Abstract
Fetal hemoglobin (HbF) is a potent genetic modifier of the severity of β-thalassemia and sickle cell anemia. We used an in vitro culture model of human erythropoiesis in which late-stage erythroblasts are derived directly from human CD34+ hematopoietic cells to evaluate HbF production. This system recapitulates expression of globin genes according to the developmental stage of the originating cell source. When cytokine-mobilized peripheral blood CD34+ cells from adults were cultured, background levels of HbF were 2% or less. Cultured cells were readily transduced with lentiviral vectors when exposed to vector particles between 48 and 72 hours. Among the genetic elements that may enhance fetal hemoglobin production is an artificial zinc-finger transcription factor, GG1-VP64, designed to interact with the proximal γ-globin gene promoters. Our data show that lentiviral-mediated, enforced expression of GG1-VP64 under the control of relatively weak erythroid-specific promoters induced significant amounts of HbF (up to 20%) in erythroblasts derived from adult CD34+ cells without altering their capacity for erythroid maturation and only modestly reducing the total numbers of cells that accumulate in culture after transduction. These observations demonstrate the potential for sequence-specific enhancement of HbF in patients with β-thalassemia or sickle cell anemia.
Introduction
All species that use hemoglobin (Hb) for oxygen transport switch the composition of Hb during development.1-3 In humans, embryonic Hbs are produced early during hematopoiesis when erythropoiesis is predominantly in the yolk sac. During the fetal period, composing the last 2 trimesters of development, fetal hemoglobin (HbF) is produced in erythroid cells populating the liver. Beginning with the perinatal period, which initiates several weeks just before the end of gestation and continues during the first year of life, HbF is progressively replaced by adult hemoglobin (HbA). All Hb molecules are composed of tetramers of 2 different types of globin chains. α-Globin encoded on chromosome 16 in humans is common to both fetal and adult Hb; the switch from HbF (α2γ2) to HbA (α2β2) reflects replacement of γ-chains in Hb tetramers with β-chains encoded by linked genes on chromosome 11. HbF production continues in adult humans at low levels with individual variation subject to genetic control.4
The level of HbF production is inherited as a quantitative trait and is of significant clinical relevance given its role in ameliorating the severity of the principal Hb disorders, sickle cell anemia and β-thalassemia.1,4 Persons homozygous for these mutations in their β-globin genes and who also have genetic characteristics leading to enhanced HbF production present with a less severe clinical syndrome than those in which HbF production is more limited. More than 5 decades of clinical studies supporting these facts have triggered an intense interest in the mechanisms that control developmental switching.1,2 These mechanistic studies have led to the identification of a number of agents that enhance HbF production in vivo.5 The most widely used drug, hydroxyurea,6 has been approved for the treatment of adult patients with sickle cell disease after a randomized clinical trial, which demonstrated its benefit.7
Transgenic mouse models have been used to study the molecular mechanisms of human Hb switching.8,9 Much has been learned from such models regarding the distribution of regulatory elements within the β-globin locus and potential influence of various transcriptional factors on the relative synthesis of γ- and β-globin.1-3 The embryonic (ϵ), duplicated γ-genes (Gγ and Aγ), the poorly expressed δ-globin gene, and the functional β-globin gene are encoded on chromosome 11 in order of their developmental expression. Upstream from this set of genes is the locus control region (LCR), which is composed of 5 hypersensitive sites, having both insulating and enhancer activity.1-3 Many transcriptional activators and repressors, most of which are neither tissue nor developmental stage specific, are known to interact with specific sequences throughout the globin loci in modulating globin gene expression.1-3
Studies in mouse models suggest that sequential expression of the individual globin genes throughout development occurs as a combination of competition between promoters regulating transcription of ϵ-, γ-, and β-globin genes for the LCR as well as autologous silencing of the ϵ gene at the end of early embryogenesis and of the γ-genes during the perinatal switch, leaving the adult β-globin gene to interact with the LCR throughout adult life.1-3,10-12 However, mouse models are limited by the fact that a mouse has no fetal globin equivalent. Indeed, embryonic Hbs are produced during mouse embryogenesis, and adult Hb production begins relatively early during fetal development and is the predominant Hb made in fetal liver (FL). Recent studies suggest that human γ-globin production in transgenic mice is limited to the embryonic erythroid compartment and that mouse FL cells lack human γ-globin derived from transgenic loci.13
These considerations have prompted us to focus on the use of human primitive hematopoietic cells from different developmental stages to derive erythroid cultures of maturing erythroblasts that can be used to evaluate the molecular mechanisms of switching. We have used a 2-stage liquid culture system14 to generate pure populations of mature erythroblasts from primitive hematopoietic cells from different developmental stages. This culture system results in very low levels of HbF production in cells derived from adult bone marrow (BM) or from cells mobilized into the peripheral blood (PB) of adults with a cytokine. Primitive hematopoietic cells from earlier developmental stages are committed to generating erythroblasts making HbF under identical culture conditions. Furthermore, we have shown that the primitive erythroid cells that expand early in culture are transduced with high efficiency by lentiviral vectors and are therefore potentially useful for evaluation of globin gene vectors and those encoding genetic elements designed to enhance HbF production.
Among the genetic elements that may enhance HbF production is an artificial transcription factor designed to interact with the proximal γ-globin gene promoters.15 Advances in the engineering of polydactyl zinc-finger transcription factors has made it possible to produce zinc-finger proteins capable of recognizing any 18-bp stretch of DNA.16 Zinc finger domains linked to a transcriptional activator domain were constructed to bind to 18-bp segments of the proximal γ-globin gene promoters.15 Of the 3 independent artificial transcriptional factors that were assembled, one binding to the 18-bp segment of the γ-promoter, which includes position −117, proved to be the most active at augmenting γ-globin production in K562 human erythroleukemia cells. The −117 position of the Aγ-promoter is the site of a naturally occurring mutation resulting in hereditary persistence of HbF17,18 ; thus, this region of the γ-globin promoter is known to be relevant to modulation of γ-globin gene expression. This artificial transcriptional activator, termed GG1-VP64, was subsequently shown to augment γ-globin production in continuously proliferating BM cells from transgenic mice harboring the entire human β-globin locus.19 These results prompted us to undertake studies designed to evaluate the impact of expression of GG1-VP64 on γ-globin expression in maturing adult erythroblasts.
Methods
Purification of human CD34+ cells
PB cells from normal volunteers were collected with a Cobe Spectra continuous flow blood cell separator after mobilization with recombinant human granulocyte-colony stimulating factor given for 4 days according to a clinical protocol approved by the Institutional Review Board of St Jude Children's Research Hospital. CD34+ cells were purified using anti-CD34+ antibodies linked to magnetic microbeads.20 Purified CD34+ cells from normal human BM, cord blood (CB), and FL were purchased commercially (Lonza Walkersville). The cells were initially cultured for expansion in Iscove modified Delbecco medium (IMDM) containing 20% fetal bovine serum (Hyclone, Thermo Scientific), human stem cell factor (10 ng/mL), human interleukin-3 (1 ng/mL), erythropoietin (2 units/mL), and dexamethasone and β-estradiol (10−6 M each). At the end of the expansion phase, the cells were pelleted and transferred into IMDM containing 20% fetal bovine serum, erythropoietin (2 units/mL), and insulin (10 ng/mL) for differentiation. Cells were incubated at 37°C in a humidified atmosphere of 5% CO2 and maintained at a density of 105 to 106 cells/mL by supplementing cultures every other day with fresh media. Cell numbers and viability were determined by trypan blue exclusion. Cell morphology was assessed by Wright-Giemsa staining of cytocentrifuge preparations, and images were acquired with an Olympus Bx41 Upright microscrope equipped with a DP70 digital camera and DP manager software (Olympus).
Plasmid constructions
Self-inactivating lentiviral vectors beginning with the plasmid, pCL20cMp-GFP, to which had been added the modified woodchuck post-transcriptional RNA processing element, were derived using components of our HIV-based lentiviral vector system previously described.21 The internal Mp promoter is a modified Murine Stem Cell Virus (MSCV) long terminal repeat (LTR) from which nonessential U5 sequences have been eliminated.
GFP vectors.
Erythroid-specific vectors encoding for expression of green fluorescent protein (GFP) were created by replacing the Mp promoter in pCL20cMp-GFP with either a minimal ankyrin-1 promoter22,23 or a human β-spectrin gene promoter,24 thereby generating pCL20cAnk-GFP or pCL20cSp-GFP, respectively. For construction of pCL20cAnk-GFP, a polymerase chain reaction (PCR) product was amplified using the primers ankyrin-F (5′-ACG CGT TTC GAA GGG GCA ACG AGG-3′) and ankyrin-R (5′-ACC GGT GGG AAT TGC CGC CGA AGG-3′) using DNA of a plasmid containing the minimal ankyrin promoter as a template.22 The resulting 281-bp product containing a MluI site 5′ and AgeI site 3′ was cloned into pCR2.1-Topo (Invitrogen) and confirmed by sequence analysis. An MluI-AgeI fragment containing the ankyrin-1 promoter was recovered from the pCR2.1-Topo clone and ligated into the same sites in pCL20cMp-GFP replacing the Mp promoter. Construction of pCL20cSp-GFP was similarly achieved by PCR amplification of the β-spectrin promoter on plasmid DNA23 using the primers spectrin-F (5′-ACG CGT TAA TTC GAA GGG AGG-3′) and spectrin-R (5′-ACC GGT GCA ATT GAC AGC GG-3′). The resulting 454-bp product flanked by introduced MluI and AgeI sites was cloned into pCR2.1-Topo and subsequently excised as MluI-AgeI fragment to replace the Mp promoter in pCL20cMp-GFP.
GG1-VP64 vectors.
A rhesus variant of GG1-VP64 was designed in anticipation of performing in vivo experiments in nonhuman primates (supplemental Figure 1, available on the Blood website; see the Supplemental Materials link at the top of the online article) and shown to be equally active as human GG1-VP64 on the human γ-promoter in the transient luciferase assay in HeLa cells (supplemental Figure 2). The rhesus variant was used in all subsequent experiments. The β-spectrin promoter was excised as a 568-bp EcoRI-EcoRI fragment from pCR2.1-Topo clone described in “GFP vectors” and cloned between MfeI and EcoRI sites of an intermediate plasmid 5′ to the internal ribosome entry site GFP sequences to create pSp-iG. This vector was linearized with EcoRI (blunt) to allow for insertion of the 803-bp GG1-VP64 coding sequences recovered as a BamHI-EcoRI fragment from a retroviral expression vector analogous to pMx-gg1-VP64-HA15 and rendered blunt with Klenow DNA polymerase generating pSp-GG1-VP64-iG. The Sp-iG or Sp-GG1-VP64-iG intermediates were excised as StuI-SnaBI fragments and cloned into pCL20cMp-GFP digested with MluI (blunt)-EcoRI (blunt) replacing the Mp-GFP cassette to create pCL20cSp-iG or pCL20cSp-GG1-VP64-iG, respectively. To construct pCL20cAnk-GG1-VP64-iG, a 1407-bp NcoI-NcoI fragment, including GG1-VP64 and internal ribosome entry site sequences, was ligated into pCL20cAnk-GFP linearized by partial digest with NcoI.
Lentiviral vector production and gene transfer
Lentiviral vector particles pseudotyped with vesicular stomatitis virus G protein were prepared using a 4-plasmid system by transient transfection of human embryonic kidney 293T cells using the calcium phosphate precipitation technique as previously described.21 Eighteen hours after transfection, cells were washed twice with phosphate-buffered saline and fresh medium was added to each plate of cells. Twenty-four hours later, the medium containing vector particles was harvested, cleared by low-speed centrifugation, and filtered through a cellulose acetate filter of 0.22 μm pore size. Viral supernatants were aliquoted and stored at −80°C until use. Vector preparations were thawed and titered on K562 human erythroleukemia cells based on GFP expression as determined by flow cytometry. Transduction of primitive hematopoietic cells was accomplished by transferring them to retronectin-coated plates (50 μg/cm2; Fisher Scientific) approximately 48 hours after the cultures were initiated. All samples were supplemented with protamine sulfate (10 μg/mL), and vector particles were added to the culture medium to achieve various multiplicities of infection (MOI). After overnight exposure to virus, the cells were harvested with enzyme-free cell dissociation solution (Millipore), washed in IMDM, and returned to the expansion medium.
Flow cytometry
Cells at various stages of differentiation were rendered into single-cell suspensions for flow cytometric analysis. Live cells were identified and gated by exclusion of 7-amino-actinomycin D (BD Biosciences) and tested for expression of GFP or cell surface receptors with antibodies specific for CD34, CD45, CD71, and CD235 conjugated to either phycoerythrin or allophycocyanin on a FACSCalibur System (BD Biosciences) using CellQuest analysis software (BD Biosciences).
DNA analysis for lentivirus VCN
The average vector copy number (VCN) in transduced CD34+ cell populations was determined by Southern blot analysis or quantitative PCR performed on DNA from cells harvested 1 to 2 days after initiation of erythroid differentiation culture conditions. Genomic DNA was isolated using the Gentra Puregene DNA Extraction kit (QIAGEN).
Southern hybridization.
Southern blotting was performed as previously described.25 DNA samples were digested with BglII, which cuts within each provirus to release a near full-length unit, electrophoresed through 0.8% agarose gel, and then blotted onto nylon membrane. Equivalent loading of lanes was confirmed by ethidium bromide staining of gels before DNA transfer to the nylon filters. A radiolabeled 761-bp fragment encoding for HIV-1 RRE element was hybridized with the blot, and the signal intensity of the hybridizing band for each DNA sample was compared with that of the DNA from a K562 clone harboring a single vector copy using a Molecular Dynamic Storm 860 Phosphorimager and its accompanying software.
Quantitative PCR analysis.
The conditions used for detecting integrated HIV vector sequences, establishing the standard curve, normalizing reactions, and calculating final VCN were conducted according to conditions previously described25 using the StepOne Plus Real-time PCR System (Applied Biosystems) and the following modifications. PCR amplification of integrated HIV vector sequences was achieved using the primers FPLV-(5′-ACC TGA AAG CGA AAG GGA AAC-3′) and RTLV-(5′-CAC CCA TCT CTC TCC TTC TAG CC-3′) and amplicon-specific probe (5′-6-FAM-AGC TCT CTC GAC GCA GGA CTC GGC-3′; Applied Biosystems). The average VCN was calculated by establishing a standard curve of K562 DNA containing a single copy of the HIV vector genome serially diluted with native K562 DNA to yield mixtures containing 1, 0.5, 0.25, 0.1, and 0.01 vector copies where DNA was normalized using primers and a probe specific for human N-RAS.26 The final VCN of each sample was adjusted by dividing the copy number by 1.5 based on the triploid nature of the K562 cell line genome.
Hb analysis
Cells (10-15 million) were harvested at various times during the differentiation phase of erythroid culture, lysed in 40 μL of hemolysate reagent (Helena Laboratories), and refrigerated overnight before centrifuging at 20 800g for 10 minutes at 4°C to remove cellular debris. The cleared supernatant was used for characterization of Hb production by cellulose acetate Hb electrophoresis or high performance liquid chromatography (HPLC) using methodologies previously established in our laboratory.25
Results
Differentiation of erythroid progenitors from various developmental stages
We initially tested our 2-stage in vitro model of human erythropoiesis for the ability to recapitulate the developmental pattern of Hb expression associated with the developmental stage of the originating primary cell source. For this purpose, approximately 105 purified CD34+ cells in the range of 94% to 99% purity, as determined by flow cytometry (data not shown), from adult PB after granulocyte-colony stimulating factor administration, adult BM, CB, or FL were established in liquid culture under conditions designed to foster expansion and differentiation (Figure 1A). After 7 days in the proliferative phase, the cells were transferred into medium designed to mediate terminal erythroid maturation. Cell division continued for 12 days and resulted in a 1000-fold expansion in the total number of cells (Figure 1B). Erythroid maturation was monitored by flow cytometry. The majority of maturing erythroid cells from PB, BM, and CB were late-stage erythroblasts reflected by nearly complete enrichment for expression of transferrin receptor (CD71; 99%) and glycophorin A (CD235; > 96%), which peaked by culture day 14 and was maintained until cultures were terminated 6 days later (Figure 1C). Although the majority of cells in cultures of FL were transferrin-positive (98%), there was a residual fraction of cells that remained glycophorin A negative (< 25%) at the end of the culture period. Morphologic evaluation after cytocentrifuge preparations and Giemsa staining indicated that the majority of cells in all of the cultures were terminally maturing erythroblasts (Figure 1D). Cellulose acetate Hb electrophoresis demonstrated that virtually all of the Hb in erythroblasts derived from PB and BM CD34+ cells was adult Hb (≥ 92%; Figure 1E-F; supplemental Figure 3). Alternatively, erythroblasts derived from CB CD34+ cells had roughly equal proportions of HbF and HbA, and the majority of Hb in erythroblasts derived from FL CD34+ cells was HbF (≥ 94%; Figure 1E-F).
Expansion and differentiation of erythroid cells from different developmental stages. (A) Schematic representation of the 2-phase erythroid culture model showing the experimental time frame and additions to the culture medium during expansion and differentiation. (B) Total cell numbers derived from 105 CD34+ cells from cytokine mobilized PB, adult BM, CB, or FL over the initial 12 days of culture. (C) Flow cytometric analysis for expression of CD71 (transferrin receptor) and CD235 (glycophorin A) where the percentages indicate the proportion of cells considered positive and (D) morphology of Wright-Giemsa–stained cytocentrifuge preparations (original magnification × 60) after 20 days of culture. (E) Cellulose acetate Hb electrophoresis of erythroblast lysates from cultured PB, BM, CB, and FL CD34+ cells and whole blood (WB) from an adult after 12 or (F) 16 days of culture.
Expansion and differentiation of erythroid cells from different developmental stages. (A) Schematic representation of the 2-phase erythroid culture model showing the experimental time frame and additions to the culture medium during expansion and differentiation. (B) Total cell numbers derived from 105 CD34+ cells from cytokine mobilized PB, adult BM, CB, or FL over the initial 12 days of culture. (C) Flow cytometric analysis for expression of CD71 (transferrin receptor) and CD235 (glycophorin A) where the percentages indicate the proportion of cells considered positive and (D) morphology of Wright-Giemsa–stained cytocentrifuge preparations (original magnification × 60) after 20 days of culture. (E) Cellulose acetate Hb electrophoresis of erythroblast lysates from cultured PB, BM, CB, and FL CD34+ cells and whole blood (WB) from an adult after 12 or (F) 16 days of culture.
Lentiviral vector-mediated transduction of adult erythroid progenitors
To evaluate the effect of lentivirus-mediated gene transfer on erythroid differentiation, dividing cells derived from mobilized PB hematopoietic progenitors were collected 48 hours after initiation of culture and 3 to 4 × 105 cells transferred to 24-well suspension tissue culture plates coated with retronectin before overnight exposure to lentiviral vector particles encoding for GFP added to the culture medium to achieve a MOI of 5 or 10 (Figure 2A). The following day, the cells were lifted from plates, returned to expansion medium, and cultured until day 7 at which point they were transferred into the differentiation medium. Greater than 1000-fold expansion of the mock-transduced cells as well as cells transduced with the GFP vector at MOIs of 5 or 10 was documented (Figure 2B). Transduction was highly efficient, with the majority of cells demonstrating expression of the GFP marker as shown by flow cytometry analysis on days 8, 11, and 14 of culture (Figure 2C). Loss of the CD34+ antigen, diminution in expression of CD45, and acquisition of expression of transferrin (CD71) and glycophorin A (CD235) were documented for both mock and transduced cell populations (Figure 2D; Surface Marker Expression). Whereas the seed cell populations were relatively large and heterogeneous in size, the average cell size diminished as the cultures progressed down the erythroid maturation pathway until the majority of cells were found in a single peak (Figure 2D; Size) consistent with maturing erythroblasts as documented by morphologic evaluation (Figure 2D; Cytospin). Furthermore, manipulation of mobilized PB CD34+ cells after vector transduction did not alter the adult pattern of Hb production (HbA > 96%; HbF < 2%) as shown in Figure 2E-F.
Lentiviral vector-mediated transduction of erythroid progenitors derived from cytokine-mobilized PB CD34+ cells. (A) Schematic representation of the lentiviral vector encoding for expression of GFP from a modified MSCV LTR sequence. (B) Expansion of cultured cells after transduction. (C) GFP expression is indicated as a function of time after transduction of cells exposed to vector particles at an MOI of 10. (D) Phenotypic comparison of cells on the day of transduction (left panels) and 14 days later at the time of termination of the cultures (right panels) by flow cytometry for expression of the CD34, CD45, CD71, and CD235 surface markers where percentages indicate the proportion of cells considered positive, cell volume, and cell morphology (Wright-Giemsa staining, original magnification × 60). (E) The results of cellulose acetate Hb electrophoresis of lysates from terminal-stage erythroblasts from 2 separate experiments (vertical lines have been inserted to indicate a repositioned gel lane) and (F) HPLC analysis of Hbs present in erythroblasts at the end of culture, which were derived from cells exposed to vector particles at an MOI of 10. The percentages indicate the proportion of Hb species.
Lentiviral vector-mediated transduction of erythroid progenitors derived from cytokine-mobilized PB CD34+ cells. (A) Schematic representation of the lentiviral vector encoding for expression of GFP from a modified MSCV LTR sequence. (B) Expansion of cultured cells after transduction. (C) GFP expression is indicated as a function of time after transduction of cells exposed to vector particles at an MOI of 10. (D) Phenotypic comparison of cells on the day of transduction (left panels) and 14 days later at the time of termination of the cultures (right panels) by flow cytometry for expression of the CD34, CD45, CD71, and CD235 surface markers where percentages indicate the proportion of cells considered positive, cell volume, and cell morphology (Wright-Giemsa staining, original magnification × 60). (E) The results of cellulose acetate Hb electrophoresis of lysates from terminal-stage erythroblasts from 2 separate experiments (vertical lines have been inserted to indicate a repositioned gel lane) and (F) HPLC analysis of Hbs present in erythroblasts at the end of culture, which were derived from cells exposed to vector particles at an MOI of 10. The percentages indicate the proportion of Hb species.
Interested in achieving erythroid-specific expression of transactivators in vivo and conscious of the potential need to have promoters of various strengths available for our planned studies of genetic elements that enhance HbF production, we also tested the minimal ankyrin-122,23 and β-spectrin24 promoters in our erythroid culture system (Figure 3A). Cells from mobilized PB were transduced as described in “Lentiviral vector production and gene transfer” and monitored for GFP expression by flow cytometry (Figure 3B). As we had found previously (Figure 2C), the MSCV LTR mediated very high expression of the GFP marker, as reflected by the 10-fold higher mean fluorescent intensity (MFI) relative to that observed with the erythroid-specific promoters at the earliest time point (day 5) after transduction with a progressive decrease in MFI as the cells diminished in size during erythroid maturation. In contrast, the β-spectrin promoter was much weaker initially but exhibited a small progressive increase in MFI over time as erythroblasts matured (Figure 3B; supplemental Figure 4). The minimal ankyrin-1 promoter gave a somewhat higher level of expression than the β-spectrin promoter at early stages of culture and also exhibited a modest (∼ 2-fold) increase in expression as erythroid maturation progressed (Figure 3B-C). Despite the lower levels of expression from the ankyrin promoter, the copy number on Southern blot was higher than with the MSCV promoter. We infer that the copy number in cells transduced with the spectrin vector was nearly equivalent based on the fact that the proportion of GFP cells was equivalent, but insufficient DNA was available for direct analysis. In all cases, gene transfer demonstrated no appreciable effect on erythroid cell development when GFP− and GFP+ fractions of bulk cell populations were monitored for coexpression of the erythroid markers CD71 and glycophorin A (supplemental Figure 4).
Marker gene expression from constitutive or erythroid-specific promoters in maturing erythroblasts. (A) Schematic representation of the lentiviral vectors encoding for expression of GFP from the MSCV, ankyrin-1, or β-spectrin promoters. The PRE element is not present in the MSCV vector. (B) GFP expression by erythroblasts derived from CD34+ cells transduced with vectors having the MSCV, spectrin, or ankyrin promoter after various times after transduction where the percentages and MFI are indicated for the proportion of cells considered positive. The percentage of glycophorin A-positive cells at the same time points are shown above the GFP profiles. (C) Southern blot analysis of genomic DNA extracted from transduced cells, digested with BglII, an enzyme that cuts twice within the vector genome and probed with an RRE fragment common to all vectors from the 5′ end of the genome. DNA size marker is shown in the leftmost lane (vertical lines have been inserted to indicate where lanes have been repositioned). Numbers below each lane on the image represent the VCN as determined by densitometry analysis, relative to controls 1.0 or 0.5, which consist of DNA from a K562 clone that contains a single copy of an integrated lentiviral vector either used directly (1.0) or diluted 1:1 with native K562 DNA to establish a sample with a copy number of 0.5.
Marker gene expression from constitutive or erythroid-specific promoters in maturing erythroblasts. (A) Schematic representation of the lentiviral vectors encoding for expression of GFP from the MSCV, ankyrin-1, or β-spectrin promoters. The PRE element is not present in the MSCV vector. (B) GFP expression by erythroblasts derived from CD34+ cells transduced with vectors having the MSCV, spectrin, or ankyrin promoter after various times after transduction where the percentages and MFI are indicated for the proportion of cells considered positive. The percentage of glycophorin A-positive cells at the same time points are shown above the GFP profiles. (C) Southern blot analysis of genomic DNA extracted from transduced cells, digested with BglII, an enzyme that cuts twice within the vector genome and probed with an RRE fragment common to all vectors from the 5′ end of the genome. DNA size marker is shown in the leftmost lane (vertical lines have been inserted to indicate where lanes have been repositioned). Numbers below each lane on the image represent the VCN as determined by densitometry analysis, relative to controls 1.0 or 0.5, which consist of DNA from a K562 clone that contains a single copy of an integrated lentiviral vector either used directly (1.0) or diluted 1:1 with native K562 DNA to establish a sample with a copy number of 0.5.
Enhanced HbF production in adult erythroid cells by the zinc finger-based transcriptional activator GG1-VP64
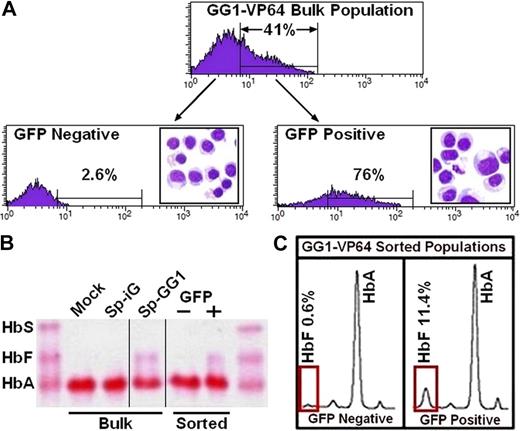
In preliminary experiments, we found that the LTR-driven GG1-VP64 transactivator consistently retarded division of erythroid cells during the expansion phase of our culture system and which was coincident with an appreciable reduction in the GFP+ cell fraction over time (supplemental Figure 5). Accordingly, GG1-VP64 vectors were constructed in which the erythroid-specific and comparably weaker ankyrin-1 or β-spectrin promoters were used to regulate transcription of the transgene (Figure 4A). Transduction of cells according to our standard experimental format between 2 and 3 days after initiation of culture with the vectors encoding GG1-VP64 under the control of the erythroid-specific promoters β-spectrin resulted in only minimal retardation of subsequent cell proliferation compared with the controls (Figure 4B). Shown are the results from 2 donors; in one, the total number of cells at the end of culture expressing the GG1-VP64 was 87% the number of cells expressing the control vector and, in the second donor, the percentage of cells expressing GG1-VP64 was 69% of control. In these experiments, approximately 50% to 60% of the cells were successfully transduced, and terminal erythroid maturation was documented at the end of culture by expression of glycophorin A (Figure 4C). Induction of HbF ranged from approximately 12% to 21% (Figure 4D-E) compared with 1% to 2% for cells transduced with the control vector. Normalization of HbF to the transduced cell population (GFP+ fraction; donor 1, Sp-GG1, 40%; and donor 2, Sp-GG1, 51%) calculates to an elevation of HbF to 30% or 41% in transduced cells, respectively. An experiment performed with mobilized PB CD34+ cells from a third adult donor gave similar results (data not shown). To demonstrate that production of HbF was present only in the fraction of cells transduced with GG1-VP64, the cells derived from mobilized PB CD34+ cells from donor 1 were sorted into GFP− and GFP+ fractions (Figure 5A inset boxes). The morphology of the 2 sorted cell populations was similar (Figure 5B-C). Significant amounts of HbF were present only in the GFP+ fraction (Figure 5C-D) as predicted.
Induction of HbF by GG1-VP64 in erythroblasts derived from adult-mobilized PB CD34+ cells. (A) Schematic diagram of the empty vector control (top) and GG1-VP64-encoding vectors (bottom) where transcription is regulated by the erythroid-specific ankyrin-1 (Ank) or β-spectrin (Sp) promoters, respectively. The results obtained with 2 separate donors are shown in panels B to E. Abbreviated designation of the vectors are spectrin-ires-GFP control vector (Sp-iG), ankyrin-GG1-VP64-ires-GFP (Ank-GG1), and spectrin-GG1-VP64-ires-GFP (Sp-GG1). (B) Cell numbers as a function of time in culture after transduction. (C) Flow cytometry analysis for expression of GFP and CD235 (glyocophorin A) in erythroblasts at the end of culture where the percentages indicate the proportion of cells considered positive. (D) Hb electrophoresis of lysates from erythroblasts at the end of culture (vertical lines have been inserted to indicate a repositioned gel lane). Numbers below each lane on the images represent the VCN as determined by Southern blot analysis and densitometry analysis or quantitative PCR, for the various cell populations. (E) HPLC analysis of lysates from erythroblasts at the end of culture where the percentage of HbF is reported for each condition.
Induction of HbF by GG1-VP64 in erythroblasts derived from adult-mobilized PB CD34+ cells. (A) Schematic diagram of the empty vector control (top) and GG1-VP64-encoding vectors (bottom) where transcription is regulated by the erythroid-specific ankyrin-1 (Ank) or β-spectrin (Sp) promoters, respectively. The results obtained with 2 separate donors are shown in panels B to E. Abbreviated designation of the vectors are spectrin-ires-GFP control vector (Sp-iG), ankyrin-GG1-VP64-ires-GFP (Ank-GG1), and spectrin-GG1-VP64-ires-GFP (Sp-GG1). (B) Cell numbers as a function of time in culture after transduction. (C) Flow cytometry analysis for expression of GFP and CD235 (glyocophorin A) in erythroblasts at the end of culture where the percentages indicate the proportion of cells considered positive. (D) Hb electrophoresis of lysates from erythroblasts at the end of culture (vertical lines have been inserted to indicate a repositioned gel lane). Numbers below each lane on the images represent the VCN as determined by Southern blot analysis and densitometry analysis or quantitative PCR, for the various cell populations. (E) HPLC analysis of lysates from erythroblasts at the end of culture where the percentage of HbF is reported for each condition.
HbF is present only in the transduced fraction of cultured erythroblasts. (A) Fluorescence-activated cell sorting of GFP− and GFP+ fraction of erythroblasts derived from adult-mobilized PB CD34+ cells transduced with SpGG1-VP64 vector where the morphology of sorted cell populations is presented as Wright-stained cytocentrifuge preparations (original magnification ×60) imbedded within histograms for GFP− and GFP+ cell populations, respectively. (B) Hb electrophoresis of lysates from various cell populations (vertical lines have been inserted to indicate a repositioned gel lane). (C) HPLC analysis of lysates from the GFP− and GFP+ cell populations.
HbF is present only in the transduced fraction of cultured erythroblasts. (A) Fluorescence-activated cell sorting of GFP− and GFP+ fraction of erythroblasts derived from adult-mobilized PB CD34+ cells transduced with SpGG1-VP64 vector where the morphology of sorted cell populations is presented as Wright-stained cytocentrifuge preparations (original magnification ×60) imbedded within histograms for GFP− and GFP+ cell populations, respectively. (B) Hb electrophoresis of lysates from various cell populations (vertical lines have been inserted to indicate a repositioned gel lane). (C) HPLC analysis of lysates from the GFP− and GFP+ cell populations.
Discussion
Human primitive hematopoietic cells from different developmental stages are already committed to production of different Hb types in developing erythroblasts when cultured under identical conditions. Erythroblasts derived from adult PB and BM cells make HbA, erythroblasts derived from CB CD34+ cells make a mixture of fetal and adult Hb, whereas erythroblasts derived from CD34+ cells from FL make HbF. We have demonstrated that early erythroid progenitors derived from cytokine-mobilized adult PB CD34+ cells can be transduced with lentiviral vectors without altering their subsequent capacity for proliferation and differentiation and pattern of Hb production. Lentiviral vector-mediated delivery of a synthetic zinc-finger transcriptional factor, GG1-VP64, under the control of relatively weak erythroid-specific promoters with various expression kinetics induced significant amounts of HbF in erythroblasts derived from transduced, mobilized PB CD34+ cells from adults without altering their capacity for erythroid maturation and only modestly reducing the total numbers of cells that accumulate in culture after transduction.
Our results with respect to the commitment of primitive hematopoietic cells that initiate erythropoiesis in culture and Hb phenotype are consistent with much earlier results obtained with clonal hematopoietic cultures in which erythroid colonies form in semisolid media.27 In these studies, erythroid colonies developed from the primitive progenitors, burst forming unit-erythroid (BFU-E), present in FL contained HbF, whereas those derived from adult hematopoietic tissue contained predominantly HbA. BFU-E from newborns generated colonies containing a mixture of HbF and HbA.27 The liquid culture system provides large numbers of differentiating erythroblasts from the different developmental stages that will be useful for comparative molecular analysis of the globin loci with respect to chromatin structure, epigenetic modifications, and transcriptional factor binding, as has recently been reported by others in erythroblasts developed from adult progenitors.28,29 Although such studies should yield important information regarding how the developmental pattern of gene expression is maintained during erythropoiesis, the initiating events that result in commitment to Hb phenotype in early progenitors may be more challenging to discern. Available evidence suggests that genes that are ultimately expressed in a lineage-restricted manner may be activated in very early progenitor cells and that lineage-specific transcriptional activators expressed at basal levels in progenitor cells may participate in gene potentiation.30,31 The order of expression of specific transcriptional factors has been shown to direct hierarchical specification of hematopoietic lineages.32 In a recent study, RUNX1 was shown to be responsible for early chromatin unfolding without assembling into a stable transcription factor complex on the PU.1 promoter.33 If such mechanisms are involved in the developmental commitment with respect to Hb phenotype in early hematopoietic cells, their discovery and elucidation may prove challenging.
Relevant to studies intending to characterize genes or microRNAs with potential for enhancing HbF expression, our culture system is characterized by a very low basal level of HbF in erythroblasts derived from adult CD34+ cells at all analyzed time points during the maturation process. Other culture conditions result in higher levels of HbF in cells developed from adult BFU-E. Early studies using immunofluorescence demonstrated that burst colonies were segmented with respect to HbF production, suggesting that commitment to produce limited amounts of HbF by these adult cells were occurring only after one or a few divisions in contrast to developmental control of HbF in which BFU-E are more fully committed with respect to Hb phenotype.34 A similar pattern of commitment during early erythropoiesis is thought to result in the heterocellular distribution of HbF in normal adults in whom up to 8% of red cells contain small and variable amounts of HbF.1,4 The increased production of HbF in adult erythroblasts under stress has been attributed to acceleration of erythroid maturation allowing for synthesis of γ-globin, which normally occurs to a limited extent in the earliest erythroblasts,35 to persist during erythroid maturation. A cell stress signaling model of HbF induction has been proposed as recently reviewed.36 Early studies demonstrated that stem cell factor (SCF) induces HbF production in cultures of purified BFU-E,37 and recent studies have shown that the combination of SCF and transforming growth factor-β consistently causes a significant increase in HbF in cultures of adult cells to levels up to 20% with a pancellular distribution.38 We have used a relatively low concentration of SCF of 10 ng/mL in our cultures to achieve a low baseline level of HbF production, whereas a concentration of 50 ng/mL is typically used when HbF synthesis is maximized.38
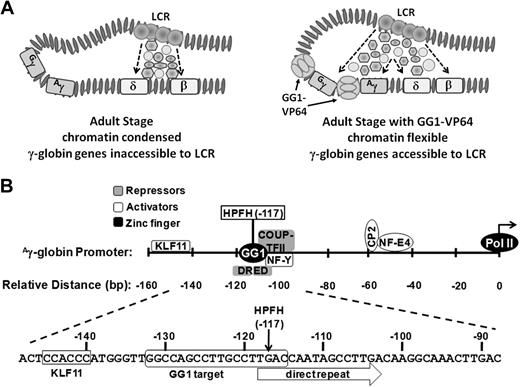
The zinc-finger transcriptional factor that we have shown induces HbF in maturing erythroblasts derived from adult CD34+ cells was designed to function as a transcriptional activator (Figure 6A); indeed, it has been shown to enhance expression from a minimal γ-promoter in a reporter assay15 (supplemental Figure 2B). However, the region in the γ-globin gene promoter to which it binds includes a sequence called the direct repeat element because it is tandemly repeated in the ϵ promoter (Figure 6B). This element has been implicated in adult-stage, γ-globin gene silencing.39 The direct repeat element interacts with nuclear receptor chicken ovalbumin upstream promoter–transcriptional factor II (COUP-TFII)40 and the direct repeat erythroid definitive binding proteins (DRED) TR2/TR4.41 Both COUP-TFII and DRED have been implicated in repression of γ-globin expression. Recent results indicate that SCF induces γ-globin gene expression by decreasing COUP-TFII expression.42 Displacement of these proteins that are involved in silencing the γ-globin gene during the adult stage of erythropoiesis by the zinc-finger transcription factor may also be involved in enhancing HbF production in maturing adult erythroblasts. Recent work in the Orkin laboratory has shown that BCL11A acts as a stage-specific repressor in silencing HbF expression in adult human erythroid cells,13 but it has been shown to bind to the intergenic region and thus may not be directly affected by expression of the zinc finger transcription factor. The mechanism for silencing the γ genes in human adult erythroid cells appears to be complex and redundant.44-46 Other proteins that interact with the region or nearby where GG1-VP64 is designed to bind (Figure 6B) are transcriptional activators, such as KLFII,47 or stage selector protein48 or may act as an activator or repressor in the case of nuclear transcription factor-Y, depending on exactly where it binds and the complexes it forms with other proteins.49
Chromatin structure and organization of the γ-globin gene promoter. (A) Potential changes in adult-stage chromatin structure from condensed (left) to flexible (right) induced by binding of GG1-VP64 (tennis ball structure) to the γ-globin gene promoters. Interactions that exist between the LCR and delta (δ)- and beta-(β) globin genes during normal adult erythroid development compared with potential new interactions between the LCR and the γ-globin genes caused by binding of GG1-VP64 are shown (adapted from Bank2 with permission). (B) Location of binding sites for selected endogenous transcriptional activators (shaded light gray) and repressors (shaded dark gray) within the proximal γ-globin gene promoter with respect to the HPFH-117 target sequence. KLF11 indicates fetal Kruppel-like factor; HPFH, hereditary persistence of HbF; NF-Y, nuclear transcription factor-Y; CP2; transcription factor CP2; and NF-E4, nuclear transcription factor-erythroid 4.
Chromatin structure and organization of the γ-globin gene promoter. (A) Potential changes in adult-stage chromatin structure from condensed (left) to flexible (right) induced by binding of GG1-VP64 (tennis ball structure) to the γ-globin gene promoters. Interactions that exist between the LCR and delta (δ)- and beta-(β) globin genes during normal adult erythroid development compared with potential new interactions between the LCR and the γ-globin genes caused by binding of GG1-VP64 are shown (adapted from Bank2 with permission). (B) Location of binding sites for selected endogenous transcriptional activators (shaded light gray) and repressors (shaded dark gray) within the proximal γ-globin gene promoter with respect to the HPFH-117 target sequence. KLF11 indicates fetal Kruppel-like factor; HPFH, hereditary persistence of HbF; NF-Y, nuclear transcription factor-Y; CP2; transcription factor CP2; and NF-E4, nuclear transcription factor-erythroid 4.
In addition to local effects (ie, direct activation of the γ-globin promoters or perturbed repressor binding at this target sequence), it is possible that GG1-VP64 makes possible long-range interactions between the LCR or transcription factor complexes and γ-globin promoter by attenuating chromatin condensation (Figure 6A). The GG1-VP64 target sequence includes the guanine residue at nucleotide position −117, which when mutated to adenine results in increased production of γ-globin in cases of Greek nondeletion hereditary persistence of HbF.17,18 Generation of transgenic mice with this mutation in the γ-globin gene resulted in persistent expression of HbF.50 Although Berry et al50 indicated that persistence of γ-globin was correlated with loss of Gata-1 binding at the γ-promoter, the precise molecular mechanism has not been fully resolved. A study analyzing DNA-protein interactions at the γ-globin promoter in K562 cells, which express γ but not β-globin, revealed that an unknown protein interacts with the promoter in the CCAAT-box located next to position −117 rendering the TTG motif sensitive to dimethyl sulfate.51 Thus, it is possible that binding of GG1-VP64 to sequences, which include the −117 position, results in retention of an open chromatin structure that allows interaction with the LCR during the course of erythroid cell maturation.
Enforced expression of the zinc-finger transcriptional activator inhibits cell proliferation in our culture system, which we have largely avoided by regulating transcription with relatively weak, erythroid-specific promoters. Our plans are to test the capacity of the zinc-finger transcriptional activator in vivo both alone and concurrently with γ-globin gene addition to augment HbF levels. For this, we will use a nonhuman primate model, the pigtailed macaque (Macaca nemestrina), the stem cells of which have been shown to be amenable to lentiviral vector-mediated gene transfer with HIV-based vector systems.52 We are also exploring the use of tamoxifen-modulated nuclear accumulation of the transcriptional activator in a further level effort to control its activity to achieve a beneficial effect with respect to HbF production while further reducing the unwanted inhibition of cell proliferation. Studies in the nonhuman primate should also provide information regarding the potential safety of this approach as a strategy for enhancing HbF in patients with thalassemia or sickle cell disease.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr David Bodine (Hematopoiesis Section, Genetics and Molecular Biology Branch, National Human Genome Research Institute, National Institutes of Health, Bethesda, MD) and Dr Patrick Gallagher (Department of Pediatrics, Yale University School of Medicine, New Haven, CT) for the ankyrin-1 and β-spectrin promoters, Qi Wang for having cloned the rhesus γ-globin gene promoter fragment, the flow cytometry laboratory of Anna Travelstead for expertise in flow cytometry studies and fluorescence-activated cell sorter analysis, and Pat Streich for help in preparing the manuscript.
A.W., P.W.H., Y.-S.K., D.A.P., and A.W.N. were supported by National Heart, Lung, and Blood Institute (PO1HL053749), the Assisi Foundation of Memphis, and the American Lebanese Syrian Associated Charities. U.T. and C.F.B. were supported by the Skaggs Institute for Chemical Biology.
National Institutes of Health
Authorship
Contribution: A.W. designed and performed research, analyzed data, and contributed to the writing of the manuscript; U.T., P.W.H., and Y.-S.K. contributed to performing the research; D.A.P., C.F.B., and A.W.N. participated in designing the research and writing the paper; and A.W.N. was responsible for the overall organization of the research effort.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for U.T. is Division of Molecular Genome Analysis, German Cancer Research Center, Heidelberg, Germany.
Correspondence: Arthur W. Nienhuis, Department of Hematology, St Jude Children's Research Hospital, 262 Danny Thomas Pl, MS#341, Memphis, TN 38105; e-mail: arthur.nienhuis@stjude.org.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal