To the editor:
There is mounting evidence from the study of animal models of human disorders of defective ribosome biogenesis, including Diamond-Blackfan anemia and Treacher Collins syndrome, that ribosomal stress leads to activation of the p53 pathway.1-3 Stabilization of p53 leads to cell-cycle arrest and apoptosis. We read with interest the manuscript by Danilova showing that deficiency of RPS19 in the zebrafish results in developmental abnormalities and defective erythropoiesis through the activation of the p53 protein family.3 The 5q− syndrome is the most distinct of all the myelodysplastic syndromes (MDS), and is defined under the World Health Organization (WHO) classification as refractory anemia with the del(5q) as the sole karyotypic abnormality [MDS with del(5q)].4,5 We have recently generated a mouse model of the human 5q− syndrome (with haploinsufficiency of RPS14) that shows the key features of the human disease, including a macrocytic anemia.6 p53 is activated in this mouse model of the human 5q− syndrome and intercrossing with p53-deficient mice completely rescued the progenitor cell defect.6 We thus hypothesized that activation of p53 and of the p53 pathway may underlie the pathogenesis of the human 5q− syndrome.
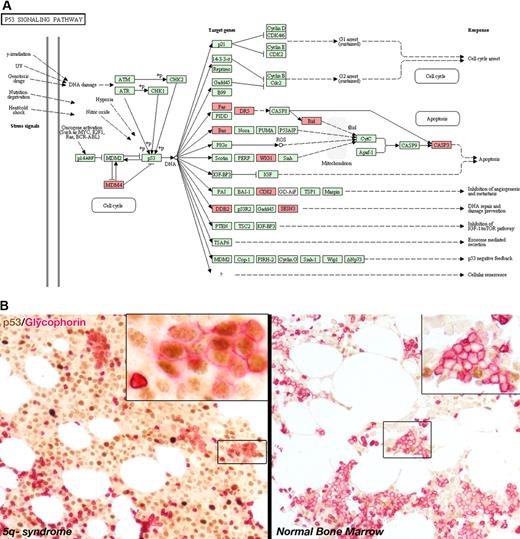
Data from several laboratories suggests that up-regulation of the p53 pathway is a common response to haploinsufficiency of ribosomal proteins. We now present an analysis of the p53 pathway in the 5q− syndrome; gene expression profiling (performed as previously described7 on Affymetrix U133 Plus2.0 arrays) was used to compare CD34+ cells from 16 patients with 5q− syndrome with CD34+ cells from 17 healthy controls, and 1570 significantly differentially expressed genes (P < .05, Benjamini-Hochberg correction) were identified. We then imported this gene list into the DAVID gene ontology application, and the p53 pathway was returned as significantly deregulated (p = .031) in the 5q− syndrome (Figure 1A). Ten genes in the p53 pathway were significantly deregulated: FAS, CD82, WIG1, CASP3, SESN3, TNFRSF10B (DR5), MDM4, BAX, DDB2 and BID. All these genes were expressed at higher levels in 5q− syndrome compared with healthy controls, with the exception of MDM4 (a negative regulator of p53) which was expressed at lower levels in 5q− syndrome patients. Moreover, 5 of the 8 most significantly up-regulated known genes in 5q− syndrome are p53 targets, including WIG1 and BAX.8 The p53 pathway was not significantly deregulated in non-5q− syndrome patients with a del(5q) (n = 30) or in non-del(5q) patients (n = 136).
Analysis of p53 in the 5q− syndrome. (A) The p53 pathway as shown by the Database for Annotation, Visualization and Integrated Discovery (DAVID) gene ontology application (http://david.abcc.ncifcrf.gov/). Genes highlighted in red are significantly differentially expressed in patients with 5q− syndrome. (B) Double immunostaining for p53 (brown) and Glycophorin A and C (red) in 5q− syndrome (×20, inset ×60) and in normal (×20, inset ×40) bone marrow trephines. Immunostaining was performed on paraffin-embedded tissue sections of normal bone marrow trephines and MDS. The monoclonal antibodies for Glycophorin A (JC159), Glycophorin C (ret40f), and p53 (DO-7) were all obtained from Dako. Images were acquired on a Nikon Eclipse E400 microscope equipped with 10×/0.30, 20×/0.50, 40×/0.75 and 60×/0.85 Plan Fluor objective lenses, using a Nikon DS-5Mc digital camera (all from Nikon) and Adobe Photoshop CS3 Version 10.0.1 image processing/manipulation software (Adobe). In 5q− syndrome, clusters of p53-positive erythroblasts (boxed area shown at higher power in top right inset) were positive for Glycophorin (mature red cells were p53-negative). In the normal bone marrow, clusters of Glycophorin-positive erythroblasts were p53-negative (boxed area shown at higher power in top right inset).
Analysis of p53 in the 5q− syndrome. (A) The p53 pathway as shown by the Database for Annotation, Visualization and Integrated Discovery (DAVID) gene ontology application (http://david.abcc.ncifcrf.gov/). Genes highlighted in red are significantly differentially expressed in patients with 5q− syndrome. (B) Double immunostaining for p53 (brown) and Glycophorin A and C (red) in 5q− syndrome (×20, inset ×60) and in normal (×20, inset ×40) bone marrow trephines. Immunostaining was performed on paraffin-embedded tissue sections of normal bone marrow trephines and MDS. The monoclonal antibodies for Glycophorin A (JC159), Glycophorin C (ret40f), and p53 (DO-7) were all obtained from Dako. Images were acquired on a Nikon Eclipse E400 microscope equipped with 10×/0.30, 20×/0.50, 40×/0.75 and 60×/0.85 Plan Fluor objective lenses, using a Nikon DS-5Mc digital camera (all from Nikon) and Adobe Photoshop CS3 Version 10.0.1 image processing/manipulation software (Adobe). In 5q− syndrome, clusters of p53-positive erythroblasts (boxed area shown at higher power in top right inset) were positive for Glycophorin (mature red cells were p53-negative). In the normal bone marrow, clusters of Glycophorin-positive erythroblasts were p53-negative (boxed area shown at higher power in top right inset).
Next, we performed an immunohistochemical analysis of p53 protein expression in bone marrow trephine sections from 3 patients with 5q− syndrome and 6 normal bone marrow samples. Strong p53 expression was found in all 3 cases of 5q− syndrome while only rare p53-positive cells were observed in normal bone marrow. By double immunostaining9 we showed that clusters of p53-positive cells with the morphologic appearance of erythroblasts were positive for Glycophorin in all 3 patients with 5q− syndrome (Figure 1B).
We have demonstrated induction of p53 and up-regulation of the p53 pathway in the human 5q− syndrome. This most probably results from haploinsufficiency of the ribosomal gene RPS14.8,10 The ribosome biogenesis checkpoint that results in activation of p53 protein and up-regulation of the p53 pathway offers a new potential therapeutic target in the human 5q− syndrome. However, this will be an option only if this intervention does not abrogate the critical tumor suppressor function of p53.
Authorship
Acknowledgments: This study was approved by the John Radcliffe Hospital (Oxford) institutional review board. Informed consent was provided according to the Declaration of Helsinki.
This work was supported by Leukaemia Research of the United Kingdom, and in part by grants from Associazione Italiana per la Ricerca sul Cancro (AIRC) and Fondazione IRCCS Policlinico San Matteo to MC.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dr Jacqueline Boultwood, University Reader at University of Oxford, Co-Director, LRF Molecular Haematology Unit, Nuffield Department of Clinical Laboratory Sciences, John Radcliffe Hospital, Oxford OX3 9DU, United Kingdom; e-mail: jacqueline.boultwood@ndcls.ox.ac.uk.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal