Abstract
The anti-CD20 antibody rituximab has substantially improved outcomes in patients with B-cell non-Hodgkin lymphomas. However, many patients are not cured by rituximab-based therapies, and overcoming de novo or acquired rituximab resistance remains an important challenge to successful treatment of B-cell malignancies. Interferon-alpha (IFNα) has potent immunostimulatory properties and antiproliferative effects against some B-cell cancers, but its clinical utility is limited by systemic toxicity. To improve the efficacy of CD20-targeted therapy, we constructed fusion proteins consisting of anti-CD20 and murine or human IFNα. Fusion proteins had reduced IFNα activity in vitro compared with native IFNα, but CD20 targeting permitted efficient antiproliferative and proapoptotic effects against an aggressive rituximab-insensitive human CD20+ murine lymphoma (38C13-huCD20) and a human B-cell lymphoma (Daudi). In vivo efficacy was demonstrated against established 38C13-huCD20 grown in syngeneic immunocompetent mice and large, established Daudi xenografts grown in nude mice. Optimal tumor eradication required CD20 targeting, with 87% of mice cured of rituximab-insensitive tumors. Gene knockdown studies revealed that tumor eradication required expression of type I IFN receptors on the tumor cell surface. Targeting type I IFNs to sites of B-cell lymphoma by fusion to anti-CD20 antibodies represents a potentially useful strategy for treatment of B-cell malignancies.
Introduction
The anti-CD20 antibody rituximab (C2B8/Rituxan; Genentech/Biogen-IDEC) has substantially improved treatment outcomes in B-cell non-Hodgkin lymphomas (NHLs), achieving high response rates in low-grade B-cell lymphomas,1 and improving survival in both indolent and aggressive lymphomas in combination with chemotherapy.2,3 However, many tumors do not respond to or relapse after rituximab-based therapies.4 Thus, new approaches are needed to improve anti-CD20 efficacy and overcome rituximab resistance.
The in vivo antilymphoma effects of rituximab are believed to be mediated by antibody dependent cell-mediated cytotoxicity (ADCC), complement-dependent cytotoxicity (CDC), induction of apoptosis in tumor cells, and recruitment of T cells responding to tumor antigens released upon antibody-mediated tumor lysis.5-7 Clinical studies have suggested that ADCC plays a dominant role in rituximab action in humans.8,9 Thus, attempts have been made to boost rituximab-mediated ADCC by activation of Fc receptor–bearing natural killer (NK) cells, monocytes/macrophages, or granulocytes via systemic administration of cytokines such as interleukin-2, interleukin-12, or granulocyte-macrophage colony-stimulating factor,10-12 with limited efficacy. None of these trials involving systemic administration of cytokines offered a clear advantage over the expected efficacy of rituximab alone, likely due to the inability of systemically administered agents to achieve high concentrations within the tumor bed.
Interferon-alpha (IFNα), a member of the type I interferon family (α, β, ω), is a pleiotropic cytokine with attractive features for combination with rituximab in treating NHL.13,14 Beneficial properties of IFNα against NHL and other cancers include direct antiproliferative and proapoptotic effects,15-17 blockade of autocrine growth factor loops,18 repression of c-myc oncogene expression,19 down-regulation of telomerase activity,20 and inhibition of angiogenesis.21 Favorable immunologic effects of IFNα for lymphoma treatment include activation of T cell, NK cell, and dendritic cell functions, as well as up-regulation of class I major histocompatibility complex and CD20 molecules on the tumor cell surface.22,23 The single-agent clinical activity of IFNα against NHL has been demonstrated in numerous early clinical trials,24-26 and in more recent series by Armitage and Coiffier27 and Armitage et al.28 Clinical studies combining systemic IFNα therapy with rituximab suggest benefits of addition of the cytokine.29,30 However, despite its potent antitumor properties, the clinical utility of IFNα in cancer therapy has been severely limited by the substantial toxicities associated with systemic administration.31 Contributing to this failure is the short serum half-life of IFNα (5 hours), and the lack of effective levels of the cytokine within tumor sites. Pharmacokinetic studies have indicated that only 0.01% of subcutaneously injected IFNα reaches the target tumor site.32 Given these limitations, it is difficult to achieve effective IFNα concentrations at sites of malignant disease without causing systemic toxicity.
The limitations of systemic IFNα therapy have led to the exploration of alternative strategies to deliver IFNα safely and effectively into the tumor vicinity. Reports have shown that intratumoral delivery of IFNα by direct injection can lead to durable complete tumor regressions of NHL.33,34 Tumor-specific IFNα delivery via transduced monocytes or adenoviral vectors is also effective in inhibition of tumor growth and angiogenesis in glioma and metastatic carcinoma tumor models.35,36 Despite promising efficacy in mouse models, clinical use of viral vectors in humans is problematic. An attractive alternative approach to improving efficacy while reducing toxicity is to deliver IFNα via an antibody fusion protein. Fusion proteins have been used extensively to deliver cytokines, radioisotopes, and toxins for cancer therapy.37 Antibody-cytokine fusion proteins use the unique targeting ability of antibodies to guide antitumor agents specifically into the tumor vicinity where these drugs can work most effectively to eradicate tumor cells. In the case of B-cell lymphoma, tumor-specific targeting of IFNα via anti-CD20 may represent a viable 2-pronged therapy.
We previously reported that an anti-HER2/neu antibody–IFNα fusion protein had in vitro and in vivo efficacy against an experimental murine tumor overexpressing HER2/neu.38 We now report the production and characterization of anti–CD20-IFNα fusion proteins carrying either murine or human IFNα.
Methods
Cell lines
HEK293T cells were purchased from ATCC and grown in Iscove modified Dulbecco medium (Invitrogen) supplemented with 2mM l-glutamine, 50μM β-mercaptoethanol, and 5% calf serum. The 38C13-huCD20 cells, which express human CD20, were previously described.39 Both 38C13 and 38C13-huCD20 were cultured in RPMI 1640 (Invitrogen) supplemented with 2mM l-glutamine, 50μM β-mercaptoethanol, and 10% fetal bovine serum. The cell line 38C13-huCD20 IFNα receptor knockdown (IFNAR KD) was produced by transducing 38C13-huCD20 cells with a lentiviral vector encoding an shRNA targeting the IFNAR1 subunit of IFNAR with the sense sequence 5′-GCGTCTACATTATAGATGACAA-3′ as previously described.40 Briefly, 38C13-huCD20 cells were infected at 0.3 multiplicity of infection and sorted for high green fluorescent protein fluorescence using fluorescence activated cell sorting (FACS). Single-cell clones were isolated by limiting dilution. Daudi cells were purchased from ATCC and grown in the same RPMI 1640 supplemented as described.
Vectors
Heavy chain and light chain variable regions of the anti–human CD20 monoclonal antibody 2B8, the murine parent of rituximab, were provided by Dr Anna Wu (University of California Los Angeles)41 and cloned into human γ3 heavy chain (pAH6180) and κ light chain (pAG3551) expression vectors, respectively, to produce anti–CD20-immunoglobulin G3 (IgG3). To construct anti–CD20-IgG3-mIFNα, polymerase chain reaction (PCR) was used to introduce a BamHI restriction enzyme site upstream and an XbaI restriction enzyme site downstream of the mature murine IFNα1 gene amplified by PCR from genomic DNA of BALB/c mice with the forward primer 5′-CGCGGATCCTGTGACCTGCCTCAGACTC-3′ and the reverse primer 5′-GCTCTAGATCATTTCTCTTCTCTCAGTCTTC-3′. The PCR product was ligated into the vector pAH9612 containing an IgG3 constant region with an anti-CD20 heavy chain variable region and a GGGGS peptide linker at the end of CH3. To construct anti–CD20-IgG1-hIFNα, PCR was used to introduce a BamHI restriction enzyme site upstream and an XbaI restriction enzyme site downstream of the mature human IFNα2 gene amplified by PCR from human genomic DNA with the forward primer 5′-CGCGGATCCTGTGATCTGCCTCAAACCCA-3′ and the reverse primer 5′-GCTCTAGATCATTCCTTACTTCTTAAACTTTC-3′. The PCR product was ligated into the vector pAH10812 containing an IgG1 constant region with an anti-CD20 heavy chain variable region and a GGGGS peptide linker at the end of CH3.
Protein production and purification
Heavy and light chain vectors were transiently transfected into HEK293T cells via polyethylenimine transfection42 and supernatants collected every other day for 2 weeks. Cell-free culture supernatants were passed through a protein A Sepharose 4B fast flow (Sigma-Aldrich) column and bound protein was eluted with 0.1M glycine, pH 2.5. Eluted fractions were neutralized immediately with 2M Tris-HCl, pH 8.0. Fractions were run on sodium dodecyl sulfate–polyacrylamide gel electrophoresis gels and stained with Coomassie blue to verify protein purity and integrity. Concentrations of proteins were determined using a standard bicinchoninic acid (BCA) assay (Pierce).
Flow cytometry
The 38C13-huCD20 cells (106) were incubated with 1 μg of anti–CD20-mIFNα, anti–DNS-IFNα, rituximab (Genentech Inc), or isotype control antibody (Sigma-Aldrich) for 1 hour at 4°C in FACS buffer (phosphate-buffered saline + 1% bovine serum albumin + 0.1% NaN3). Binding of fusion protein was detected using biotinylated rat anti–human κ, followed by streptavidin–phycoerythrin (PE; BD Biosciences). IFNAR expression was detected using a biotinylated anti–mouse IFNAR antibody (clone MAR1-5A3; Leinco Technologies) followed by streptavidin-PE. Data were acquired using a FACScan flow cytometer (Becton Dickinson) and analyzed using FlowJo software (TreeStar Inc).
Inhibition of proliferation assay
The 38C13-huCD20 cells were incubated with various treatments at 37°C for 48 hours. Cell viability was quantified using MTS (3-(4,5 dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl-2-(4-sulfophenyl)-2H-tetrazolium) solution (Promega) by measuring absorbance at 490 nm using a Synergy HT Microplate Reader. Data were analyzed using KC4 software (Bio-Tek). Percentage of inhibition of proliferation was calculated as [1 − (ODexp/ODuntreated)] × 100. Activity of IFNα was compared at the dosage required for 50% growth inhibition based on the best fit curve. Recombinant murine IFNα reference standard was obtained from the National Institutes of Health.
Apoptosis assay
The 38C13-huCD20 or Daudi cells were incubated with various treatments at 37°C for 48 or 72 hours, respectively. Cells were stained with annexinV–fluorescein isothiocyanate (FITC) and propidium iodide (PI) to distinguish populations of early apoptotic (annexinV+/PI−), late apoptotic (annexinV+/PI+), and necrotic (annexinV−/PI+) cells using Vybrant Apoptosis Kit #2 (Molecular Probes). The percentage of apoptotic cells was calculated as the sum of the percentages of early apoptotic cells and late apoptotic cells.
In vivo antitumor activity against murine B-cell lymphoma
Female (6-8 weeks old) C3Hf/Sed/Kam mice were bred and housed at the University of California Los Angeles (UCLA) Defined Pathogen Colony according to institutional guidelines. All animal procedures were approved by the UCLA Animal Research Committee. 38C13-huCD20 was thawed 3 days before tumor challenge and split the day before use. For tumor challenge, cells were washed twice in Hanks balanced salt solution (HBSS) and diluted to the appropriate concentration in HBSS. Challenge inocula consisted of 5 × 103 38C13-huCD20 cells injected subcutaneously above the base of the tail. Experimental groups were treated with 10 μg of anti–CD20-mIFNα fusion protein intravenously 1, 2, and 3 or 5, 6, and 7 days after tumor challenge. In some experiments, fusion protein–treated mice received additional 30-μg doses 12 and 19 days after tumor challenge. In some cases, mice surviving initial tumor challenge were rechallenged subcutaneously at day 60 with 5 × 103 parental 38C13 cells. Mice were followed for survival and killed when tumors reached 1.4 cm in diameter. Bidirectional tumor growth measurements were taken throughout the experiments.
In vivo antitumor activity against human B-cell lymphoma
Female nude mice (6-8 weeks old) were bred and housed as described for C3Hf/Sed/Kam mice. Challenge inocula consisted of 1.2 × 107 Daudi cells resuspended in a total of 200 μL of HBSS, injected subcutaneously above the base of the tail. Mice (32% of total number inoculated) with established tumors greater than 0.5 cm in diameter after 30 days were randomly assigned to treatment groups. Groups were treated intravenously with either 30 μg of anti–CD20-hIFNα fusion protein, equimolar amounts of rituximab (20 μg), or HBSS 3 times at weekly intervals beginning 30 days after tumor challenge. Tumor volume was calculated using a modified ellipsoid formula43 where tumor volume = 0.52 × (length × width2).
Statistical analysis
Apoptosis data were compared using the unpaired, 2-tailed Student t test. Survival differences among groups of mice were assessed using the Kaplan-Meier method with the log-rank test using Prism software (GraphPad Software). P values were considered statistically significant at P less than .05.
Results
Production and characterization of anti–CD20-mIFNα and anti–CD20-hIFNα
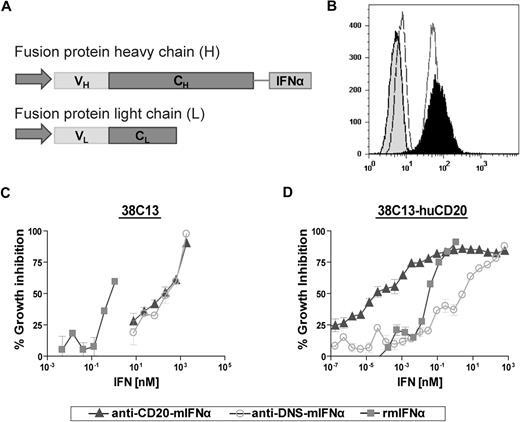
We chose to construct anti–CD20-IFNα fusion proteins using the variable regions from the rituximab parent monoclonal antibody 2B8,44 so that our recombinant fusion proteins could be directly compared with this prototypical anti-CD20 therapeutic. To generate the fusion proteins, the N-terminus of either mature murine IFNα1 (mIFNα) or mature human IFNα2a (hIFNα) was fused via a Gly4Ser linker to the C-terminus of the heavy chain of anti-CD20 (Figure 1A). Nontargeted anti–DNS-mIFNα (antidansyl) and anti–DNS-hIFNα were also produced, along with unfused anti-CD20 IgG3. Proteins were purified using protein A Sepharose and eluted fractions analyzed by sodium dodecyl sulfate–polyacrylamide gel electrophoresis. Under nonreducing conditions, the molecular weight of anti–CD20-mIFNα was approximately 210 kDa (data not shown), indicating proper assembly of an H2L2 molecule. After treatment with β-mercaptoethanol, the fusion protein migrated as heavy chains attached to murine IFNα (∼ 80 kDa) and light chains (∼ 25 kDa; data not shown). Similar results were seen with anti–CD20-hIFNα (data not shown).
Production and characterization of anti–CD20-mIFNα. (A) The heavy chain and light chain variable regions of anti-CD20 2B8 were cloned into human γ3 heavy chain and human κ light chain expression vectors. Mature murine IFNα was inserted downstream of a Gly4Ser (GlySer) linker following the CH3 domain of the constant region gene. (B) Flow cytometry using 38C13-huCD20 cells demonstrates that the fusion protein retains the ability to bind human CD20. Cells were incubated with anti–CD20-mIFNα (black peak), rituximab (solid line), isotype control for IgG1 (dashed line), or isotype control for IgG3 (shaded peak). Anti–human kappa-PE was used to detect cell-bound antibodies. (C) Comparative biologic activity of IFNα fusion protein and recombinant mIFNα as measured by inhibition of growth of wild-type 38C13 (hu-CD20 negative) cells. (D) Antiproliferative activity of fusion protein against 38C13-huCD20 cells. Cells were incubated with the indicated proteins for 48 hours. Cell proliferation was measured using the MTS assay and the data are represented as mean ± SD of triplicate values.
Production and characterization of anti–CD20-mIFNα. (A) The heavy chain and light chain variable regions of anti-CD20 2B8 were cloned into human γ3 heavy chain and human κ light chain expression vectors. Mature murine IFNα was inserted downstream of a Gly4Ser (GlySer) linker following the CH3 domain of the constant region gene. (B) Flow cytometry using 38C13-huCD20 cells demonstrates that the fusion protein retains the ability to bind human CD20. Cells were incubated with anti–CD20-mIFNα (black peak), rituximab (solid line), isotype control for IgG1 (dashed line), or isotype control for IgG3 (shaded peak). Anti–human kappa-PE was used to detect cell-bound antibodies. (C) Comparative biologic activity of IFNα fusion protein and recombinant mIFNα as measured by inhibition of growth of wild-type 38C13 (hu-CD20 negative) cells. (D) Antiproliferative activity of fusion protein against 38C13-huCD20 cells. Cells were incubated with the indicated proteins for 48 hours. Cell proliferation was measured using the MTS assay and the data are represented as mean ± SD of triplicate values.
Anti–CD20-mIFNα and anti–CD20-hIFNα retain their antigen-binding abilities
The binding of the fusion proteins to human CD20 was assessed by flow cytometry against the human CD20–expressing tumor lines 38C13-huCD20 and Daudi. Anti–CD20-mIFNα bound 38C13-huCD20 (Figure 1B) in a manner similar to that of native rituximab. Anti–CD20-hIFNα bound Daudi, a human B-cell lymphoma cell line (data not shown). These data show that C-terminal fusion of IFNα to anti-CD20 did not affect the ability of the antibody to bind its antigen.
In vitro targeting of IFNα via anti-CD20 significantly enhances its efficacy against human CD20+ murine B-cell lymphoma 38C13-huCD20
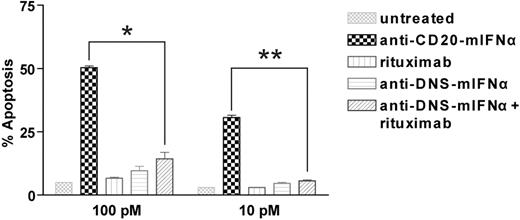
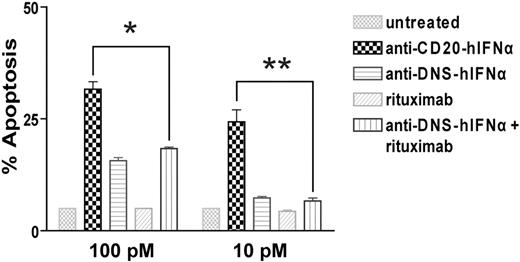
To quantify the IFNα bioactivity of the anti–CD20-mIFNα fusion protein, MTS assays measuring cell viability were performed on non–CD20-expressing parental 38C13 cells, which are sensitive to growth inhibition by IFNα.45 Anti–CD20-mIFNα and anti–DNS-mIFNα had equivalent ability to inhibit the proliferation of 38C13; however, their activities were 300-fold reduced compared with recombinant mIFNα (Figure 1C). In contrast, anti–CD20-mIFNα had 105-fold higher antiproliferative activity than nontargeted anti–DNS-mIFNα against 38C13 cells expressing human CD20,39 indicating that targeting of IFNα markedly enhances its efficacy (Figure 1D). Compared with recombinant murine IFNα (rmIFNα), anti–CD20-mIFNα had 103-fold higher antiproliferative activity against 38C13-huCD20 despite its reduced IFNα activity (Figure 1D). Over an equivalent range of concentrations (from 1 to 100pM), native rituximab did not exhibit any antiproliferative effects against this tumor (data not shown). When proapoptotic activity was assayed in 38C13-huCD20 after 48 hours of treatment with 100pM concentrations of fusion proteins, anti–CD20-mIFNα induced significantly higher levels of apoptosis (50.3% annexinV-positive cells) than equivalent amounts of the nontargeted anti–DNS-mIFNα (9.7%), and the combination of anti–DNS-mIFNα + rituximab (14.3%; P = .001; Figure 2). A similar trend was observed at a lower concentration of anti–CD20-mIFNα (10pM), with apoptosis induced in 30.7% of the treated cells, whereas the other treatments caused significantly less apoptosis. Taken together, these data show that targeting of IFNα was essential for the efficacy of this fusion protein because the combination of rituximab and a nontargeted IFNα fusion protein was significantly less effective than anti–CD20-mIFNα.
Anti–CD20-mIFNα potently induces apoptosis of 38C13-huCD20 cells in a dose-dependent manner. 38C13-huCD20 cells were incubated with various concentrations of the indicated proteins for 48 hours. Staining with annexinV-FITC and PI was performed to distinguish necrotic (annexin−PI+), early apoptotic (annexin+PI−), and late apoptotic (annexin+PI+) cell populations. The percentage of total apoptotic cells was quantified for each sample as the sum of early apoptotic and late apoptotic cells (bottom panel). Experiments were performed in triplicate, and error bars indicate mean ± SD. *P = .001. **P < .001.
Anti–CD20-mIFNα potently induces apoptosis of 38C13-huCD20 cells in a dose-dependent manner. 38C13-huCD20 cells were incubated with various concentrations of the indicated proteins for 48 hours. Staining with annexinV-FITC and PI was performed to distinguish necrotic (annexin−PI+), early apoptotic (annexin+PI−), and late apoptotic (annexin+PI+) cell populations. The percentage of total apoptotic cells was quantified for each sample as the sum of early apoptotic and late apoptotic cells (bottom panel). Experiments were performed in triplicate, and error bars indicate mean ± SD. *P = .001. **P < .001.
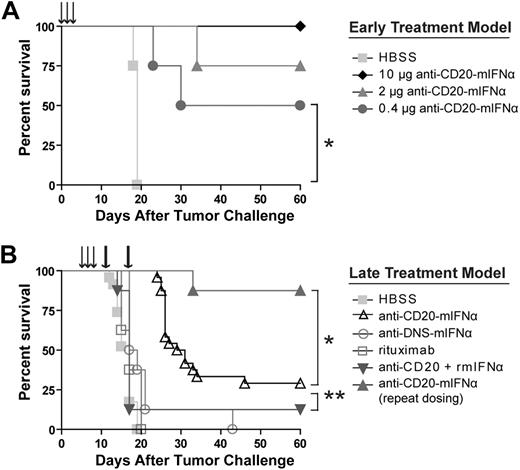
Anti–CD20-mIFNα prevents growth of 38C13-huCD20 tumors in vivo without apparent systemic toxicity
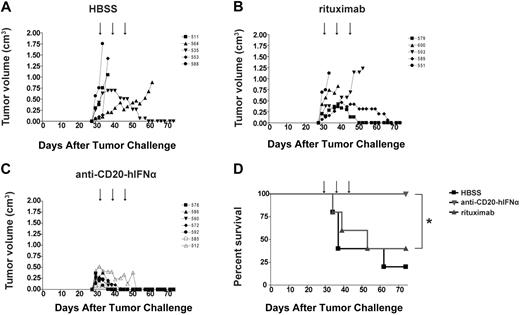
We next chose to evaluate our anti–CD20-mIFNα in vivo using an immunocompetent, syngeneic mouse model, in which the species of the fused mIFNα moiety matched that of the tumor host, permitting evaluation of potential IFN toxicity. The 38C13-huCD20 tumor line, which expresses physiologic levels of human CD20, is a model of rituximab-insensitive lymphoma when injected subcutaneously into C3H mice. After 3 or more days of establishment, 38C13-huCD20 tumors cannot be eradicated, even by a large dose (500 μg) of single-agent rituximab.39 Initial experiments in this model demonstrated that an early treatment regimen consisting of 0.4-μg, 2-μg, or 10-μg doses of anti–CD20-mIFNα given 1, 2, and 3 days after tumor inoculation could eradicate tumors from 50%, 75%, and 100% of the animals, respectively (P ≤ .01 for all groups vs HBSS treated), illustrating potent, dose-dependent activity of the fusion protein in vivo (Figure 3A). The anti–CD20-mIFNα fusion protein was also effective at treating more established tumors (Figure 3B). When 5-day established tumors were treated with three 10-μg doses of anti–CD20-mIFNα given 5, 6, and 7 days after tumor inoculation, the overall survival of the fusion protein–treated mice was higher compared with HBSS-treated mice (P < .001) and mice treated with a combination of anti-CD20 and rmIFNα given at equivalent molar doses (P = .007; Figure 3B). Consistent with our in vitro results, targeting of the fusion protein played a significant role in its efficacy given the improved overall survival of the anti–CD20-mIFNα group versus the nontargeted anti–DNS-mIFNα group (P < .001). In this treatment model, rituximab failed to prolong survival of any animals. Indications of systemic toxicity such as lethargy, ruffled fur, or weight loss were not observed in any of the animals.
Anti–CD20-mIFNα treatment can eradicate established, rituximab-insensitive human CD20+ B-cell lymphoma. (A) Early treatment model. Mice (n = 4 per group) were treated with the indicated doses of fusion protein or saline (HBSS) 1, 2, and 3 days after tumor inoculation (arrows). *P = .01. (B) Late treatment model. Mice (n = 8 per group) were treated with 10 μg of fusion protein or equivalent molar concentration of the indicated proteins 5, 6, and 7 days after tumor inoculation (arrows). Mice in the repeat dosing group were given 2 additional 30-μg doses of fusion protein 12 and 19 days after tumor inoculation (thick arrows). *P = .001. **P = .007. The data from 3 independent experiments, each of which included HBSS and fusion protein groups, were combined.
Anti–CD20-mIFNα treatment can eradicate established, rituximab-insensitive human CD20+ B-cell lymphoma. (A) Early treatment model. Mice (n = 4 per group) were treated with the indicated doses of fusion protein or saline (HBSS) 1, 2, and 3 days after tumor inoculation (arrows). *P = .01. (B) Late treatment model. Mice (n = 8 per group) were treated with 10 μg of fusion protein or equivalent molar concentration of the indicated proteins 5, 6, and 7 days after tumor inoculation (arrows). Mice in the repeat dosing group were given 2 additional 30-μg doses of fusion protein 12 and 19 days after tumor inoculation (thick arrows). *P = .001. **P = .007. The data from 3 independent experiments, each of which included HBSS and fusion protein groups, were combined.
Repeat dosing with anti–CD20-mIFNα efficiently eradicates established tumors
Although a single round of CD20-targeted IFNα significantly prolonged the survival of mice, some animals eventually developed tumors. To test whether efficacy could be improved by repeat dosing, 5-day established 38C13-huCD20 tumors were treated with either a single round of anti–CD20-mIFNα or with a repeat dosing schedule that included 2 additional doses of 30 μg of fusion protein 12 and 19 days after tumor inoculation (Figure 3B). At the time of administration of the first repeat dose on day 12, tumors were palpable in the HBSS-treated mice, but not in the fusion protein–treated animals. Whereas the single round of therapy with fusion protein eradicated tumors from 29% of mice, the 3 weekly rounds of therapy cured 87% of animals (P < .001 vs HBSS, P = .001 vs single round of therapy). No animals showed any signs of toxicity during therapy, highlighting the safety of this approach in immunocompetent hosts.
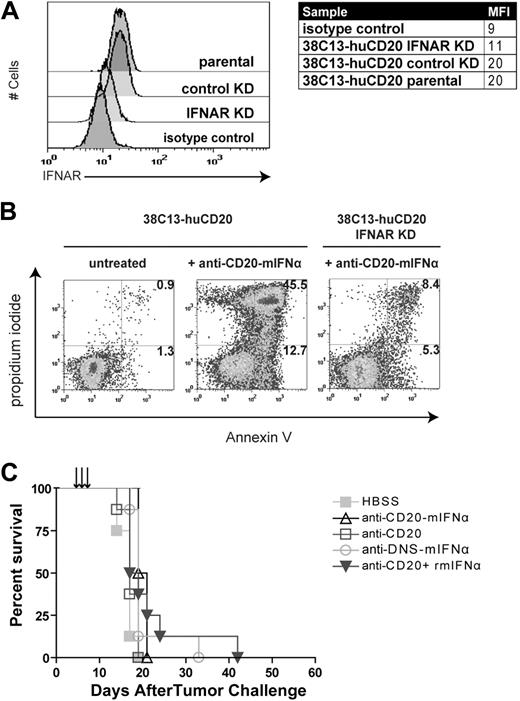
Fusion protein efficacy requires IFNAR expression on tumor cells
IFNα can act on tumor cells directly by inducing apoptosis upon binding to its receptor IFNAR on the cell surface, or indirectly by recruiting host immune cells such as NK cells into the tumor microenvironment to promote tumor killing.13 To distinguish between these possibilities, we used shRNA to generate 38C13-huCD20 IFNAR KD, a cell line with decreased expression of IFNAR (Figure 4A; mean fluorescence intensity = 11) compared with 38C13-huCD20 (mean fluorescence intensity = 20). Knockdown of IFNAR did not affect CD20 expression as determined by flow cytometry (data not shown), and the in vivo growth kinetics of 38C13-huCD20 IFNAR KD were similar to those of 38C13-huCD20 (data not shown). In vitro apoptosis studies showed that 38C13-huCD20 IFNAR KD had decreased sensitivity to fusion protein treatment. At 48 hours after treatment with 1000pM anti–CD20-mIFNα, 58.2% of parental 38C13-huCD20 cells were apoptotic compared with only 13.7% of the 38C13-huCD20 IFNAR KD cells (Figure 4B). In animal studies, the treatment regimen that had previously been effective against 5-day established 38C13-huCD20 tumors strikingly failed to delay or prevent tumor onset in mice inoculated with 38C13-huCD20 IFNAR (Figure 4C). Thus, IFNAR expression at the tumor cell surface is required for anti–CD20-mIFNα–mediated activity in vivo, suggesting that direct tumor cytotoxicity is the primary mechanism of action of the fusion protein in this model.
Antitumor efficacy of anti–CD20-mIFNα requires IFNAR expression on tumor cells. (A) Flow cytometric analysis of IFNAR expression on indicated cell lines. 38C13-huCD20 transduced with IFNAR-specific shRNA (38C13-huCD20 IFNAR KD), 38C13-huCD20 transduced with nonspecific shRNA (38C13-huCD20 control), and 38C13-CD20 parental cells were stained with anti–IFNAR-biotin primary antibody (clone MAR1-5A3) and detected with streptavidin-PE. Biotinylated IgG1 isotype–stained control is also shown. (B) Apoptosis assay using parental 38C13-huCD20 and 38C13-huCD20 IFNAR KD. Cells were treated with 1000pM anti–CD20-mIFNα and stained with annexinV-FITC and PI 48 hours later. Flow cytometry patterns and gate frequencies in percentages are shown in the upper right hand corner of the annexin+ gates. (C) Effective in vivo treatment of 38C13-huCD20 with anti–CD20-mIFNα requires IFNAR expression on tumor cells. Mice (n = 8 per group) were treated 5, 6, and 7 days after tumor inoculation with 10 μg of anti–CD20-mIFNα or the molar equivalent of the indicated proteins. Mice were followed for survival and killed when tumors reached 1.4 cm in diameter as per institutional guidelines. Mice treated with HBSS were used as control.
Antitumor efficacy of anti–CD20-mIFNα requires IFNAR expression on tumor cells. (A) Flow cytometric analysis of IFNAR expression on indicated cell lines. 38C13-huCD20 transduced with IFNAR-specific shRNA (38C13-huCD20 IFNAR KD), 38C13-huCD20 transduced with nonspecific shRNA (38C13-huCD20 control), and 38C13-CD20 parental cells were stained with anti–IFNAR-biotin primary antibody (clone MAR1-5A3) and detected with streptavidin-PE. Biotinylated IgG1 isotype–stained control is also shown. (B) Apoptosis assay using parental 38C13-huCD20 and 38C13-huCD20 IFNAR KD. Cells were treated with 1000pM anti–CD20-mIFNα and stained with annexinV-FITC and PI 48 hours later. Flow cytometry patterns and gate frequencies in percentages are shown in the upper right hand corner of the annexin+ gates. (C) Effective in vivo treatment of 38C13-huCD20 with anti–CD20-mIFNα requires IFNAR expression on tumor cells. Mice (n = 8 per group) were treated 5, 6, and 7 days after tumor inoculation with 10 μg of anti–CD20-mIFNα or the molar equivalent of the indicated proteins. Mice were followed for survival and killed when tumors reached 1.4 cm in diameter as per institutional guidelines. Mice treated with HBSS were used as control.
Anti–CD20-hIFNα is effective against the human B-cell lymphoma Daudi
We next sought to confirm the efficacy of our anti–CD20-IFNα fusion protein strategy against human B-cell lymphoma. We generated a human IFNα fusion protein, anti–CD20-hIFNα, and compared its antitumor activity against the human B-cell lymphoma line Daudi with that of rituximab, nontargeted anti–DNS-hIFNα, and a combination of anti–DNS-hIFNα and rituximab. Addition of 100pM rituximab to Daudi cells did not induce significant apoptosis (5.0%) compared with untreated cells (5.0%; data not shown; Figure 5). In contrast, cells treated with 100pM anti–CD20-hIFNα were significantly more apoptotic (31.7%) than rituximab-treated cells (5.0%, P < .001). Targeting was important for fusion protein activity as equimolar amounts of anti–DNS-hIFNα induced significantly lower levels of apoptosis (15.7%) compared with anti–CD20-hIFNα (P = .001). Anti–CD20-hIFNα was also more effective at inducing apoptosis than the combination of rituximab and anti–DNS-hIFNα (P = .001). When the treatment dose was decreased to 10pM, similar results were obtained, with anti–CD20-hIFNα inducing significantly more apoptosis than the combination of anti–DNS-hIFNα and rituximab (P = .003). Consistent with the results obtained in our studies with the murine IFNα fusion protein, anti–CD20-hIFNα has potent activity against human lymphoma cells that is dependent on targeting of IFNα to the tumor cell surface.
Anti–CD20-hIFNα fusion protein has proapoptotic activity against the human B-cell lymphoma Daudi. (A) Daudi lymphoma cells were incubated with various concentrations of the indicated proteins for 72 hours. Staining with annexinV-FITC and PI was performed to distinguish necrotic (annexin−PI+), early apoptotic (annexin+PI−), and late apoptotic (annexin+PI+) cell populations. The percentage of total apoptotic cells was quantified for each sample as the sum of early apoptotic and late apoptotic cells. Experiments were performed in triplicate, and error bars indicate mean ± SD. *P = .001. **P = .003.
Anti–CD20-hIFNα fusion protein has proapoptotic activity against the human B-cell lymphoma Daudi. (A) Daudi lymphoma cells were incubated with various concentrations of the indicated proteins for 72 hours. Staining with annexinV-FITC and PI was performed to distinguish necrotic (annexin−PI+), early apoptotic (annexin+PI−), and late apoptotic (annexin+PI+) cell populations. The percentage of total apoptotic cells was quantified for each sample as the sum of early apoptotic and late apoptotic cells. Experiments were performed in triplicate, and error bars indicate mean ± SD. *P = .001. **P = .003.
Anti–CD20-hIFNα can cure well-established human lymphoma xenograft tumors
To extend our in vitro results against human lymphoma cells to an in vivo setting, we used a xenograft model of established Daudi tumors in nude mice. Treatment with 3 weekly 30-μg doses of anti–CD20-hIFNα, equimolar doses of rituximab or HBSS were initiated only after tumors reached at least 0.5 cm in diameter (Figure 6A-C). In 7 of 7 mice treated with anti–CD20-hIFNα, there was rapid and uniform tumor regression. In comparison, only 2 of 5 mice treated with rituximab experienced tumor regression. Spontaneous tumor regression was seen in 1 of 5 mice treated with HBSS. The overall survival of the fusion protein–treated mice was significantly better than that of mice treated with rituximab (P = .02), and all fusion protein–treated mice remained tumor free after 60 days (Figure 6D). In contrast, the rituximab-treated mice did not exhibit enhanced survival compared with mice treated with HBSS (P = .5).
Anti–CD20-hIFNα completely cures established human xenograft tumors. (A-C) Tumor growth in mice (n = 5-7 per group) inoculated subcutaneously with Daudi cells and treated as indicated with 3 weekly doses of 30 μg of fusion protein, the equivalent molar concentration of rituximab, or HBSS. Treatment was administered 30, 37, and 44 days after tumor inoculation (arrows) to mice with tumors at least 0.5 cm in diameter. HBSS was injected as a control. Symbols represent individual mice. (D) Survival curves for the mice whose tumor growth is shown in panels A-C. *P = .02.
Anti–CD20-hIFNα completely cures established human xenograft tumors. (A-C) Tumor growth in mice (n = 5-7 per group) inoculated subcutaneously with Daudi cells and treated as indicated with 3 weekly doses of 30 μg of fusion protein, the equivalent molar concentration of rituximab, or HBSS. Treatment was administered 30, 37, and 44 days after tumor inoculation (arrows) to mice with tumors at least 0.5 cm in diameter. HBSS was injected as a control. Symbols represent individual mice. (D) Survival curves for the mice whose tumor growth is shown in panels A-C. *P = .02.
Discussion
The use of systemically administered IFNα in treating B-cell lymphoma has been problematic because of the toxicity of this potent antitumor agent. In the present study, we demonstrated that the targeting of IFNα to lymphomas by an antibody specific for the B-cell differentiation antigen CD20 allows concentrations of the cytokine to reach effective levels at the tumor site, leading to the efficient cure of tumor-bearing mice in the absence of systemic toxicity. The anti–CD20-IFNα fusion protein was significantly more effective against CD20-expressing tumors than both single-agent rituximab and the combination of anti-CD20 and systemic IFNα. Thus, IFNα, an agent limited in practice by its toxicity, has here been exploited for its advantageous antitumor properties to potent effect when targeted via anti-CD20.
In vitro treatment of lymphoma cells expressing human CD20 with anti–CD20-IFNα induced higher levels of apoptosis than did rituximab, a nonspecific antibody-IFNα fusion (anti–DNS-IFNα), or a combination of the two (Figures 2,5). This finding predicted an advantage when treating tumors in vivo, which was indeed the case. For our initial in vivo studies, we chose the 38C13-huCD20 model, a syngeneic human CD20–expressing murine B-cell lymphoma grown in immunocompetent C3H mice.39 The 38C13 lymphoma is a highly aggressive tumor model in which mice develop micrometastases in the spleen, lymph nodes, and bone marrow within 6 to 9 days of subcutaneous tumor inoculation,46 thus representing a stringent setting in which to evaluate novel therapeutics. This setting not only allows recruitment of the full range of host innate and adaptive immune effector mechanisms, but also assessment of IFNα toxicity. In vivo treatment of mice bearing 38C13-huCD20 tumors with anti–CD20-IFNα resulted in significant delay in tumor growth and/or cure in small and established tumors. The low dose of 10 μg of anti–CD20-mIFNα given on 3 consecutive days completely prevented growth of small tumors when administered as an early treatment regimen beginning 1 day after tumor inoculation. Treatment with equivalent doses of rituximab had no effect, consistent with the antitumor activity being mediated through IFNα and not via ligation of CD20. Indeed, earlier studies reported that 500 μg of rituximab given as a single dose 1 day after tumor inoculation cured only 38% of small tumors.39 In a treatment model of established 38C13-huCD20 tumors, 3 daily 10-μg doses of anti–CD20-mIFNα administered in a late treatment regimen beginning 5 days after tumor challenge resulted in significant delay of tumor growth in all animals and 29% cure. Animals treated with rituximab did not experience any delay in tumor growth or enhancement in survival compared with HBSS-treated mice. To improve upon the 29% cure rate of animals with established tumors, mice were retreated at weekly intervals when tumor cells surviving the initial treatment would have proliferated significantly. This repeat treatment regimen resulted in 87% cure with no observed toxicity. Thus, repeat administration of anti–CD20-mIFNα has the potential to completely cure lymphoma with none of the associated systemic toxicity that has been an obstacle in IFNα-based cancer therapy. Tumor-specific targeting of mIFNα was critically important in the action of anti–CD20-mIFNα as the nontargeted anti–DNS-mIFNα fusion protein did not prolong or promote survival. This demonstrates that delivery of IFNα via anti-CD20 directly into the tumor vicinity is superior to systemic IFNα administration and is a viable approach to increasing the therapeutic index of this potent antitumor agent. Because of the multifunctional nature of the anti–CD20-IFNα fusion protein, the mechanism by which it prevents tumor growth is of interest. In vivo experiments using 38C13-huCD20 IFNAR KD, a cell line with decreased IFNAR expression, resulted in complete abrogation of fusion protein–mediated tumor killing. These data suggest that the antitumor effect of anti–CD20-mIFNα is mediated primarily and possibly exclusively through the induction of tumor cell death via a direct interaction between targeted IFNα and its receptor on the surface of tumor cells.
ADCC,8,9 CDC,47 and direct induction of apoptosis48 have been shown to contribute to the antitumor activities of rituximab. However, in the present studies, ADCC and CDC do not appear to play a significant role in fusion protein–mediated protection against tumor growth, although their involvement cannot be excluded. Given that in most cases only 10-μg doses of fusion protein were used in our in vivo studies, these results are not surprising. The low doses of fusion protein used in treatments would not be expected to saturate the CD20 molecules present on tumor cell surfaces. This lack of saturation would limit activities such as ADCC and CDC, which require binding of effector cells or complement, respectively, to closely spaced Fc regions of an antibody.
To extend our observations in 38C13-huCD20 to a human tumor model, an anti-CD20 human IFNα fusion protein (anti–CD20-hIFNα) was generated. When its activity was evaluated using the human lymphoma Daudi, consistent with what had been observed in the murine tumor model, anti–CD20-hIFNα had far higher proapoptotic activity than rituximab or the combination of rituximab and hIFNα. Importantly, the fusion protein was effective at very low doses where rituximab treatment did not induce significant levels of apoptosis. In a xenograft model of large, well-established Daudi tumors, low doses of fusion protein treatment resulted in rapid regression of 100% of tumors. In marked contrast, tumor regression was seen in only 40% of the mice treated with equimolar concentrations of rituximab. Fusion protein–treated mice also had enhanced survival compared with rituximab-treated mice, and all were tumor-free 60 days after the initial tumor challenge. These results indicate that the fusion protein can eliminate bulky tumors and may be an effective therapeutic against established human lymphoma.
Recently, Rossi et al reported a CD20-targeted tetrameric human IFNα construct that showed efficacy against human lymphoma xenografts in severe combined immunodeficient mice in vivo.49 The molecule in this study was generated using a “dock and lock” method whereby 4 molecules of IFNα2b were attached to an antibody targeting CD20. The resulting conjugates showed potent interferon activity comparable with that of PEG-intron (Peginterferon alpha-2b). Although this construct displayed promising activity against several human lymphoma xenografts, its high-level interferon activity raises the concern of systemic toxicity against nontumor cells that could not be evaluated in xenograft models, as mouse cells are insensitive to human IFNα2b. In our studies, using a syngeneic immunocompetent murine model, no toxicity was observed with the species-appropriate mouse IFNα fusion protein. Importantly, the fusion protein demonstrated powerful efficacy against lymphoma despite a reduction in its IFNα activity. Thus, a targeted therapeutic in which the activity of the attached interferon is reduced may have a higher therapeutic index.
Anti–CD20-hIFNα has the potential to be a versatile therapeutic. It could be administered in combination with rituximab and/or chemotherapy to augment antitumor activity. As a monotherapy, anti–CD20-hIFNα may provide a viable alternative to rituximab in the clinic for the subpopulation of patients in whom rituximab-refractory tumors arise by unknown mechanisms. Various hypotheses such as loss or down-regulation of CD20 and the presence of circulating CD20 have been proposed to explain how resistance to rituximab arises. A study by Jazirehi et al50 reported that this phenomenon was independent of diminished CD20 expression, but in fact resulted from aberrant expression of signaling molecules or dysregulation of signaling pathways. Because anti–CD20-IFNα is essentially a 2-pronged attack whereby both CD20 and IFNAR signaling pathways can be activated to induce tumor cell apoptosis, it may remain efficacious in instances when CD20 signaling pathways become dysregulated in patients with rituximab-resistant tumors. It would be of interest to examine the effect of fusion protein treatment on rituximab-refractory lymphomas in future studies.
IFNα has potent biologic activities against B-cell malignancies, but its clinical utility is currently limited by systemic toxicities. We hypothesized that targeting IFNα directly to tumor cells via fusion to an anti-CD20 antibody could be a valuable agent for treating lymphomas. Our results indicate that anti–CD20-IFNα fusion proteins possess a markedly improved therapeutic index, exhibiting the ability to eradicate both murine and human lymphomas in vivo using a small fraction of the IFNα that would be used in conventional systemic dosing. Therefore, we propose that the anti–CD20-hIFNα fusion protein warrants further evaluation as a lymphoma therapeutic.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the National Institutes of Health (RO1 GM074051) and the Leukemia & Lymphoma Society Translational Research Program (grant 6098). C.X. was supported by the UCLA Dissertation Year Fellowship.
National Institutes of Health
Authorship
Contribution: C.X. designed and performed experiments, analyzed data, and wrote the paper; K.K.S. designed and performed experiments, reviewed data, and reviewed the paper; and J.M.T. and S.L.M designed experiments, reviewed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Sherie L. Morrison, Professor of Microbiology, Immunology and Molecular Genetics, 247 BSRB, 615 Charles E. Young Dr East, Los Angeles, CA 90095; e-mail: sheriem@microbio.ucla.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal