Abstract
Previous observational studies suggest that rituximab may be useful in the treatment of primary immune thrombocytopenia (ITP). This randomized trial investigated rituximab efficacy in previously untreated adult ITP patients with a platelet count of 20 × 109/L or less. One hundred three patients were randomly assigned to receive 40 mg/d dexamethasone for 4 days with or without 375 mg/m2 rituximab weekly for 4 weeks. Patients who were refractory to dexamethasone alone received salvage therapy with dexamethasone plus rituximab. Sustained response (ie, platelet count ≥ 50 × 109/L at month 6 after treatment initiation), evaluable in 101 patients, was greater in patients treated with dexamethasone plus rituximab (n = 49) than in those treated with dexamethasone alone (n = 52; 63% vs 36%, P = .004, 95% confidence interval [95% CI], 0.079-0.455). Patients in the experimental arm showed increased incidences of grade 3 to 4 adverse events (10% vs 2%, P = .082, 95% CI, −0.010 to 0.175), but incidences of serious adverse events were similar in both arms (6% vs 2%, P = .284, 95% CI, −0.035 to 0.119). Dexamethasone plus rituximab was an effective salvage therapy in 56% of patients refractory to dexamethasone. The combination of dexamethasone and rituximab improved platelet counts compared with dexamethasone alone. Thus, combination therapy may represent an effective treatment option before splenectomy. This study is registered at http://clinicaltrials.gov as NCT00770562.
Introduction
Adult primary immune thrombocytopenia (ITP) is an acquired autoimmune disease characterized by increased platelet destruction, impaired megakaryocyte maturation with reduced platelet production, and possible hemorrhagic complications. The incidence is nearly 30 new cases per 1 million annually.1 ITP is a chronic condition presenting most commonly in women and elderly patients, often with an insidious onset and varying severities of thrombocytopenia.
Glucocorticoids, the standard first line of treatment for patients with symptomatic disease,2 enable the recovery of platelet count in 70% to 80% of cases; however, in most cases, steroid tapering or withdrawal is followed by a drop in platelet count and the need for additional treatment. Splenectomy still represents the standard salvage therapy, with the caveat that approximately 40% of patients either do not respond or relapse after surgery,3 approximately 10% experience perioperative or delayed infections, and some develop surgical complications, although rarely fatal.3 Recently, romiplostim and eltrombopag, 2 new thrombopoietin receptor agonists, have shown potent activity in ITP4,5 ; however, these agents do not act on the underlying disease mechanism, and therapeutic efficacy is dependent on continual administration. Recently, the use of high-dose dexamethasone given in a single 4-day course (40 mg/d orally) as first-line treatment for adult ITP patients resulted in an 85% initial response and, more importantly, a 42% sustained response (SR) rate (ie, platelet count >50 × 109/L at 6 months after initial treatment).6
Rituximab (MabThera; Roche) is a chimeric monoclonal antibody7 highly specific for the CD20 antigen that is expressed only by mature B cells. Its administration is accompanied by marked, although transient, B-cell depletion,8 and this effect has been exploited for the treatment of several autoimmune disorders with encouraging results.9-15 The authors of various reports16-25 have described the activity of rituximab administered as a weekly infusion of 375 mg/m2 for 4 consecutive weeks as a single-agent salvage treatment in ITP. Despite differences in patient characteristics, results from these studies showed 40% to 70% overall early response rates with 20% to 50% complete response rates, 20% to 40% medium-term relapse rates, nearly 40% SR rates, and splenectomy-sparing capacity in a substantial number of patients.26 These studies also reported possible significant toxicities, including death (not necessarily attributable to rituximab) in nearly 3% of cases.25
The principal mechanism of rituximab action in ITP appears strictly related to B-cell depletion and the consequent inhibition of several B-cell pathologic activities, such as the production of autoantibodies specific for platelet and megakaryocyte glycoproteins (glycoproteins IIb/IIIa and Ib/IX),27-29 cytokine secretion, and antigen-presenting cell activity. These effects may explain the rapid kinetics of platelet recovery observed in the majority of responders. Recent observations30,31 of the reversion of several baseline T-cell abnormalities in ITP patients who responded to rituximab suggest that rituximab may have an indirect regulatory effect that may help explain its long-term therapeutic effect.
To date, no prospective randomized studies evaluating rituximab efficacy in ITP have been performed. We report the results of the first prospective phase 3 study in which we compared the efficacy of dexamethasone plus rituximab versus dexamethasone alone in treatment-naive adult ITP patients.
Methods
Study design
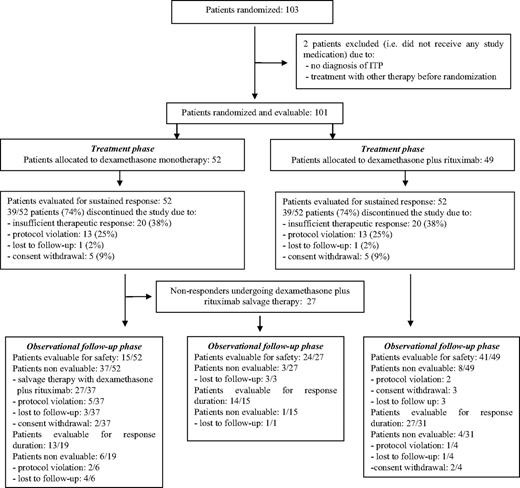
This randomized, open-label, phase 3, multicenter study began in June 2005; enrollment was stopped in June 2007. The study consisted of 2 phases: the treatment phase (ML18542 study) lasted from the time of enrollment up to month 6, and an observational follow-up phase lasted from months 6 to 36 (Figure 1).
Consolidated Standards of Reporting Trials (CONSORT) flow diagram of progress through the study phases.
Consolidated Standards of Reporting Trials (CONSORT) flow diagram of progress through the study phases.
Patients
Twenty-two Italian academic centers or hospitals participated in this study. The study protocol was approved by the institutional review boards of each participating center, and written informed consent was obtained from all patients before study entry in accordance with the Declaration of Helsinki. We enrolled treatment-naive patients (any previous dose of corticosteroids or other immune-suppressive agents was an exclusion criteria), 18 years or older, with a diagnosis of ITP according to the guidelines of the American Society of Hematology2 and a platelet count of 20 × 109/L or less. No patient stratification was performed. All patients underwent baseline immunophenotypic assessments of peripheral blood lymphocytes (CD3, CD4, CD8, CD19, CD20); serum levels of immunoglobulin A (IgA), immunoglobulin G (IgG), and immunoglobulin M (IgM); and HIV, hepatitis C virus, and hepatitis B virus evaluation.
Exclusion criteria included a positive pregnancy test; congenital or acquired cell or humoral immunologic deficit; positive HIV, hepatitis B virus, or hepatitis C virus tests; history of malignancies within 3 years of study entry; concomitant immunosuppressive or cytotoxic treatment; active infection requiring systemic therapy; and non-ITP immune thrombocytopenias. Positive tests for antibodies to nuclear DNA, cardiolipin, or ENA; direct Coombs test; lupus anticoagulant not associated with a specific clinical history; or the presence of signs and symptoms of other autoimmune disorders were not criteria for exclusion. Treatment-naive patients with previously asymptomatic, chronic ITP that subsequently worsened were included in the study. The study population consisted mainly of patients with newly diagnosed ITP (85 patients; Table 1) assessed according to recently published criteria32 but also included treatment-naive patients with persistent or chronic disease (16 patients; Table 1).
Baseline demographic and disease characteristics in the ITT and safety population (101 patients)
| Parameter . | Dexamethasone . | Dexamethasone plus rituximab . | P . |
|---|---|---|---|
| Number of patients | 52 | 49 | |
| Male/female | 19/33 (37%/63%) | 22/27 (45%/55%) | .39 |
| Ethnic origin | 98% white | 100% white | .98 |
| 2% Asian | |||
| Age, y (mean ± SD) | 47 ± 19 | 49 ± 16 | .60 |
| Months from diagnosis, median, n (range)* | 0 (0-103) | 0 (0-100) | .14 |
| Patients with newly diagnosed ITP, n (%) | 44 (85) | 41 (84) | |
| Patients with persistent or chronic ITP, n (%) | 8 (15) | 8 (16) | |
| Platelets 0-10 × 109/L, n (%) | 24 (46) | 22 (45) | .80 |
| Platelets 10-20 × 109/L, n (%) | 27 (52) | 25 (51) | |
| Platelets > 20 × 109/L, n (%)† | 1 (2)‡ | 2 (4)§ |
| Parameter . | Dexamethasone . | Dexamethasone plus rituximab . | P . |
|---|---|---|---|
| Number of patients | 52 | 49 | |
| Male/female | 19/33 (37%/63%) | 22/27 (45%/55%) | .39 |
| Ethnic origin | 98% white | 100% white | .98 |
| 2% Asian | |||
| Age, y (mean ± SD) | 47 ± 19 | 49 ± 16 | .60 |
| Months from diagnosis, median, n (range)* | 0 (0-103) | 0 (0-100) | .14 |
| Patients with newly diagnosed ITP, n (%) | 44 (85) | 41 (84) | |
| Patients with persistent or chronic ITP, n (%) | 8 (15) | 8 (16) | |
| Platelets 0-10 × 109/L, n (%) | 24 (46) | 22 (45) | .80 |
| Platelets 10-20 × 109/L, n (%) | 27 (52) | 25 (51) | |
| Platelets > 20 × 109/L, n (%)† | 1 (2)‡ | 2 (4)§ |
ITT indicates intention to treat; and ITP, primary immune thrombocytopenia.
Some patients had a previous diagnosis of ITP initially not requiring treatment.
Three patients, 1 in the dexamethasone arm and 2 in the dexamethasone plus rituximab arm, were randomized despite a platelet count greater than 20 × 109/L.
Platelet count 21 × 109/L.
Platelet count 21 × 109/L and 22 × 109/L.
Treatment
Patients were randomized 1:1 to receive dexamethasone with or without rituximab. A daily dose of 40 mg of oral dexamethasone was administered to both arms for 4 consecutive days (days 1-4). Patients in the experimental arm received 375 mg/m2 intravenous rituximab (regardless of the initial response to dexamethasone) on days 7, 14, 21, and 28. In the case of lack of response (ie, platelet count ≤ 20 × 109/L or bleeding during the first 28 days after the start of treatment), patients in both arms could receive rescue therapy with corticosteroids at the minimum effective dose and/or a single course of 2 g/kg intravenous immunoglobulin (IVIG) over the course of 2 days according to protocol. When rescue therapy was administered beyond day 28, this was considered failure of response to study treatment. Salvage therapy with dexamethasone plus rituximab (by use of the regimen described previously) was administered to patients initially assigned to the dexamethasone arm exhibiting platelet counts 20 × 109/L or less at any time point from day 28 up to the end of month 6. The necessity for salvage therapy was a criterion for nonresponse to the study treatment. Patients in the dexamethasone plus rituximab arm who failed to achieve SR were treated according to the standard practice of the study center.
Assessments and outcome measures during the treatment phase
The primary objective of the study was to compare the rates of SR (defined as platelet count ≥ 50 × 109/L at month 6). We selected platelet count instead of clinical signs of bleeding as the main efficacy parameter to eliminate any potential bias in assigning the grade of bleeding. Platelet count was evaluated weekly during the first month and subsequently at biweekly intervals until the end of month 6.
Patients who failed therapy, that is, they required further treatments in the interval between day 28 and month 6, were considered failures. The same efficacy parameters also were adopted to evaluate clinical efficacy in those patients who underwent dexamethasone plus rituximab salvage therapy. In this group, the onset of salvage therapy was considered as day 1.
The secondary objectives of the study were the following: hematologic response (platelet count ≥ 100 × 109/L [SR 100] and ≥ 150 × 109/L [SR 150]); safety profile (adverse event [AE] incidence up to 6 months from the beginning of therapy, according to National Cancer Institute Common Toxicity Criteria version 3.0); early response (platelet count ≥ 50 × 109/L at day 28); the efficacy of dexamethasone plus rituximab salvage therapy in patients not responding to dexamethasone monotherapy; the effect of treatment on cellular and humoral immune response; and the identification of clinical and laboratory parameters predictive of SR.
To evaluate the levels of B-cell depletion and changes in immunoglobulin levels during the study period, the following analyses were performed at baseline and at monthly intervals: peripheral blood immune-phenotypic analysis of the CD20 lymphoid marker and serum levels of IgG, IgA, and IgM.
Assessments and outcome measures during the observational follow-up phase
To monitor the effect of treatment beyond month 6, patients were monitored at least every 4 months up to the end of year 3 from the time of enrollment, with the study authors documenting the occurrence of any delayed side effects, response status, and need for further treatment. During this period of observation, treatment efficacy parameters included the duration of response (RD), that is, the time interval between achievement of SR and relapse, the relapse rate (number of patients who lost SR status), and the need of additional ITP therapy beyond month 6.
Statistical analysis
On the basis of the results from Cheng et al,6 this study was designed for an 80% probability to detect an improvement in SR from 40% of patients treated with dexamethasone and up to 60% of patients treated with dexamethasone plus rituximab, with a 1-sided overall significance level of 5%. To account for the evaluation of toxicity, the sample size was adjusted from 154 to an overall size of 198 patients, assuming a 10% dropout rate, to capture greater than 11% grade 3 to 5 AEs in the experimental arm in comparison with an expected 2% in the dexamethasone arm. Three interim analyses were performed with every 50 enrolled patients to assess the necessity for early stopping for safety and efficacy reasons. The study would be stopped when a 50% relative difference in SR was demonstrated (α 0.017; Bonferroni adjustment) or if a greater-than-expected toxicity was observed in the experimental arm (> 26%, > 16%, > 12.5% at each safety checkpoint, respectively).
Results were analyzed according to the intention to treat (ITT) population, which included all randomized patients who received the first dose of dexamethasone. In the cases of missing platelet count for premature discontinuations, the last observation carried forward approach was adopted. Patients who switched to salvage therapy in the dexamethasone arm and any patient requiring steroids or IVIG therapy after day 28 in both arms were counted as SR failures. Differences in SR and toxicity rates were assessed with the χ2 or the Fisher test, depending on the assumption check. An exploratory logistic regression model was applied to the SR data, including the effect of treatment and rescue therapy as covariates; results were expressed as odds ratios and 95% confidence intervals (95% CI). Analysis of potential predictive factors was performed by the use of logistic regression, and results were expressed as odds ratios and corresponding 95% CI. The following clinical and laboratory factors were considered: age; sex; baseline platelet count (< or ≥ 10 × 109/L); total and CD20-positive lymphocyte counts and IgG serum levels; baseline versus week 24 changes in the levels of IgG, IgA, IgM; and CD20-positive lymphocytes.
The early response assessment was performed on the ITT population. Those patients requiring additional treatment with steroids or IVIG during the first month were considered as early response failures, independent of the platelet count achieved at the day 28 visit.
Safety, response to salvage therapy, and cellular and humoral immune responses were analyzed by the use of descriptive statistics. The Kaplan-Meier method and log-rank test were used to evaluate the RD during the observational follow-up phase. The relapse rate and the need for additional ITP therapy after month 6 were assessed by the use of the χ2 or Fisher test, depending on the assumption check.
Results
Treatment phase
Accrual and clinical characteristics.
Enrollment was stopped in advance in June 2007 after 103 patients had been accrued, when results of the first interim analysis indicated that the primary efficacy goal had already been achieved (SR advantage for the experimental arm: 81% vs 29%, Δ = 52%, P < .001, 98.3% CI, 0.228-0.804).33 One-hundred one patients (52 in the dexamethasone arm and 49 in the experimental arm) comprised the safety and ITT populations. Two patients could not be assessed because they did not receive any study drug after randomization (1 patient in the experimental arm because of no ITP diagnosis and 1 patient in the dexamethasone arm because of previous treatment received). Both arms were balanced with respect to baseline characteristics (Table 1).
Efficacy.
Compliance and additional therapy.
Mean compliance to dexamethasone was 100% in both arms. Five patients in the experimental arm discontinued the study before taking the first dose of rituximab. For the remaining 44 patients, mean and median compliance was 95% and 100%, respectively (range, 13%-110%).
A total of 37 of 101 patients, 14 (27%) in the dexamethasone arm and 23 (47%) in the experimental arm, received further steroids and/or IVIG therapy up to day 28. A total of 9 of 14 patients in the dexamethasone arm and 8 of 23 patients in the experimental arm needed to continue the therapy beyond day 28 and were therefore considered failures for SR. One additional failure occurred in the experimental arm (patient who began additional therapy after day 28). Both groups exhibited differences in mean platelet counts at day 7 (98 ± 87 × 109/L in the dexamethasone arm and 74 ± 61 × 109/L in the combination arm).
SR.
Analysis of the ITT population indicated that SR was greater among patients in the experimental arm compared with those in the dexamethasone arm (63% vs 36%, P = .004, difference = 27%, 95% CI, 0.079-0.455; Table 2). A similar pattern was observed for SR 100 and SR 150 (Table 2). The SR rate was 29 of 44 (66% vs 36% for the dexamethasone arm; P = .004) when the 5 patients who did not receive rituximab were eliminated. The administration of rescue therapy during the first 28-day period was not associated with the SR rate (odds ratio, 0.48; 95% CI, 0.20-1.18), thus confirming the expected short-term effects of low-dose steroids and IVIG therapy. The effect of treatment was significant (P < .004), and the odds ratio of the experimental versus dexamethasone arms was 3.58 (95% CI, 1.51-8.47). None of the potential predictive clinical or laboratory parameters of SR (baseline platelet count, total and CD20-positive lymphocyte count, and baseline vs week 24 changes in serum IgG levels) were found to be significant when the logistic regression model was used. Treatment was the only predictive factor for better response (P = .004). In both arms, the response rate was similar among patients with newly diagnosed or persistent/chronic ITP (dexamethasone arm: 3/8, 37.5%, vs 16/44, 36.3%, P = .951; dexamethasone plus rituximab arm: 4/8, 50%, vs 27/41, 65.8%, P = .395, respectively).
Effects of treatment with dexamethasone or dexamethasone plus rituximab on the rates of sustained response (overall SR: platelet count ≥ 50 × 109/L; SR 100: platelet count ≥ 100 × 109/L; SR 150: platelet count ≥ 150 × 109/L)
| Patients . | SR . | SR 100 . | SR 150 . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Platelets 50 × 109/L or greater . | Platelets 100 × 109/L or greater . | Platelets 150 × 109/L or greater . | |||||||
| Dexamethasone . | Dexamethasone plus rituximab . | P . | Dexamethasone . | Dexamethasone plus rituximab . | P . | Dexamethasone . | Dexamethasone plus rituximab . | P . | |
| Evaluable | 52 | 49 | 52 | 49 | 52 | 49 | |||
| Responders, n (%) | 19 (36) | 31 (63) | .004 | 17 (33) | 26 (53) | .019 | 13 (25) | 21 (43) | .029 |
| Patients . | SR . | SR 100 . | SR 150 . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Platelets 50 × 109/L or greater . | Platelets 100 × 109/L or greater . | Platelets 150 × 109/L or greater . | |||||||
| Dexamethasone . | Dexamethasone plus rituximab . | P . | Dexamethasone . | Dexamethasone plus rituximab . | P . | Dexamethasone . | Dexamethasone plus rituximab . | P . | |
| Evaluable | 52 | 49 | 52 | 49 | 52 | 49 | |||
| Responders, n (%) | 19 (36) | 31 (63) | .004 | 17 (33) | 26 (53) | .019 | 13 (25) | 21 (43) | .029 |
SR indicates sustained response; SR 100, SR of platelet count ≥ 100 × 109/L; and SR 150, SR of platelet count ≥ 150 × 109/L.
Early response.
During the early response assessment (ITT population), 28 (53.8%) of 52 patients in the dexamethasone arm were considered nonresponders (11 with platelet count < 50 × 109/L; 17 receiving salvage therapy or taking concomitant steroids and/or IVIG); and 31 (63.2%) of 49 patients were nonresponders in the experimental arm (8 with platelet count < 50 × 109/L, 23 taking concomitant steroids and/or IVIG).
Salvage therapy with dexamethasone plus rituximab.
Twenty-seven patients (51.9%) initially allocated to receive dexamethasone monotherapy who failed to achieve SR received salvage treatment with dexamethasone plus rituximab, 18 in month 2, 7 in month 3, and 2 in month 5 from the start of treatment. In this subgroup, compliance to dexamethasone and rituximab was 100%. The rates of SR, SR 100, and SR 150 were 56%, 44%, and 37%, respectively (Table 3).
Effects of treatment with dexamethasone plus rituximab salvage therapy in 27 patients previously allocated to dexamethasone monotherapy who failed to achieve sustained response (overall SR: platelet count ≥ 50 × 109/L; SR 100: platelet count ≥ 100 × 109/L; SR 150: platelet count ≥ 150 × 109/L)
| . | SR . | SR 100 . | SR 150 . |
|---|---|---|---|
| Platelets 50 × 109/L or greater . | Platelets 100 × 109/L or greater . | Platelets 150 × 109/L or greater . | |
| Patients | 27 | 27 | 27 |
| Responders, n (%) | 15 (56%) | 12 (44%) | 10 (37%) |
| . | SR . | SR 100 . | SR 150 . |
|---|---|---|---|
| Platelets 50 × 109/L or greater . | Platelets 100 × 109/L or greater . | Platelets 150 × 109/L or greater . | |
| Patients | 27 | 27 | 27 |
| Responders, n (%) | 15 (56%) | 12 (44%) | 10 (37%) |
SR indicates sustained response; SR 100, SR of platelet count ≥ 100 × 109/L; and SR 150, SR of platelet count ≥ 150 × 109/L.
Safety.
Because of the early stopping of the trial, the safety evaluation was performed during a shorter period than initially planned. Table 4 summarizes the occurrence of AEs in both treatment arms. Overall, study treatments were well tolerated, and no grade 5 toxicity, hemorrhaging, or deaths occurred. Patients in the experimental arm exhibited a greater incidence of grade 3 to 4 AEs (10% vs 2%, P = .082, 95% CI, −0.010 to 0.175) and drug-related AEs (4% vs 0%, P = .149, 95% CI, −0.015 to 0.096) but no significant increase in serious AEs (6% vs 2%, P = .284, 95% CI, −0.035 to 0.119; Table 4). In the experimental arm, 1 patient required hospitalization because of platelet decrease and/or bleeding unrelated to study drug during salvage therapy; another patient required hospitalization 1 month after the end of rituximab treatment for interstitial pneumonia with a probable relation to study drug. No specific microbiologic agents were detected, and the patient recovered after treatment with levofloxacin plus ceftriaxone and azithromycin. One patient experienced supraventricular tachycardia during the first administration of rituximab, and 1 patient experienced seizure during the salvage treatment: both were probably related to study drug, and both patients were discontinued from the study. One patient with a previous history of vascular disease had a transitory ischemic attack not related to study drug during salvage therapy. All AEs were resolved during the study period.
Most frequent AEs occurring in patients treated with dexamethasone alone, dexamethasone plus rituximab, or dexamethasone plus rituximab salvage therapy
| Therapy . | Patients, n (%) . | ||
|---|---|---|---|
| Dexamethasone . | Dexamethasone plus rituximab . | Dexamethasone plus rituximab salvage therapy . | |
| Safety population | 52 | 49 | 27 |
| Patients with any AE | 26 (50) | 37 (76) | 18 (67) |
| Patients with serious AEs | 1 (2) | 3 (6) | 3 (11) |
| List of serious AEs | |||
| Hemorrhagic disorder | 0 | 1 | 0 |
| Platelet count decreased | 0 | 0 | 1 |
| Supraventricular tachycardia | 0 | 1 | 0 |
| Pneumonia | 0 | 1 | 0 |
| Rib fracture | 1 | 0 | 0 |
| Convulsion | 0 | 0 | 1 |
| Transitory ischemic attack | 0 | 0 | 1 |
| Patients with grade 3-4 AEs | 1 (2) | 5 (10) | 6 (22) |
| List of AEs | |||
| Hemorrhagic disorder | 0 | 1 | 0 |
| Platelet count decreased | 1 | 0 | 3 |
| Supraventricular tachycardia | 0 | 1 | 0 |
| Fever | 0 | 2 | 0 |
| Hypersensitivity | 0 | 1 | 0 |
| Pneumonia | 0 | 1 | 0 |
| Transaminases increased | 0 | 2 | 0 |
| Arthralgia | 0 | 0 | 1 |
| Convulsion | 0 | 0 | 1 |
| Nephrolithiasis | 0 | 0 | 1 |
| Patients with grade 3-4 AEs related to study drug | 0 | 2 (4) | 2 (7) |
| List of AEs | |||
| Supraventricular tachycardia | 0 | 1 | 0 |
| Fever | 0 | 1 | 0 |
| Pneumonia | 0 | 1 | 0 |
| Transaminases increased | 0 | 1 | 0 |
| Arthralgia | 0 | 0 | 1 |
| Convulsion | 0 | 0 | 1 |
| Therapy . | Patients, n (%) . | ||
|---|---|---|---|
| Dexamethasone . | Dexamethasone plus rituximab . | Dexamethasone plus rituximab salvage therapy . | |
| Safety population | 52 | 49 | 27 |
| Patients with any AE | 26 (50) | 37 (76) | 18 (67) |
| Patients with serious AEs | 1 (2) | 3 (6) | 3 (11) |
| List of serious AEs | |||
| Hemorrhagic disorder | 0 | 1 | 0 |
| Platelet count decreased | 0 | 0 | 1 |
| Supraventricular tachycardia | 0 | 1 | 0 |
| Pneumonia | 0 | 1 | 0 |
| Rib fracture | 1 | 0 | 0 |
| Convulsion | 0 | 0 | 1 |
| Transitory ischemic attack | 0 | 0 | 1 |
| Patients with grade 3-4 AEs | 1 (2) | 5 (10) | 6 (22) |
| List of AEs | |||
| Hemorrhagic disorder | 0 | 1 | 0 |
| Platelet count decreased | 1 | 0 | 3 |
| Supraventricular tachycardia | 0 | 1 | 0 |
| Fever | 0 | 2 | 0 |
| Hypersensitivity | 0 | 1 | 0 |
| Pneumonia | 0 | 1 | 0 |
| Transaminases increased | 0 | 2 | 0 |
| Arthralgia | 0 | 0 | 1 |
| Convulsion | 0 | 0 | 1 |
| Nephrolithiasis | 0 | 0 | 1 |
| Patients with grade 3-4 AEs related to study drug | 0 | 2 (4) | 2 (7) |
| List of AEs | |||
| Supraventricular tachycardia | 0 | 1 | 0 |
| Fever | 0 | 1 | 0 |
| Pneumonia | 0 | 1 | 0 |
| Transaminases increased | 0 | 1 | 0 |
| Arthralgia | 0 | 0 | 1 |
| Convulsion | 0 | 0 | 1 |
AE indicates adverse event.
Immunologic assessment.
The baseline levels of IgG, IgA, IgM, and CD20-positive lymphocytes were similar in both arms. After therapy, a slight reduction in mean IgG was observed in the dexamethasone arm (baseline vs week 24: 13.29 ± 5.12 g/L vs 9.33 ± 1.96 g/L, P < .001); however, there were no significant changes in IgA, IgM, or CD20-positive lymphocytes. Patients in the experimental arm experienced a significant reduction in IgG, IgA, and IgM levels (IgG baseline vs week 24: 12.04 ± 9.92 g/L vs 9.72 ± 3.56 g/L, P < .001; IgA baseline vs week 24: 2.23 ± 0.91 g/L vs 1.51 ± 1.01 g/L, P < .001; IgM baseline vs week 24: 1.16 ± 0.78 g/L vs 0.82 ± 0.58 g/L, P < .001). As expected, B-cell depletion in this group was observed from the very first control time point 4 weeks after the start of therapy and persisted until the last scheduled visit at week 24 (mean CD20-positive lymphocyte level, 0.00 ± 0.01 × 109/L).
Observational follow-up phase
Eighty patients (15 in the dexamethasone arm, 41 in the experimental arm, and 24 in the dexamethasone plus rituximab salvage therapy group) were followed-up beyond month 6 for a median observation period of 20 months (range, 4-40 months). Twenty-one patients were not evaluable for this part of the study because 9 were lost to follow-up during the study period.
Safety.
One case of herpes zoster reactivation was documented at month 22 in a patient who initially was allocated to the dexamethasone arm who then received dexamethasone plus rituximab salvage therapy. No other infectious, delayed toxic events, or deaths were registered.
Efficacy.
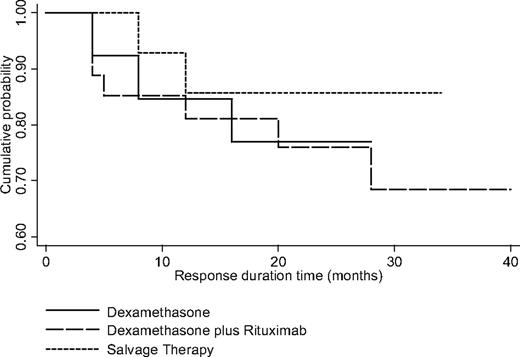
Fifty-four of 80 patients achieved a SR and could be evaluated for relapse rate and RD beyond month 6 (Figure 2). This group included 13 patients from the dexamethasone arm who were observed after month 6 for a median period of 20 months (range, 4-28 months), 27 from the experimental arm observed for a median period of 20 months (range, 4-40 months), and 14 from the dexamethasone plus rituximab salvage therapy group observed for a median period of 20 months (range, 8-34). The rate of relapse (ie, platelets < 50 × 109/L) in the 3 groups was 23% (3/13), 26% (7/27), and 14% (2/14), respectively (Fisher test; P = .837). The time to loss of SR was at months 4, 8, and 16 in the dexamethasone arm; months 4, 4, 4, 5, 12, 20, and 28 in the experimental arms; and months 8 and 12 in the dexamethasone plus rituximab salvage therapy. All relapsed patients underwent additional specific ITP therapy. The 30-month estimated probability of RD for each of the aforementioned treatment groups was 77%, 69%, and 85% (log-rank test, P = .783), respectively. During the period of observation, 4 (5%) of 80 of the evaluable patients underwent splenectomy.
Kaplan-Meier response duration curves after month 6 in patients who achieved sustained response (SR) after dexamethasone monotherapy, dexamethasone plus rituximab, and dexamethasone plus rituximab salvage therapy.
Kaplan-Meier response duration curves after month 6 in patients who achieved sustained response (SR) after dexamethasone monotherapy, dexamethasone plus rituximab, and dexamethasone plus rituximab salvage therapy.
Discussion
We report results from the first randomized clinical trial in which we evaluated the efficacy of rituximab in ITP. Our results suggest that the addition of rituximab to a single course of dexamethasone therapy improves outcomes in treatment-naive patients with ITP when used as first-line or salvage therapy. The inclusion of rituximab to the dexamethasone-based treatment regimen resulted in improved SR rates compared with dexamethasone alone (63% vs 36%). Furthermore, the dexamethasone-rituximab regimen was effective as salvage therapy (SR 56%) in the subgroup of patients who were refractory to dexamethasone monotherapy.
We chose to assess the efficacy of rituximab in treatment-naive patients to minimize any potential bias in patient baseline characteristics with respect to previous therapies. A single course of dexamethasone treatment (40 mg daily for 4 days) was selected as the comparator treatment on the basis of the data of Cheng et al.6 Indeed, comparison of the data obtained from our dexamethasone arm with those of Cheng et al6 revealed similar SR rates (36% vs 42%, respectively). A phase 2 study run by the Italian Group for Hematological Diseases (Gruppo Italiano Malattie Ematologiche dell'Adulto [GIMEMA]) performed in treatment-naive adult ITP patients and published in 2007 (our study began in 2005) suggested that 4 courses of 4-day, biweekly administrations of 40 mg of dexamethasone may yield better results than a single course, with response rates of 85%, 60% relapse-free survival at 15 months, and good safety profile.34 However, these findings need to be confirmed.
We administered rituximab at the standard dosage of 375 mg/m2 weekly for 4 weeks, according to the regimen adopted for non-Hodgkin lymphoma, previous findings in ITP, and other autoimmune disorders. The first rituximab infusion was administered on day +7 to allow study physicians to confirm the diagnosis and establish platelet count increase or stabilization upon dexamethasone treatment.
The authors of previous studies16-26 have demonstrated the therapeutic activity of rituximab and its long-term effects in patients with relapsed/refractory ITP. However, it is difficult to compare these results with those from our study because of differences in study methodology, including patient selection criteria and the parameters used to assess response. Stasi et al17 showed that 10 of 25 patients responded to rituximab, and this response was maintained for 6 months or longer in 7 (28%) patients. Garcia-Chavez et al23 reported a 67% SR rate in 18 pretreated ITP patients; Godeau et al26 reported promising 1-year responses in 40% of patients. With 1 exception,23 our data compare well with other studies in terms of long-term response (63% vs nearly 40%), corroborating the hypothesis that better long-term effects of rituximab can be achieved when administered earlier in the course of the disease.21,22 In contrast to published findings,20,26 we could not confirm the prognostic significance of age or any other clinical or biologic factors, including sex, platelet count, lymphocyte count, or immunoglobulin level.
Of the 52 patients initially treated with dexamethasone alone, 27 received dexamethasone plus rituximab salvage therapy because of nonresponsiveness, and of these 15 (55.5%) achieved SR. This proportion of responders is similar to that of the dexamethasone plus rituximab arm (63%). This finding suggests that a short course of dexamethasone therapy does not negate the effects of subsequent rituximab treatment; on the contrary, it could be useful for identifying patients who may not require further treatment with rituximab.
A greater proportion of patients in the experimental arm received rescue therapy up to day 28 after the start of treatment. This discrepancy may be related to the open-label nature of the study. Nevertheless, this variable did not confound the significance of the primary end point, as evidenced by the finding that low-dose steroids or a single course of IVIG had only transient and no long-term effects on response outcome.35
No significant differences in the relapse rate, RD, and the need for additional ITP therapy were observed in patients who achieved SR after dexamethasone monotherapy or dexamethasone plus rituximab (either front-line or salvage therapy). The risk of relapse after SR was low (∼20%), in agreement with the observations of Cheng et al6 and the relapse-free survival data of Mazzucconi et al34 Therefore, relapse rate appears to be a potential surrogate of SR rather than of the treatment regimen.
This study has several limitations. The follow-up analysis is brief in comparison with those after splenectomies,3 thus limiting the evaluation of the long-term effects of rituximab in disease stabilization and its long-term splenectomy-sparing potential. The early interruption of patient accrual resulted in insufficient power for detecting subtle differences in clinical events between the groups, including safety outcomes. With this in mind, the experimental arm was associated with an increased incidence of any and grade 3 to 4 AEs but not of serious AEs. Most of the grade 3 to 4 AEs were drug related, all AEs were resolved, and no deaths were recorded. Long-term follow-up revealed only 1 delayed toxic event (a case of herpes zoster), confirming the long-term safety findings for rituximab previously reported in non-Hodgkin lymphoma and ITP.36
Overall, little is known regarding the long-term effects of rituximab in ITP. In our study, 1 early serious infectious event (interstitial pneumonia) was recorded, which developed after the end of rituximab treatment. The single case of herpes zoster reactivation developed at month 22 after dexamethasone plus rituximab salvage therapy. The collective experience from this and other studies shows no evidence of increased risk for infectious complications with rituximab treatment. A recent review of case descriptions and published literature for the development of progressive multifocal leukoencephalopathy in rituximab-treated patients highlighted the development of progressive multifocal leukoencephalopathy in 1 ITP patient treated with rituximab.37 The long-term effects of rituximab treatment in ITP need to be confirmed in further clinical studies in this patient cohort.
In addition to safety, little is known of how rituximab acts in ameliorating the symptoms of ITP. Better characterization of the immune pathways involved in the pathogenesis of ITP and the corresponding patient pharmacogenetic profiles will aid in the selection of candidates for rituximab therapy. Further studies are warranted to confirm the effects of low-dose rituximab38 and the clinical activity of novel anti-CD20 antibodies that will be available in the near future.
In conclusion, results from this study suggest that the combination of dexamethasone and rituximab improves patient outcomes without compromising the safety profile. This outcome does not appear to be affected by a previous single course of dexamethasone monotherapy. The relatively sustained duration of response observed in some patients suggests a beneficial effect on the underlying disease. This treatment regimen provides an option for second-line therapy, particularly in patients not responding to steroids and as an alternative to splenectomy.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Katia De Luzio and Silvia Amigoni from OPIS-Italy for their support, and Carlo Brugnara, Karen Yeow, and Damian Page for editing the manuscript.
Roche sponsored the study, supplying dexamethasone and rituximab and covering the costs of the Contract Research Organization OPIS (Desio, Milan, Italy), patients' insurance, and all other costs of the trial. The sponsor did not influence or impose any specific restriction on study design, collection, analysis, or interpretation of data.
Authorship
Contribution: F.Z., M.B., and R.F. conceived and designed the study; P.M., M.B., L.G., A.Z., N.V., M.D., A.T., S.A., S.C., F.F., E.A., E.U., S.C., G.V., A.F., R.R., V.D.S., F.C., and M.L.B. collected and assembled data; F.Z, M.I., and F.S. analyzed and interpreted data; F.Z. and M.I. wrote the manuscript; E.G. was responsible for all study arrangements and planning; and all authors provided final approval of the manuscript.
Conflict-of-interest disclosure: F.Z. has received honoraria and financial support for the costs of travel to ASH annual meetings from Roche. E.G. is an employee of Roche. The remaining authors declare no competing financial interests.
A complete list of trial participants appears in the Appendix.
Correspondence: Francesco Zaja, MD, Clinica Ematologica, Azienda Ospedaliero Universitaria, Piazzale S Maria della Misericordia, 15, 33100 Udine, Italy; e-mail: zaja.francesco@aoud.sanita.fvg.it.
Appendix
In addition to the authors, other participants in this trial were as follows: M. De Simone, Department of Hematology, Ospedale Cardarelli, Napoli; A. L. Molinari, Department of Hematology, Ospedale Santa Maria delle Croci, Ravenna; A. Gallamini, Department of Hematology, Cuneo; M. G. Mazzucconi, Department of Hematology, La Sapienza, Roma; S. Mirto and S. Magrin, Department of Hematology, Palermo; D. Veneri, Department of Hematology, University of Verona; G. Semenzato, Department of Hematology, University of Padova; G. Rossi and C. Carbone, Department of Hematology, Brescia; E. Morra, Department of Hematology, Niguarda, Milano; G. Fioritoni, Department of Hematology, Pescara; V. Liso, Department of Hematology, University of Bari; and G. Leone, Department of Hematology, Catholic University, Roma.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal