCL Younger) or 2 different maintenance therapies (MCL Elderly). Inclusion criteria were: patients up to 65 years of age in the MCL Younger trial and older than 60 years in the MCL Elderly trial with previously untreated, advanced Ann-Arbor stage II to IV MCL. The histologic diagnosis was confirmed by a central pathology review at one of the designated pathology reference centers (European MCL Pathology Panel). Both protocols, including the incorporated MRD analyses, had been approved by the institutional review boards of all participating institutions and were conducted according to the updated declaration of Helsinki, and are listed under www.clinicaltrials.gov (MCL Younger NCT00209222, MCL Elderly NCT00209209).
Prospective quantitative MRD monitoring was a predefined secondary objective of the current trials of the EU-MCL Network. However, participation in the MRD program was not a prerequisite for randomization in the clinical trials. MRD analysis was performed in national reference laboratories. For logistical reasons, MRD assessment was mainly performed in Germany and France. Because this analysis was performed within ongoing trials, clinical and molecular data could not be collected concordantly in all cases.
Histologic, immunhistochemical, and cytogenetic analyses
The diagnosis of MCL was established according to World Health Organization criteria.29 Conventionally and immunohistochemically stained paraffin sections of lymph node biopsies were reviewed. Minimal requirements for immunohistochemistry included: positivity for Cyclin D1, CD20, and CD5, and negativity for CD23 and CD10. The histologic slides and immunohistochemical stainings (CD20, CD5, CD23, and CyclinD1) were evaluated according to the Annecy criteria,21 and MCL were subclassified according to the different cytologic subtypes. Histologic slides were reviewed by members of the European Mantle Cell Lymphoma Study Group.
The presence of a t(11;14)(q13;q32) translocation was investigated by PCR, fluorescence in situ hybridization, or conventional cytogenetics in diagnostic peripheral blood (PB) and/or bone marrow (BM) samples.
Treatment of patients younger than 65 years and eligible for ASCT
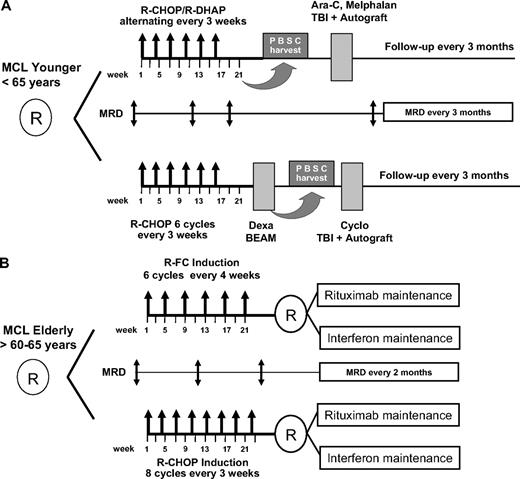
After initial randomization, patients received either 6 cycles (once every 3 weeks) rituximab with cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) followed by stem cell mobilization with DexaBEAM and myeloablative radiochemotherapy with autologous blood stem cell support according to a previously published protocol of the EU-MCL Study Group14 or a total of 6 cycles of alternating R-CHOP/R-DHAP (rituximab with high-dose cytarabine and cisplatin) regimens followed by a high-dose Ara-C containing myeloablative radio-chemotherapy and ASCT.15,16 After myeloablative therapy, no further antilymphoma treatment was applied until clinical relapse (Figure 1).
Diagram of the 2 randomized EU-MCL network trials. (A) Mantle cell lymphoma (MCL) Younger and (B) MCL Elderly with the respective minimal residual disease (MRD) sampling time points. MRD is assessed until clinical relapse or death. Maintenance treatment in both arms of the elderly protocol is given until progression or death.
Diagram of the 2 randomized EU-MCL network trials. (A) Mantle cell lymphoma (MCL) Younger and (B) MCL Elderly with the respective minimal residual disease (MRD) sampling time points. MRD is assessed until clinical relapse or death. Maintenance treatment in both arms of the elderly protocol is given until progression or death.
Treatment of patients 60 years of age or older and ineligible for ASCT
Patients were randomized to induction treatment of either 8 cycles (once every 3 weeks) of R-CHOP or 6 cycles (once every 4 weeks) of rituximab, fludarabine, cyclophosphamide (R-FC) chemotherapy. After a second randomization, all patients in clinical remission received maintenance treatment with either interferon-α (3 × 3M IU) or PegIntron 1 μg/kg weekly) or rituximab maintenance (rituximab 375 mg/m2) at 2-monthly intervals. Maintenance treatment was given until clinical relapse (Figure 1).30
Flow cytometry
Four-color flow cytometry (4C-FC) was performed to assess the proportion of MCL cells in diagnostic blood and BM samples.
This 4C-FC assay had been previously standardized and tested in 281 PB and BM samples from 98 MCL patients of the current EU-MCL trials and demonstrated a high specificity and sensitivity for MCL cell quantification. The principles of staining protocols for flow cytometry, gating strategies, and specificity and sensitivity have been recently published by our group in detail.31
The degree of lymphoma involvement of the diagnostic sample was subsequently used to establish standard dilution series of the diagnostic specimen for RQ-PCR for each individual patient.
Clonality assessment and PCR-based MRD analysis
DNA from PB, peripheral blood mononuclear cells or BM was extracted with a standard proteinase K digestion and a phenol-chloroform extraction or the QIAGEN Blood Mini Kit (QIAGEN). Samples were analyzed by t(11;14) (BCL1-IGH) PCR and IGH multiplex PCR as published to assess the clonal rearrangement.32,33 Gene scanning and sequence analyses were performed on an ABI PRISM 377 automated sequencer (Applied Biosystems). Sequencing of clonal rearrangements for allele-specific RQ-PCR was performed using the BigDye Terminator Cycle Sequencing Ready Reaction Kit (Applied Biosystems).
Quantitative PCR with allele-specific oligonucleotides was performed as described previously.26 The assays were established to reach a sensitivity of 10−5, tested by analyzing 10-fold serial dilutions from diagnostic samples in polyclonal DNA derived from pooled mononuclear cells of healthy donors. For determining the quantitative MRD levels, target copy numbers were related to the number of target copies at diagnosis.
MRD levels were given as fraction of numbers of MCL cells per total number of mononuclear BM or PB cells analyzed per PCR assay.
Response criteria and evaluation
Clinical response was assessed after midterm induction therapy (3 or 4 cycles of induction therapy according to the protocol), at restaging after completion of induction therapy (∼ 4 weeks after the last cycle of induction) and for follow-up every 3 months after ASCT (MCL Younger) and every 2 months during maintenance (MCL Elderly). As this analysis has been performed within ongoing clinical trials, randomized arms were pooled and analyzed together without any unblinding of the treatment arms.
Response was defined according to the International Working Group criteria.36 Response duration (RD) was defined only for patients who achieved at least a partial response (PR) after induction treatment and was calculated as period from the completion of induction to documented progression or death from any cause, which were both considered as an event. OS was defined as the interval between trial registration and death from any cause.
Definition of MR and sampling time points for MRD analysis
PB and/or BM samples were collected at diagnosis and at follow-up according to clinical staging time points. Sampling time points corresponded to clinical response assessment and included: midterm staging (after 3 or 4 cycles of induction therapy according to the protocol), restaging after completion of induction therapy (∼ 4 weeks after the last cycle of induction with R-CHOP or R-CHOP/R-DHAP or R-FC before DexaBEAM and ASCT or maintenance treatment) and postinduction monitoring at 3-monthly intervals after ASCT for MCL Younger patients and at 2- to 3-monthly intervals during maintenance follow-up for MCL Elderly patients (Figure 1). MRD monitoring was intended to be performed until clinical relapse in both trials.
MRD status at a certain time point was assigned using the MRD information from MRD analysis in PB or BM and, if available, both. In case of parallel investigation of PB and BM, MRD was judged positive if at least 1 of both samples was positive by RQ-PCR. In case of MRD positivity in parallel samples, the higher MRD value was used for calculation.
MR was defined as MRD negativity investigated by allele-specific RQ-PCR with an assay sensitivity of at least 10−4. MR was assigned in parallel analyzed PB and BM samples if both were MRD negative.
The MRD status within the postinduction period (implying for MCL Younger patients the first 12 months after ASCT and for MCL Elderly patients the first 12 months after end of induction/start of maintenance) was judged MRD-positive if at least 1 sample demonstrated MRD positivity by RQ-PCR.
Samples collected at or subsequent to documented clinical relapse were not included in statistical analysis.
Statistical analysis
To describe quantitative MRD values, median and ranges of MRD levels were assessed. Quantitative MRD values were compared between groups according to baseline characteristics using the Mann-Whitney U test. Quantitative MRD values in PB and BM were compared with Pearson r and the concordance correlation coefficient.37 Quantitative MRD values at different time points during induction were compared by Wilcoxon rank-sum test. Cross-tables together with exact Fisher tests were calculated to assess the association of the achievement of an MR with categorical clinical parameters and with clinical response. MR after induction and during the first year of follow-up was compared in paired samples by McNemar test. RD according to clinical or MR was analyzed by Kaplan-Meier estimates and compared using the log-rank test. Follow-up time was estimated using the reversed Kaplan-Meier method. Multiple Cox regression was performed to analyze the adjusted prognostic value of MR in a model together with clinical remission status and MIPI prognostic score at diagnosis. The significance level was 5%. Statistical analyses were performed using SAS 9.1 (SAS Institute).
Results
Patients and samples
From January 1, 2004 until October 16, 2008, 760 patients with central review-confirmed MCL were randomized within the intergroup trials of the MCL net, 600 of these in Germany (n = 356) or France (n = 244; supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Sample recruitment for MRD (at least 1 sample) composed 90% of all study patients recruited in Germany and France. In the missing 10%, samples were not sent according to lacking center or patient compliance because MRD assessment was not a prerequisite for study enrollment. As of October 2008, 259 patients with a molecular marker and at least 2 samples from different time points had been analyzed for MRD, composing 160 patients treated within the European MCL Younger trial and 99 patients within the MCL Elderly trial (supplemental Figure 2).
The detection of a suitable molecular marker for RQ-PCR was possible in 90% of all patients with material available for MRD. In case of patients with diagnostic samples not informative for molecular follow-up, this was principally the result of failure to identify a clonal IGH population. MRD was assessed with quantitative allele-specific IGH-RQ-PCR (n = 245) or allele-specific BCL1-IGH-RQ-PCR (n = 14).
Patients analyzed for MRD did not differ from those of the complete study cohort with respect to clinical characteristics (Table 1). Overall, 315 samples before treatment (210 PB, 105 BM) and 1324 follow-up samples (907 PB, 417 BM) were investigated. All 315 baseline samples demonstrated lymphoma infiltration by RQ-PCR.
Baseline clinical characteristics
| Variable . | Whole cohort analyzed for MRD (n = 259) . | MCL Younger (n = 160) . | MCL Elderly (n = 99) . |
|---|---|---|---|
| Median age, y (range) | 61 (33-81) | 55 (33-65) | 70 (60-81) |
| Sex male, % | 77 | 81 | 71 |
| Stage, % | |||
| 2 | 2 | 1 | 4 |
| 3 | 14 | 15 | 12 |
| 4 | 84 | 84 | 84 |
| B-symptoms, % | 39 | 39 | 39 |
| LDH, elevated, % | 39 | 38 | 41 |
| Extranodal > 1, % | 34 | 37 | 29 |
| Bone marrow, % | 81 | 83 | 79 |
| MIPI risk, % | |||
| Low | 43 | 65 | 8 |
| Intermediate | 34 | 23 | 52 |
| High | 23 | 12 | 40 |
| Variable . | Whole cohort analyzed for MRD (n = 259) . | MCL Younger (n = 160) . | MCL Elderly (n = 99) . |
|---|---|---|---|
| Median age, y (range) | 61 (33-81) | 55 (33-65) | 70 (60-81) |
| Sex male, % | 77 | 81 | 71 |
| Stage, % | |||
| 2 | 2 | 1 | 4 |
| 3 | 14 | 15 | 12 |
| 4 | 84 | 84 | 84 |
| B-symptoms, % | 39 | 39 | 39 |
| LDH, elevated, % | 39 | 38 | 41 |
| Extranodal > 1, % | 34 | 37 | 29 |
| Bone marrow, % | 81 | 83 | 79 |
| MIPI risk, % | |||
| Low | 43 | 65 | 8 |
| Intermediate | 34 | 23 | 52 |
| High | 23 | 12 | 40 |
MRD indicates minimal residual disease; MCL, mantle cell lymphoma; LDH, lactate dehydrogenase; and MIPI, MCL International Prognostic Index.
Comparability of PB and BM for MRD detection
With regard to the ease of access to MRD samples in clinical routine, we addressed the question of comparability of PB and BM for MRD assessment. At diagnosis, 95 paired BM and PB samples demonstrated similar levels of lymphoma cells with a median level of 7.1 × 10−2 in BM and 5.6 × 10−2 in PB (Pearson r = 0.82, concordance correlation coefficient c = 0.81).
After induction, 31 of 108 paired samples were concordantly positive in PB and BM and 50 were negative in both. In 21 paired samples, discordant results were obtained with MRD− PB but low-level MRD detectable in the corresponding BM. In contrast, only 6 BM samples failed to demonstrate persistent disease when the corresponding PB sample was MRD+. Thus, PB analysis after induction underestimated MRD in approximately 19% of patients.
Kinetics of MRD
Circulating lymphoma cells (CLCs) were assessed using 4C-FC in 157 of 210 patients with available PB at diagnosis. Because of different sample processing, PB samples of 53 patients were not directly accessible for CLCs by 4C-FC.
Although the majority had no leukemic MCL by clinical parameters, all 157 patients showed CLC at a median level of 6.3 × 10−2 (range, 2.0 × 10−4 to 8.3 × 10−1). Levels of CLC correlated significantly with the following parameters: stage (median 5.4 × 10−3 stage II, 2.4 × 10−2 stage III, and 7.6 × 10−2 stage IV, P = .002), elevated LDH (1.2 × 10−1 vs 4.7 × 10−2, P = .002), histologic BM infiltration (7.8 × 10−2 vs 1.9 × 10−2, P < .001), and MIPI prognostic index (2.7 × 10−2 vs 7.9 × 10−2 vs 3.3 × 10−1 for low, intermediate, and high risk, respectively, P < .001; supplemental Figure 3).
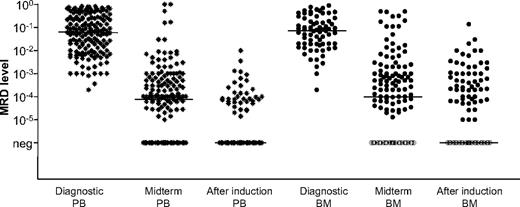
Monitoring of MRD kinetics during induction was possible in 190 patients and showed that induction treatment with combined immunochemotherapy protocols rapidly reduced the tumor cell load. At midterm staging, 59 of 190 (31%) patients achieved an MR corresponding to a median 3-log tumor cell reduction (Figure 2). The median lymphoma cell levels before treatment were comparable in PB and BM samples (6.2 × 10−2 vs 7.1 × 10−2; range, 2.0 × 10−4 to 9.0 × 10−1 in BM and 8.3 × 10−1 in PB) and were significantly reduced to a median of 1.0 × 10−4 in PB and 1.2 × 10−4 in BM at midterm staging (P < .001) and to MRD negativity at end of induction in both (P < .001; Figure 2).
MRD quantification by RQ-PCR of 190 patients before, during, and after induction. MRD levels at diagnosis, at midterm staging, and after induction are given. Combined immunochemotherapy resulted in a reduction of lymphoma cells of 3 orders of magnitude in peripheral blood (PB) and bone marrow (BM) from 6.2 × 10−2 in PB and 7.0 × 10−2 in BM prior treatment to 1.0 × 10−4 in PB and 1.2 × 10−4 in BM at midterm staging (P < .001) and the median of samples were MRD negative after end of induction (before DexaBEAM/autologous stem cell transplantation or maintenance). Black lines indicate the median MRD level at the stated time point.
MRD quantification by RQ-PCR of 190 patients before, during, and after induction. MRD levels at diagnosis, at midterm staging, and after induction are given. Combined immunochemotherapy resulted in a reduction of lymphoma cells of 3 orders of magnitude in peripheral blood (PB) and bone marrow (BM) from 6.2 × 10−2 in PB and 7.0 × 10−2 in BM prior treatment to 1.0 × 10−4 in PB and 1.2 × 10−4 in BM at midterm staging (P < .001) and the median of samples were MRD negative after end of induction (before DexaBEAM/autologous stem cell transplantation or maintenance). Black lines indicate the median MRD level at the stated time point.
Clinical response to treatment
A total of 207 of 259 patients with MRD data were evaluable for clinical response after induction with 65 patients (31%) achieving a complete response (CR) and 135 patients achieving a complete response unconfirmed (CRu) or PR (overall response rate = 97%). For patients of the MCL Younger and MCL Elderly trial, the CR rate was 32% and 31%, and the overall response rate = 99% and 95%, respectively. To date, 27 patients relapsed (12 in MCL Younger and 15 in MCL Elderly) and 5 patients died in remission (1 in MCL Younger and 4 in MCL Elderly). With a median observation time of 17 months, the median RD has not been reached with 75% patients in remission at 2 years (MCL Younger: median RD not reached, patients in remission at 2 years 84%; MCL Elderly median RD 37 months, patients in remission at 2 years 58%). Of 225 patients evaluable for overall survival, 26 have died, with median OS not reached and 2-year OS of 86%.
Achievement of MR
MR after induction treatment was achieved by 106 of 190 patients with MRD data (56%). Interestingly, MCL Elderly patients achieved an MR (54 of 81, 67%) more frequently compared with MCL Younger patients (MR, 52 of 109, 48%; P = .012) despite a higher number of patients with an adverse MIPI score (MIPI high risk, 40% vs 12%).
MRD persistence after induction correlated with the following pretreatment parameters: stage (0%, 36%, and 47% positive for stages II, III, and IV, respectively, P = .04), the presence of B-symptoms (53% vs 38%, P = .051), LDH above normal level (55% vs 38%, P = .024), and BM infiltration (49% vs 25%, P = .015). Patients achieving a clinical CR and CRu had a higher probability of obtaining an MR (both 70%) than patients achieving a PR (42%, P = .002).
Prognostic relevance of MR
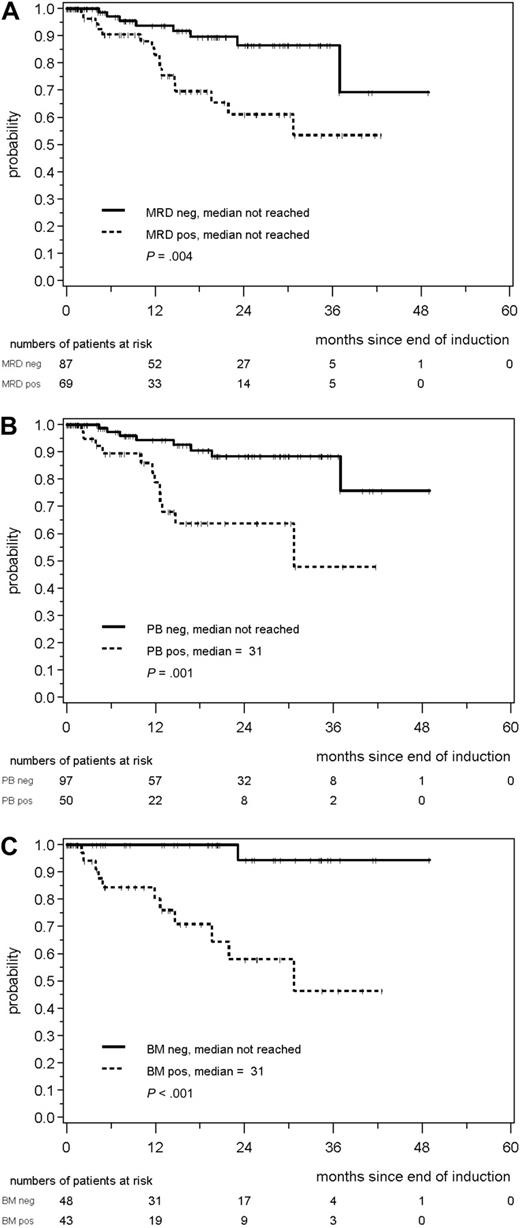
A total of 156 patients with MRD data and a documented clinical remission after induction were evaluable for assessment of the prognostic impact of MRD (supplemental Figure 2). Patients achieving an MR after induction (n = 87) demonstrated a significantly improved RD compared with patients with residual disease (n = 69; patients in remission at 2 years, 87% vs 61%, P = .004; Figure 3A).
Response duration (RD) according to MRD status after combined immunochemotherapy. (A) RD according to MRD status in PB and/or BM after end of induction in MCL Younger and MCL Elderly patients. RD duration according to MRD status assessed in the PB (B) or BM (C) after induction treatment in both trials.
Response duration (RD) according to MRD status after combined immunochemotherapy. (A) RD according to MRD status in PB and/or BM after end of induction in MCL Younger and MCL Elderly patients. RD duration according to MRD status assessed in the PB (B) or BM (C) after induction treatment in both trials.
The high impact of MR on RD was also confirmed when only PB was analyzed (147 patients, patients in remission at 2 years: 88% for MRD− patients compared with 64% of MRD+ patients, P = .001; Figure 3B). Eight of 97 (8%) patients in the PB-MRD− cohort relapsed compared with 12 of 50 (24%) patients in the MRD+ group. The impact of MRD was even more prominent when only BM was assessed (n = 91, patients in remission at 2 years 94% in MRD− vs 58% in MRD+ patients, P < .001; Figure 3C). Only 1 patient relapsed in the BM-MRD− group compared with 11 patients with detectable residual disease.
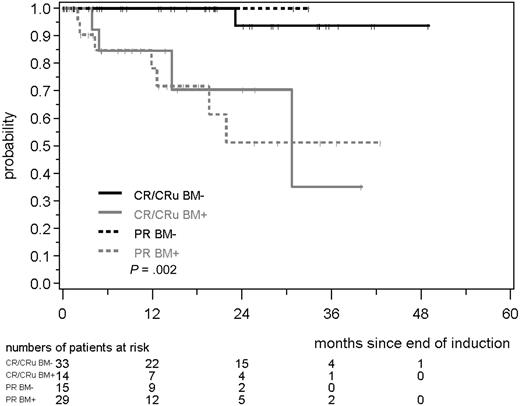
We also compared the significance of MRD in PB and BM within the clinical response groups after induction (CR/CRu and PR). Notably, MRD status in PB and BM was a much better predictor for RD than the clinical response status (CR/CRu/PR). MR in the BM correlated with a significant prolongation of RD compared with MRD+ CR/CRu or MRD+ PR patients (patients in remission at 2 years: 94% in MRD− CR/CRu and 100% in MRD− PR, compared with 71% in MRD+ CR/CRu and 51% in MRD+ PR, P = .002; Figure 4). This was also reproducible when PB alone was investigated (patients in remission at 2 years: 92% in MRD− CR/CRu and 83% in MRD− PR, compared with 85% in MRD+ CR/CRu and 55% in MRD+ PR, P = .003; data not shown).
RD according to MRD status and clinical remission (CR/CRu/PR). MRD was assessed in the BM after induction with combined immunochemotherapy in MCL Younger and MCL Elderly patients.
RD according to MRD status and clinical remission (CR/CRu/PR). MRD was assessed in the BM after induction with combined immunochemotherapy in MCL Younger and MCL Elderly patients.
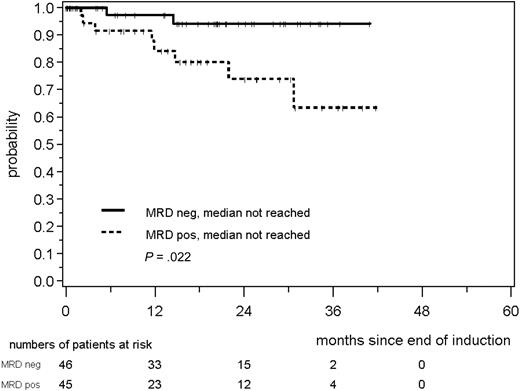
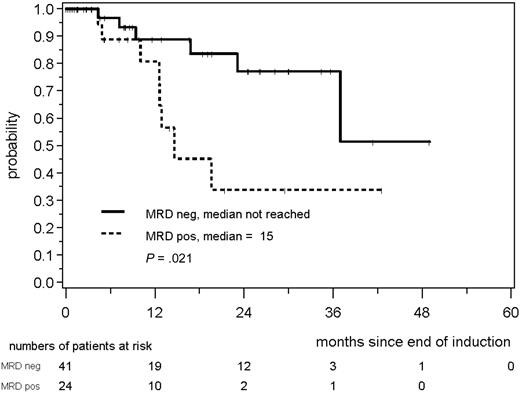
MRD was prognostically significant independent of assignment to the MCL Younger or MCL Elderly trial. MRD negativity after induction was reached in 46 of 91 evaluable MCL Younger patients and 41 of 65 MCL Elderly patients and was associated with a better prognosis in both cohorts (MCL Younger: patients in remission at 2 years: 94% vs 74%, P = .022; Figure 5; MCL Elderly: 77% vs 34%, P = .021; Figure 6). In both trials, the impact of MRD on prognosis was confirmed when PB and BM were analyzed (supplemental Figures 4-7).
RD according to MRD status assessed in PB and/or BM after induction with combined immunochemotherapy in MCL Younger patients.
RD according to MRD status assessed in PB and/or BM after induction with combined immunochemotherapy in MCL Younger patients.
RD according to MRD status assessed in PB and/or BM after induction with combined immunochemotherapy in MCL Elderly patients.
RD according to MRD status assessed in PB and/or BM after induction with combined immunochemotherapy in MCL Elderly patients.
A Cox regression model was used to evaluate the prognostic significance of MR together with achievement of CR/CRu versus PR and pretreatment clinical variables summarized in the continuous MIPI score. Achievement of an MR after induction (hazard ratio [HR] =0.4; 95% confidence interval [CI], 0.1-0.9; P = .028) turned out to be an important prognostic factor for RD independent from MIPI (HR = 3.2; 95% CI, 1.8-5.6; P < .001) and achievement of CR/CRu (HR = 0.7; 95% CI, 0.2-2.0; P = .49).
MRD assessment in the postinduction period
Within both trials, MRD assessments were performed during the first 12 months of the postinduction period (after ASCT in MCL Younger patients and during maintenance in MCL Elderly patients) to evaluate the prognostic impact of a sustained MR. MRD data were pooled from a median of 3 samples (range, 1-8), and the MRD status was judged as MRD+ if at least 1 sample was positive by RQ-PCR.
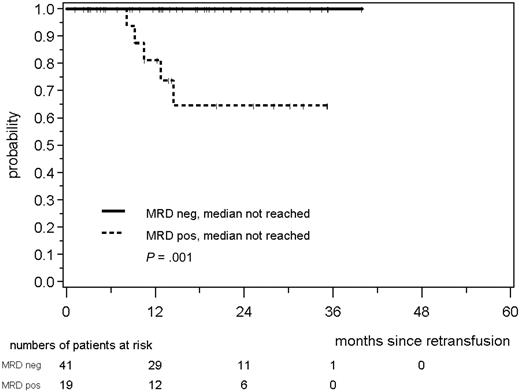
Among 60 MCL Younger patients, all 41 patients with a consistently negative MRD status within the first year after ASCT remained in continuous clinical remission, whereas 5 of 19 patients with at least 1 MRD+ sample within this period relapsed (patients in remission at 2 years, 100% vs 65%, medians not reached, P = .001; Figure 7). This was also confirmed when either only PB (n = 57, P = .004) or only BM (n = 37, P = .014) was assessed for the presence of MRD (data not shown).
RD according to MRD status assessed in PB and/or BM within the first 12 months after ASCT in MCL Younger patients. The MRD status was judged as MRD+ if at least 1 sample of a median of 3 was positive.
RD according to MRD status assessed in PB and/or BM within the first 12 months after ASCT in MCL Younger patients. The MRD status was judged as MRD+ if at least 1 sample of a median of 3 was positive.
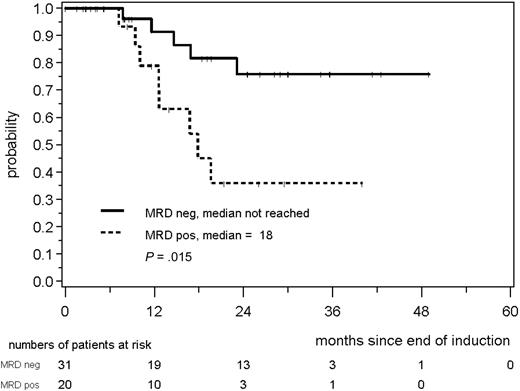
Similarly, in 51 MCL Elderly patients, a consistently negative MRD status during the first year of maintenance was associated with a prolonged RD; 76% of patients in MR were in clinical remission at 24 months compared with only 36% of the patients with residual disease (P = .015; Figure 8).
RD according to MRD status assessed in PB and/or BM during the first year of maintenance in MCL Elderly patients.
RD according to MRD status assessed in PB and/or BM during the first year of maintenance in MCL Elderly patients.
For younger and elderly patients, the impact of sustained MRD negativity could be confirmed in landmark analysis. Only patients in ongoing clinical remission at the respective time point 3, 6, and 12 months after induction treatment (MCL Elderly trial) or ASCT (MCL Younger trial) were included in this analysis, demonstrating that during all 3 time periods a sustained MR retains its high clinical significance (supplemental Figures 8-13).
Hence, these results demonstrate that a consistently negative MRD status in patients achieving clinical remission has a strong impact on prognosis independent from the treatment regimen that was applied to achieve it.
Impact of high-dose treatment on MR
A direct comparison of the impact of high-dose treatment followed by ASCT on tumor cell reduction could be performed in 67 MCL Younger patients with DNA available after induction treatment and after ASCT. Among 67 MCL Younger patients, 37 were MRD− after induction treatment (55%). High-dose treatment followed by ASCT increased the MR rate to 72% (48 of 67, P = .012, McNemar test), demonstrating a significant impact of high-dose treatment on tumor reduction.
Discussion
Today, a spectrum of highly effective but potentially toxic treatment modalities is available for patients with MCL. This makes sensitive and reliable assessment of treatment efficacy allowing individual estimation of RD desirable to optimize patient care as well as trial design. Previous retrospective data from our group suggested that quantitative measurement of MRD during and after treatment may provide an excellent tool to achieve this objective.26
Those earlier MRD data were in keeping with the notion that persistence of residual lymphoma cells is considered to be the principal reason for relapse.21,23 However, in those earlier series, patients did not receive rituximab as a part of induction treatment. We hypothesized that MRD assessment as a dynamic parameter might contribute to the prediction of prognosis, independently from pretreatment risk profile (MIPI score). This analysis was particularly important as previous data addressed this question retrospectively in small patient cohorts only.21,23,26
The aim of our study was therefore (1) to assess the prognostic relevance of MRD in the context of 2 large prospective trials investigating various immunochemotherapy regimens with different consolidation treatments, and (2) to study the individual effects of different treatment components on response kinetics and tumor load in MCL patients.
Because of easier access and patient comfort, MRD analysis of PB would be preferable to BM assessment. This is of particular importance as rituximab-based treatment protocols induce profound peripheral B-cell depletion that might lead to a discordant PB MRD status compared with the clinical disease status and prognosis may be altered by prior rituximab use because of a preferential clearance of disease from the PB compartment.
Therefore, we addressed the question of the comparability of both sources for MRD assessment before and after combined immunochemotherapy. By analyzing 95 paired PB and BM pretreatment samples, we found comparable median CLC levels in PB and BM. However, the situation changes when paired samples after the start of immunochemotherapy were compared. Analysis of PB alone failed to demonstrate persistent lymphoma cells in approximately 19% of patients who were simultaneously positive in BM. We thus demonstrate herein that rituximab-based immunochemotherapy more effectively clears lymphoma cells from PB than from BM. This phenomenon has been reported for alemtuzumab administered in chronic lymphocytic leukemia38 but is in contrast to our earlier findings in MCL demonstrating a similar predictive value of MRD assessment in PB or BM after rituximab-free treatment.26 Accordingly, in the present study, BM MR predicted more accurately for an event-free clinical course than PB MR, although even the latter was associated with a clearly superior outcome. Therefore, source of material for MRD assessment remains an important issue that must be taken into account when designing clinical trials or using MRD results as basis for consolidation or preemptive treatment decisions.
In this study, MRD assessment allowed, for the first time, direct evaluation of the impact of individual treatment elements on tumor cell reduction within a multimodal protocol in patients with MCL. Immunochemotherapy rapidly caused a median MRD reduction of 3 log by midterm staging and induced MR in 56% of the cases after end of induction. This is in marked contrast to our previous work showing that 4 to 6 cycles of CHOP alone did not significantly reduce MRD levels, and none of those patients achieved MR after induction.26 This observation correlates with the superior clinical response rate of R-CHOP over CHOP in MCL5 but can also be influenced by the potential effect of the different induction regimens that are tested within the 2 trials (R-DHAP and R-FC). Thus, our data suggest that rituximab improves the efficacy of CHOP chemotherapy that on its own shows only limited activity in MCL.
With regard to prediction of prognosis, achievement of MR after induction was highly correlated with prolonged RD independent of the study protocol applied. A favorable outcome could be predicted early by MRD assessment of PB or preferably BM at a single time point after induction. Thus, achieving MR after induction is clearly a desirable goal in the treatment of patients with MCL. It remains unclear, however, if the better sensitivity to cytotoxic treatment as documented by achievement of MR simply reflects a more “benign” biologic profile of the tumor in individual patients or if MR has prognostic impact per se.
Our results are in contrast to published data suggesting that achievement of MR in MCL after rituximab and CHOP or other chemotherapy schemes has no impact on prognosis.39 Although in the study by Howard et al39 9 of 25 patients with PCR-detectable disease at diagnosis achieved an MR, no differences in outcome according to MRD status could be demonstrated. This can in part be explained by the small numbers of patients but also by technical aspects. Most previous reports on MRD detection within multimodal treatment protocols were based on qualitative PCR approaches that appear less reliable for PCR-based risk stratification in MCL because of various sensitivity and the lack of standardized evaluation. Thus, our results underline once more the importance of highly standardized, sensitive, and quantitative RQ-PCR assays for MRD assessment,26 as this has already been documented previously by our group.
Quality of clinical remission after treatment is thought to be one of the strongest parameters for prognosis in patients with MCL.5,13,40 Of note, in this series, the prognostic impact of MR in responding patients exceeded that of clinical remission status. Patients achieving an MRD− CR/CRu or PR had an RD that was superior to that of patients with an MRD+ CR/CRu or PR. This implies that prediction of outcome by MRD is much more meaningful than by quality of clinical remission and strongly suggests that future treatment protocols should incorporate MRD assessment in response evaluation of MCL treatment.
Similar observations on the prognostic impact of MRD have recently been reported in chronic lymphocytic leukemia where an MRD− status was the strongest predictor of clinical outcome superior to quality41,42 of response or low level of MRD during and after induction was identified as prognostic parameter for progression-free survival.43 In addition, in MCL it will be useful to investigate the prognostic value of different MRD cut-offs in PCR+ patients to precisely define clinical risk groups.
In a multivariate analysis, including the parameters MIPI and quality of clinical response in addition to MRD, the achievement of MR after induction turned out to be an independent prognostic factor for RD. These results document, for the first time, the independent prognostic value and high clinical relevance of MRD after combined immunochemotherapy in MCL. Comparable results in a prospective series have only been published in patients in follicular lymphoma undergoing treatment with 6 courses of CHOP followed by rituximab (CHOP-R) or supplemented high-dose sequential chemotherapy with autografting (R-HDS). In this entity, MR was achieved in 44% of R-CHOP and 80% of R-HDS patients (P < .001),25 representing the strongest predictor of outcome.
MRD kinetics was also analyzed in the postinduction period, after ASCT consolidation in MCL Younger patients and during maintenance treatment in MCL Elderly patients.
In younger patients, MRD kinetics clearly demonstrated that high-dose radio-chemotherapy followed by ASCT can further reduce the tumor load even if applied immediately after immunochemotherapy. The rate of MR increased from 55% after induction to 72% within the first year after ASCT. These data impressively demonstrate the antilymphoma activity of high-dose radio-chemotherapy in MCL, as already inferred from clinical data9,10,13,14 and our previous observations.26 On the other hand, patients in whom neither rituximab containing induction nor the high-dose consolidation-induced MR had a significantly inferior outcome. This might suggest that for future clinical trials the post-ASCT rather than the postinduction MRD status is an excellent tool for identifying patients in need of further treatment intensification or consolidation. However, the postinduction MRD status has the advantage of providing the prognostic information more timely, thereby allowing for better planning of potential postconsolidation treatment intensification or maintenance treatment.
In conclusion, the addition of rituximab into the treatment of MCL has clearly improved the outcome of patients with MCL, as shown by several trials.10-12 The present study demonstrates, for the first time, that prospective longitudinal monitoring of MRD after combined immunochemotherapy can be a powerful predictor of treatment outcome in patients with MCL, allowing an early individual risk assessment already during treatment. Accordingly, MRD assessment should be integrated into future clinical trial concepts to evaluate new treatment strategies and to serve as an early surrogate marker that can be used for risk-adapted treatment.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the European Community within the European MCL Network (LSHC-CT 2004-503351; C.P., E.M., M.-H.D.-L., and R.S.), the Lymphoma Research Foundation (Correlative Research grant; C.P., S.B., M.D.), Association de Recherche Contre le Cancer (Subvention number 3730; M.-H.D.-L.), Fondation de France Comité Leucémie (Subvention number 2004004029; M.-H.D.-L.), and Association de Recherche sur le Traitement, la Genétique et l'Immunologie des Lymphomes (M.-H.D.-L.).
Authorship
Contribution: C.P. guided MRD analysis and interpretation and drafted the manuscript; E.H. conducted the statistical analysis of clinical and MRD data; C.P., M.-H.D.-L., K.B., N.A., and E.M. were responsible for sample and data collection and interpretation; J.J.M.v.D. assisted in data interpretation; S.B., V.A., and A.P. were responsible for flow cytometry; W.K. and F.B. performed the pathologic panel review; R.S. and E.C.-B. were responsible for cytogenetic and molecular cytogenetic data collection and interpretation; M.U. is the responsible statistician of the EU-MCL Study Group; M.D. is the coordinator of the European MCL trials; V.R., A.L.v.H., M.T., J.W., W.H., H.C.K.-N., O.H., and M.D. were responsible for the clinical trial design and conduct of the European MCL trials; P.D. participated in data interpretation and manuscript preparation; and M.K. was responsible for the overall conduct of the MRD study and participated in manuscript preparation.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Christiane Pott, Second Medical Department, University Hospital Schleswig-Holstein, Campus Kiel, Chemnitzstr 33, 24116 Kiel, Germany; e-mail: c.pott@med2.uni-kiel.de.