Abstract
Inhibition of integrins αvβ3 and αvβ5 in human brain microvascular endothelial cells (HBMECs) by the function-blocking peptide RGDfV induces loss of spreading on vitronectin, cell detachment, and apoptosis. We demonstrate that cell detachment is not required for apoptosis because plating on bovine serum albumin–blocked poly-L-lysine (allows attachment, but not integrin ligation and cell spreading) also induced apoptosis. Latrunculin B (LatB), which inhibits F-actin polymerization, induced transient loss of HBMEC spreading on vitronectin, but not their detachment, and induced apoptosis despite recovery of cell spreading. However, LatB did not cause apoptosis in 5 tumor cell lines. In HBMECs, both LatB and RGDfV induced transient Y412 and Y245 phosphorylation of endogenous c-Abl, a nonreceptor tyrosine kinase that reciprocally regulates F-actin. LatB also induced nuclear translocation of c-Abl in HBMECs. STI-571 (imatinib), a targeted therapy for BCR-ABL1+ leukemias and inhibitor of c-Abl, platelet-derived growth factor receptor, and c-Kit, decreased endothelial apoptosis. LatB-induced HBMEC apoptosis, and its inhibition by STI-571 also occurred in a 3-dimensional collagen model, supporting physiologic relevance. Last, siRNA to c-Abl (but not nonspecific siRNA) also inhibited RGDfV- and LatB-induced apoptosis. Thus, endogenous c-Abl mediates endothelial apoptosis induced by inhibition of integrins αvβ3/αvβ5 or by LatB-induced disruption of F-actin.
Introduction
Angiogenesis and endothelial cell responses are essential processes in diseases, such as cancer, and ischemic conditions. Integrins, heterodimeric cell-surface receptors composed of α and β subunits, are central regulators of angiogenesis and endothelial cell functions.1 Integrins enable cells to adhere to extracellular matrix (ECM), migrate over ECM substrates, and respond to ECM contact by proliferation, differentiation, and protection from apoptosis mediated by regulation of a number of intracellular signaling pathways.1-3 Inhibition of integrins αvβ3 and αvβ5, which are preferentially expressed and activated on angiogenic endothelial cells, induces endothelial apoptosis and impairs tumor angiogenesis.4,5 αvβ3/αvβ5-integrin signaling is mediated through interactions with an arginine-glycine-aspartic acid (RGD) peptide sequence in matrix proteins, such as vitronectin (VN), and can be abrogated by soluble function-blocking RGD peptides, such as cyclic RGDfV.4 Indeed, inhibitors of integrin αvβ3 are undergoing clinical trials in cancer patients, and cilengitide (EMD 121974; Merck KGaA), an integrin αvβ3/αvβ5 function-blocking RGDfV peptide, has encouraging activity in phase 1 and 2 trials against brain tumors in children and adult cancer patients.6,7
Integrin αvβ3 mechanism of action is complex. αvβ3 participates in pathologic angiogenesis,4,8 supporting its development as a target for therapy. To mediate its function, integrin αvβ3 requires activation and phosphorylation of the β3 integrin tail on Tyr747 and Tyr459, which signal downstream to pathways involving, among others, Src, FAK, Shc, p53, and p21WAF.8,9 Complicating matters, integrin αvβ3 cosignals with growth factor receptors, such as vascular endothelial growth factor receptor-2 and others.8 The intracellular signaling events mediating outside-in and inside-out signaling are complex and depend on the context of activation of the integrin and the cell type studied. Therefore, it is not surprising that the precise molecular mechanisms induced by engagement, crosstalk, or inhibition of αvβ3 integrin remain only partially understood.
Engagement of integrins with the ECM allows cells to adhere and spread, inducing changes in the actin cytoskeleton. Actin is an abundant cytoskeletal protein important in cell spreading and motility.10,11 Engagement of integrins with the ECM generates complex bidirectional signaling cascades between integrins and the actin cytoskeleton, which serve to transmit both force and biochemical signals. The interaction of integrins with actin is mostly through a number of intermediary proteins that can be cell-specific and/or stimulus-specific.11 A critical molecule that interacts with F-actin in fibroblasts is c-Abl. c-Abl integrates multiple signals to coordinate F-actin dynamics, whereas F-actin itself has an inhibitory effect on c-Abl kinase activity.12-14 Therefore, it is anticipated that actin-dependent signaling, including that by c-Abl, may mediate at least some of the phenotypes regulated by integrin signaling in endothelial cells.
In the work presented here, we investigated endothelial apoptosis induced by inhibition of integrins αvβ3/αvβ5 (RGDfV) and by the inhibitor of F-actin polymerization, latrunculin B (LatB). Both stimuli induced phosphorylation of c-Abl that could be inhibited by STI-571 (imatinib; Gleevec). Importantly, STI-571 and c-Abl siRNA protected endothelial cells from apoptosis induced by both RGDfV and LatB, demonstrating, for the first time, a role for c-Abl in mediating endothelial apoptosis induced by inhibition of integrin αvβ3/αvβ5.
Methods
Additional details on methods used are in supplemental data (available on the Blood website; see the Supplemental Materials link at the top of the online article).
Apoptosis assays
Apoptosis was evaluated by flow cytometry of cells (adherent and nonadherent) to detect terminal deoxynucleotidyltransferase activity as incorporation of fluorescein isothiocyanate–deoxyuridine triphosphate (FITC-dUTP) compared with propidium iodide (PI) using the Apo-Direct kit (BD Biosciences Pharmingen) according to the manufacturer's instructions. Apoptosis in human brain microvascular endothelial cells (HBMECs) grown in 3-dimensional (3D) collagen was assessed using the annexin V FITC fluorescence microscopy kit (BD Biosciences Pharmingen) according to the manufacturer's instructions.
Caspase-3 and caspase-8 activation
Caspase activity was measured using the ApoTarget Caspase-3/CPP32 or Caspase-8/FLICE Colorimetric Protease Assay (BioSource) and was determined in 200 μg of lysate protein suspended in 50 μL of extraction buffer. Absorbance at 400/405 nm was determined after 16 hours of incubation (37°C) with substrate.
Cell adhesion and 3D culture in collagen
Three-dimensional (3D) culture of HBMECs in collagen type I was performed according to Alavi and Stupack.15 After treatment, HBMECs were stained using the annexin V detection kit according to the manufacturer's instructions (BD Biosciences Pharmingen). Fluorescent images were acquired on an Olympus CKX41 microscope and photographed using PictureFrame 2.1 software (Optronics; original magnification ×400).
Cell culture
HBMECs of 2 to 4 different isolates were used for experiments.16,17 Tumor cell lines used were D293MED and UW228-2 (medulloblastoma), D54MG and D645MG (glioblastoma multiforme) from Dr Darrell Bigner (Duke University, Durham, NC) and CHLA-25518 (neuroblastoma). For all experiments with RGDfV and where indicated, cells were seeded on VN-coated plates that were blocked with heat-denatured bovine serum albumin (HD-BSA). Unless indicated, experiments were performed in serum-free medium containing 0.4% BSA (non-HD).
Immunofluorescence microscopy
Cells seeded onto VN-coated/HD-BSA–blocked chamber slides were fixed with 4% paraformaldehyde and incubated with c-Abl antibody (8E9; BD Biosciences) followed by AlexaFluor 488 rabbit anti–mouse (Invitrogen) and then AlexaFluor 568 phalloidin and 4,6-diamidino-2-phenylindole (DAPI; Invitrogen), or phalloidin/DAPI alone. Mounting medium (ProLong Gold Antifade Reagent; Invitrogen) was applied and coverslips sealed with clear nail polish. Fluorescent images were acquired at room temperature on a Zeiss Axiovert 200M inverted confocal microscope with a 40× Plan Neofluar objective using IP Lab 4.0 software (Scanalytics). Photomicrographs (0.5-μm z-sections taken through the nuclei and adjacent to the plane of adhesion to the plate) were acquired using a Hamamatsu ORCA ER HAL100 digital camera (original magnification ×400).
Mitochondrial membrane depolarization (ΔΨm)
Cells were collected and resuspended in 0.5 mL of serum-free culture medium containing 10 μg/mL JC-1 (5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolyl-arbpcyanine iodide) and assessed by flow cytometry. Mitochondrial depolarization is indicated by cells that shift from red to green fluorescence. Bandpass filters were 525 nm (± 25 nm) for JC-1 green emission and 610 nm (± 10 nm) for JC-1 red emission.
Reagents
RGDfV cyclic peptide was from the Drug Synthesis and Chemistry Branch, Developmental Therapeutics Program, Division of Cancer Treatment and Diagnosis, National Cancer Institute (NSC#707544; cilengitide, cyclo-[Arg-Gly-Asp-DPhe-NMeVal]) and from BIOMOL Research Laboratories (AM#100; cyclo-[Arg-Gly-Asp-DPhe-Val]). BOC-D-FMK (BOC-Asp(OMe)-fluoromethyl ketone) was from BIOMOL Research Laboratories. Z-VAD-FMK (Z-Val-Ala-Asp-FMK) was from Calbiochem. LatB was purchased from Alexis. STI-571 (imatinib mesylate) was a kind gift from Novartis. VN was made as described.19 Primary antibodies for Western blots were: anti–c-Abl 8E9, mouse monoclonal, 1:1000 (BD Biosciences); anti–c-Abl K-12, rabbit polyclonal, 1:1000 (Santa Cruz Biotechnology); antipoly(adenosine diphosphate-ribose) polymerase (PARP), rabbit polyclonal, 1:1000 (Cell Signaling Technology); anti-GAPDH, mouse monoclonal, 1:20 000 (Santa Cruz Biotechnology); anti–c-Abl-phospho-Y245, rabbit polyclonal, 1:1000 (Abcam); ant–c-Abl-phospho-Y412, rabbit polyclonal, 1:1000 (Abcam); anti–caspase-8, mouse monoclonal, 1:1000 (Cell Signaling Technology). All other reagents were from Sigma-Aldrich unless stated otherwise.
RNA interference
siRNA specific for c-Abl (5′-GAAGGGAGGGUGUACCAUUtt-3′) was purchased from Ambion. A nonspecific nonsilencing siRNA (AllStars Negative Control siRNA; QIAGEN) was used as negative control. HBMECs were transfected 5 hours with 100pM c-Abl siRNA, or the negative control siRNA, or no siRNA (mock), using Lipofectamine 2000 (Invitrogen).
Results
Apoptosis induced by lack of endothelial integrin αvβ3/αvβ5 engagement in HBMECs does not require cell detachment
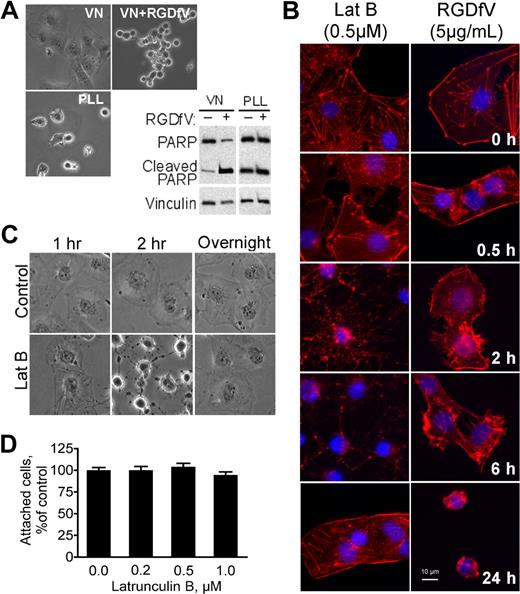
HBMECs spread well on VN (Figure 1A),19 a matrix protein that is a ligand for integrins αvβ3 and αvβ5, which are abundantly expressed and activated on endothelial cells.20,21 When treated with RGDfV, an integrin αvβ3/αvβ5 cyclic peptide inhibitor, HBMECs lost their spreading and detached from the matrix (Figure 1A).17 RGDfV-induced detachment from VN was accompanied by cell rounding and disruption of the F-actin cytoskeleton (Figure 1B). We and others have shown that inhibition of integrins αvβ3/αvβ5 by RGDfV was associated with robust apoptosis, which in HBMECs was also associated with PARP cleavage (Figure 1A).17 Apoptosis (PARP cleavage) also occurred when HBMECs were seeded on poly-L-lysine (PLL), which was blocked with HD-BSA to prevent endogenous matrix deposition by the cells (Figure 1A),17 and apoptosis in HBMECs on the HD-BSA–blocked PLL was not enhanced by RGDfV (Figure 1A). Because HBMECs can attach to the HD-BSA–blocked PLL (provides a nonintegrin-binding, positively charged matrix) but are unable to engage their integrins and spread, this suggests that lack of integrin engagement and/or loss of cell spreading, but not cell detachment, contributed to the apoptosis of the HBMECs.
LatB transiently disrupts spreading of HBMECs but does not induce cell detachment. Endothelial apoptosis induced by RGDfV does not require cell detachment. (A) HBMECs were seeded on VN or PLL, both blocked with HD-BSA, and incubated for 24 hours. Where indicated, RGDfV (5 μg/mL) was added after cells were spread for 4 hours. Cells were photographed (original magnification ×400) or harvested, and lysates were subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and Western blotting for PARP cleavage. Vinculin served as loading control. (B) HBMECs (3000 cells/well) were allowed to adhere and spread for 2 hours on VN-coated/HD-BSA–blocked 8-well chamber slides. RGDfV (5 μg/mL) or LatB (0.5μM) was added in 0.4% BSA/RPMI 1640 for 0.5, 2, 6, or 24 hours. Cells were permeabilized and stained with phalloidin, counterstained with DAPI, and photographed (original magnification ×400). (C) HBMECs were seeded on VN-coated/HD-BSA–blocked 6-well plates in 0.4% BSA/RPMI 1640. LatB (0.2μM) or vehicle was then added for 1, 2, or 20 hours (overnight), and plates were photographed. Shown are representative cells (all cells underwent the transient shape change after LatB treatment; original magnification ×400). (D) HBMECs (104 cells/well) were seeded on VN-coated/HD-BSA–blocked 48-well plates and allowed to adhere and spread for 2 hours before LatB (0.2μM) in 0.4% BSA/RPMI 1640 was added. After overnight incubation, nonadherent cells were washed off and the adherent cells were enumerated using methyl-thiazol-tetrazolium assay. Data are mean ± SD; n = 16 for each mean. P > .05.
LatB transiently disrupts spreading of HBMECs but does not induce cell detachment. Endothelial apoptosis induced by RGDfV does not require cell detachment. (A) HBMECs were seeded on VN or PLL, both blocked with HD-BSA, and incubated for 24 hours. Where indicated, RGDfV (5 μg/mL) was added after cells were spread for 4 hours. Cells were photographed (original magnification ×400) or harvested, and lysates were subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and Western blotting for PARP cleavage. Vinculin served as loading control. (B) HBMECs (3000 cells/well) were allowed to adhere and spread for 2 hours on VN-coated/HD-BSA–blocked 8-well chamber slides. RGDfV (5 μg/mL) or LatB (0.5μM) was added in 0.4% BSA/RPMI 1640 for 0.5, 2, 6, or 24 hours. Cells were permeabilized and stained with phalloidin, counterstained with DAPI, and photographed (original magnification ×400). (C) HBMECs were seeded on VN-coated/HD-BSA–blocked 6-well plates in 0.4% BSA/RPMI 1640. LatB (0.2μM) or vehicle was then added for 1, 2, or 20 hours (overnight), and plates were photographed. Shown are representative cells (all cells underwent the transient shape change after LatB treatment; original magnification ×400). (D) HBMECs (104 cells/well) were seeded on VN-coated/HD-BSA–blocked 48-well plates and allowed to adhere and spread for 2 hours before LatB (0.2μM) in 0.4% BSA/RPMI 1640 was added. After overnight incubation, nonadherent cells were washed off and the adherent cells were enumerated using methyl-thiazol-tetrazolium assay. Data are mean ± SD; n = 16 for each mean. P > .05.
LatB transiently disrupts endothelial cell spreading
To uncouple integrin binding from cell spreading, we turned to LatB, a marine sponge toxin that inhibits actin polymerization in a highly specific manner. LatB functions by binding purified G-actin to form a nonpolymerizable 1:1 complex that inhibits polymerization of G-actin and promotes depolymerization of F-actin.22 Here we used LatB to disrupt the actin cytoskeleton of HBMECs that were spread on VN without causing their detachment, thereby maintaining at least part of their integrin-mediated matrix attachment.
Within 1 to 2 hours of LatB exposure, HBMECs that were spread on VN attained a mostly round morphology, with persistence of only remnants of residual membrane protrusions (Figure 1C). Despite loss of spreading induced by LatB, cells maintained their adhesion to VN and did not detach (Figure 1C-D). Interestingly, although loss of spreading induced by LatB was complete within 1 to 2 hours, it was transient: respreading of the cells began 8 to 12 hours after start of exposure despite the continued presence of LatB in the medium. HBMEC respreading was complete after overnight incubation with LatB (Figure 1C). The changes in F-actin as visualized by phalloidin staining during the early exposure of HBMECs to LatB and to RGDfV appeared similar (Figure 1B). As early as 30 minutes after addition of LatB (0.5μM) or RGDfV (5 μg/mL), cell spreading was disrupted. At 2 to 6 hours, cells were still attached but clearly lost their spreading. Phalloidin staining showed near-complete disruption of F-actin with LatB and partial disruption with RGDfV (Figure 1B-C). F-actin fiber formation was restored after overnight incubation with LatB despite its continued presence, corresponding to respreading of the cells we observed. As expected, cell spreading remained impaired in the now-detached RGDfV-treated HBMECs at 24 hours.
Loss of endothelial spreading induced by LatB is associated with apoptosis
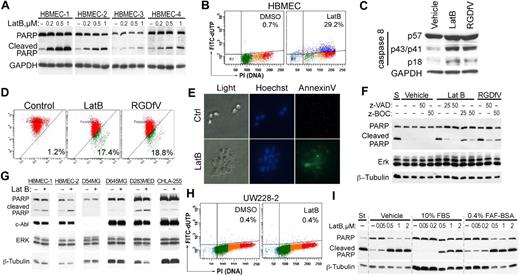
As shown in Figure 1A, HBMECs plated on the non–integrin-binding HD-BSA–blocked PLL matrix were unable to spread and underwent apoptosis. LatB, which disrupted the actin cytoskeleton and cell spreading, also induced HBMEC apoptosis, manifested by PARP cleavage and increased TdT-mediated dUTP nick end labeling (TUNEL) staining (Figure 2A-B). PARP cleavage was dependent on the dose of LatB and occurred despite HBMECs retaining at least partial αv-integrin–mediated attachment to VN during their transient loss of spreading, and despite their successful respreading by 24 hours. Other indicators of apoptosis induced by LatB were cleavage of pro-caspase-8 (57 kDa) into its p43/p41 and p18 subunits and depolarization of mitochondrial membrane potential as determined by the JC-1 mitochondrial probe, both of which were induced similarly by LatB and by RDGfV (Figure 2C-D). LatB also induced 1.72 plus or minus 0.17-fold increase in caspase-3 activity after 16 hours of incubation with 0.5μM LatB compared with vehicle (n = 5, P < .003; see also Figure 6D). Last, LatB also increased annexin V on the outer face of HBMECs in the 3D collagen drop model (Figure 2E), supporting this cell death as physiologically relevant apoptosis. We did not detect cleavage of caspase-9 or of LC3 with either LatB or RGDfV (data not shown). The pan-caspase inhibitors, zVAD-FMK and zBOC-FMK, effectively inhibited LatB-induced PARP cleavage (Figure 2F) but did not prevent the associated change in cell shape (data not shown), indicating that caspase activation was either downstream of LatB-induced cell shape change or was activated independently of disruption of actin. Apoptosis induced by the combination LatB (0.5μM) plus RGDfV (5 μg/mL; 14.7% ± 2.6%) was similar in magnitude to apoptosis by each alone (LatB, 12.6% ± 3.0%; RGDfV, 14.4% ± 5.9%; TUNEL, flow cytometry, 40-hour incubation, mean ± SEM, n = 3).
LatB induces caspase-dependent endothelial apoptosis. (A,G) Isolates of HBMECs from 4 (A) or 2 (G) different donors, or tumor cells (4 × 105/well) were allowed to spread on VN-coated/HD-BSA–blocked 6-well plates and then incubated for 24 hours with 0 to 1μM (A) or 0.2μM (G) LatB in 0.4% BSA/RPMI 1640. Whole-cell lysates were resolved on 10% SDS-PAGE and analyzed by Western blotting (PARP cleavage and c-Abl). (A) The blot of PARP cleavage for HBMECs-3 and HBMECs-4 is shown at longer exposure than for HBMECs-1 and HBMECs-2 resulting from lower PARP expression in HBMECs-3 and HBMECs-4. GAPDH or ERK 1/2 and β-tubulin served as loading controls. (B) HBMECs (2 × 105 cells/well) seeded on VN-coated/HD-BSA–blocked 6-well plates in 0.4% BSA/RPMI 1640 were treated with vehicle or LatB (0.5μM) for 48 hours. Apoptosis was assessed by flow cytometry using the Apo-Direct kit, measuring FITC-dUTP and PI content. Percentage of apoptotic cells (FITC-dUTP+ cells in top quadrants) is indicated for each condition. Shown is a representative panel from 1 experiment of 12 with similar results. (C) HBMECs (2 × 105 cells/well) were allowed to spread on VN-coated/HD-BSA–blocked 6-well plates and then incubated for 24 hours with 0.5μM LatB or 5 μg/mL RGDfV in 0.4% BSA/RPMI 1640. Whole-cell lysates were resolved on 12.5% SDS-PAGE and analyzed by Western blotting for caspase-8 cleavage. GAPDH served as loading control. (D) HBMECs treated as in panel C were analyzed by flow cytometry for effect on mitochondrial polarization. Loss of mitochondrial membrane potential (ΔΨm) was measured by flow cytometry using the JC-1 mitochondrial probe. The transition of red fluorescence to green indicates mitochondrial membrane depolarization by the drug(s). Indicated are the percentages of mitochondrial membrane-depolarized cells. (E) HBMECs (5 × 104 cells/mL) were cultured in 3D collagen as described in supplemental Data and treated with LatB (0.5μM) for 18 hours. Cells were stained with Hoechst and annexin V–FITC and photographed by light microscopy or fluorescence. Shown are representative fields from each condition (original magnification ×400). (F) HBMECs spread on VN in serum-free RPMI 1640 were preincubated with the caspase inhibitors zVAD-FMK or zBOC-FMK (25μM or 50μM) or vehicle control for 2 hours, and then LatB (0.05μM) or RGDfV (5 μg/mL) was added for another 24 hours. Whole-cell lysates were resolved on 10% SDS-PAGE and analyzed by Western blotting (PARP cleavage, ERK, β-tubulin). (H) UW228-2 medulloblastoma cells (2 × 105 cells/well) seeded on 6-well plates were treated for 48 hours with LatB (0.5μM) as in panel A, and apoptosis was analyzed by flow cytometry as in panel B. (I) HBMECs (4 × 105/well) were cultured in 6-well plates in RPMI 1640 without additions, or in the presence of 10% FBS or 0.4% FAF-BSA. After cells were spread, 0 to 2μM LatB was added for 24 hours as indicated, and lysates were processed as indicated in panel A.
LatB induces caspase-dependent endothelial apoptosis. (A,G) Isolates of HBMECs from 4 (A) or 2 (G) different donors, or tumor cells (4 × 105/well) were allowed to spread on VN-coated/HD-BSA–blocked 6-well plates and then incubated for 24 hours with 0 to 1μM (A) or 0.2μM (G) LatB in 0.4% BSA/RPMI 1640. Whole-cell lysates were resolved on 10% SDS-PAGE and analyzed by Western blotting (PARP cleavage and c-Abl). (A) The blot of PARP cleavage for HBMECs-3 and HBMECs-4 is shown at longer exposure than for HBMECs-1 and HBMECs-2 resulting from lower PARP expression in HBMECs-3 and HBMECs-4. GAPDH or ERK 1/2 and β-tubulin served as loading controls. (B) HBMECs (2 × 105 cells/well) seeded on VN-coated/HD-BSA–blocked 6-well plates in 0.4% BSA/RPMI 1640 were treated with vehicle or LatB (0.5μM) for 48 hours. Apoptosis was assessed by flow cytometry using the Apo-Direct kit, measuring FITC-dUTP and PI content. Percentage of apoptotic cells (FITC-dUTP+ cells in top quadrants) is indicated for each condition. Shown is a representative panel from 1 experiment of 12 with similar results. (C) HBMECs (2 × 105 cells/well) were allowed to spread on VN-coated/HD-BSA–blocked 6-well plates and then incubated for 24 hours with 0.5μM LatB or 5 μg/mL RGDfV in 0.4% BSA/RPMI 1640. Whole-cell lysates were resolved on 12.5% SDS-PAGE and analyzed by Western blotting for caspase-8 cleavage. GAPDH served as loading control. (D) HBMECs treated as in panel C were analyzed by flow cytometry for effect on mitochondrial polarization. Loss of mitochondrial membrane potential (ΔΨm) was measured by flow cytometry using the JC-1 mitochondrial probe. The transition of red fluorescence to green indicates mitochondrial membrane depolarization by the drug(s). Indicated are the percentages of mitochondrial membrane-depolarized cells. (E) HBMECs (5 × 104 cells/mL) were cultured in 3D collagen as described in supplemental Data and treated with LatB (0.5μM) for 18 hours. Cells were stained with Hoechst and annexin V–FITC and photographed by light microscopy or fluorescence. Shown are representative fields from each condition (original magnification ×400). (F) HBMECs spread on VN in serum-free RPMI 1640 were preincubated with the caspase inhibitors zVAD-FMK or zBOC-FMK (25μM or 50μM) or vehicle control for 2 hours, and then LatB (0.05μM) or RGDfV (5 μg/mL) was added for another 24 hours. Whole-cell lysates were resolved on 10% SDS-PAGE and analyzed by Western blotting (PARP cleavage, ERK, β-tubulin). (H) UW228-2 medulloblastoma cells (2 × 105 cells/well) seeded on 6-well plates were treated for 48 hours with LatB (0.5μM) as in panel A, and apoptosis was analyzed by flow cytometry as in panel B. (I) HBMECs (4 × 105/well) were cultured in 6-well plates in RPMI 1640 without additions, or in the presence of 10% FBS or 0.4% FAF-BSA. After cells were spread, 0 to 2μM LatB was added for 24 hours as indicated, and lysates were processed as indicated in panel A.
The LatB-induced dose-dependent apoptosis occurred in 4 independent HBMEC isolates we examined (Figure 2A). However, we did not observe LatB-induced apoptosis (PARP cleavage, TUNEL stain) in 5 tumor cell lines (neuroblastoma, medulloblastomas, gliomas, Figure 2G-H; supplemental Figure 1), indicating that, although LatB-induced apoptosis in 4 of 4 isolates of microvascular endothelial cells, this was not universal in all cell types.
We next determined whether serum could protect HBMECs from LatB-induced PARP cleavage (Figure 2I). Serum starvation itself was accompanied by only low-level PARP cleavage in HBMECs seeded on VN, which was inhibited by inclusion of 10% fetal bovine serum (FBS) in the culture medium (Figure 2I). Although 10% FBS somewhat diminished LatB-induced PARP cleavage, FBS could not prevent it. Replacing FBS with 0.4% fatty acid-free (FAF) BSA (non–heat-denatured) in the medium to provide drug-binding capacity consistent with that provided by 10% FBS did not prevent PARP cleavage induced by LatB (Figure 2I; and data not shown). Therefore, all subsequent experiments were performed in the presence of 0.4% fatty acid-free BSA.
These data indicate that, despite adhesion to the integrin αvβ3/αvβ5-binding VN matrix, transient disruption of actin microfilament organization and transient loss of cell spreading induced by LatB were associated with endothelial cell apoptosis.
c-Abl is transiently phosphorylated and translocated to the nucleus during LatB and RGDfV treatment
In view of the known reciprocal interactions between actin and c-Abl,13,14,23 we next asked whether c-Abl was involved in endothelial apoptosis induced by LatB and/or by RGDfV-induced αvβ3/αvβ5-integrin inhibition. We first examined the effect of LatB and RGDfV on phosphorylation of tyrosines Y412 and Y245 of c-Abl in HBMECs spread on VN.
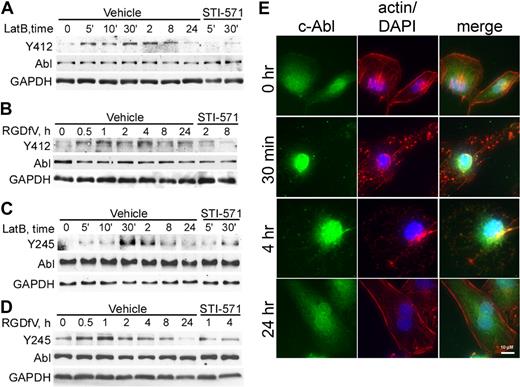
Tyrosine Y412 is located within the c-Abl kinase domain, is phosphorylated in oncogenic forms of Abl, and is the site of BCR-ABL1 inhibition by the targeted therapy for chronic myeloid leukemia, STI-571. Tyrosine Y245 is located in the SH2-kinase-domain linker and contributes to the autoactivation of c-Abl.13 Both Y412 and Y245 of c-Abl showed minimally detectable baseline phosphorylation in HBMEC spread on VN (Figure 3A-D), which increased as early as 5 minutes after addition of 0.5μM LatB (Figure 3A,C). Treatment with RGDfV also increased tyrosine phosphorylation of c-Abl, but after a longer delay (0.5-1 hour; Figure 3B,D). Delay of c-Abl phosphorylation induced by RGDfV compared with LatB may be related to the slower effect RGDfV may have on integrin ligation or to the slower loss of cell spreading triggered by RGDfV (2-6 hours) compared with LatB (1-2 hours; Figure 1B). This c-Abl phosphorylation increased in a time-dependent manner, peaked (30 minutes for LatB and 1-4 hours for RGDfV; Figure 3A-D), and then declined.
c-Abl is phosphorylated and undergoes subcellular localization change in the presence of LatB and RGDfV. (A-D) HBMECs (2 × 105 cells/well) were seeded on VN-coated/HD-BSA–blocked 6-well plates in 0.4% BSA/RPMI 1640 and treated with LatB (0.5μM; A,C) or RGDfV (5 μg/mL; B,D) for the times indicated. (A,C) The short time points (5-30 minutes) are labeled as 5′, 10′, and 30′, and the remainder of the lanes were incubated for 2 to 24 hours. Where indicated, STI-571 (5μM) was also included in the medium, starting 30 minutes before LatB or RGDfV. Whole-cell lysates were resolved on 10% SDS-PAGE and analyzed by Western blotting for c-Abl (SH2 domain, 8E9 antibody) and tyrosine phosphorylation, with either phospho-c-Abl-Y412 (A-B) or phospho-c-Abl-Y245 (C-D). GAPDH served as loading control. (E) To examine c-Abl translocation, HBMECs (3000 cells/well) were allowed to adhere and spread overnight on VN-coated/HD-BSA–blocked 8-well chamber slides. LatB (0.2μM) was added in 0.4% BSA/RPMI 1640 for 0.5, 4, or 24 hours. Cells were permeabilized and stained with phalloidin (actin, red), c-Abl (8E9, green), counterstained with DAPI (nucleus, blue), and photographed. Shown is representative field (original magnification ×400).
c-Abl is phosphorylated and undergoes subcellular localization change in the presence of LatB and RGDfV. (A-D) HBMECs (2 × 105 cells/well) were seeded on VN-coated/HD-BSA–blocked 6-well plates in 0.4% BSA/RPMI 1640 and treated with LatB (0.5μM; A,C) or RGDfV (5 μg/mL; B,D) for the times indicated. (A,C) The short time points (5-30 minutes) are labeled as 5′, 10′, and 30′, and the remainder of the lanes were incubated for 2 to 24 hours. Where indicated, STI-571 (5μM) was also included in the medium, starting 30 minutes before LatB or RGDfV. Whole-cell lysates were resolved on 10% SDS-PAGE and analyzed by Western blotting for c-Abl (SH2 domain, 8E9 antibody) and tyrosine phosphorylation, with either phospho-c-Abl-Y412 (A-B) or phospho-c-Abl-Y245 (C-D). GAPDH served as loading control. (E) To examine c-Abl translocation, HBMECs (3000 cells/well) were allowed to adhere and spread overnight on VN-coated/HD-BSA–blocked 8-well chamber slides. LatB (0.2μM) was added in 0.4% BSA/RPMI 1640 for 0.5, 4, or 24 hours. Cells were permeabilized and stained with phalloidin (actin, red), c-Abl (8E9, green), counterstained with DAPI (nucleus, blue), and photographed. Shown is representative field (original magnification ×400).
Involvement of c-Abl was also seen when examining c-Abl subcellular localization in LatB-treated HBMECs (Figure 3E). In resting well-spread HBMECs, c-Abl was localized in both cytoplasm and nucleus. Within 30 minutes of adding LatB, cytoplasmic c-Abl decreased and nuclear c-Abl increased and by 24 hours, when HBMECs have respread, c-Abl was again abundant in both nucleus and cytoplasm. Interestingly, 2 medulloblastoma cell lines (UW-228-2 and D283MED), which were resistant to LatB-induced apoptosis, did not show nuclear accumulation of c-Abl after LatB (supplemental Figure 1; Figure 2H).
STI-571 is a pharmacologic inhibitor of several kinases, including Abl kinase. It binds to the inactive conformation of Abl and occupies the adenosine triphosphate-binding site in a manner competitive with adenosine triphosphate. Because Y412 is the target for STI-571 in c-Abl, we examined whether STI-571 could inhibit LatB- and RGDfV-induced phosphorylation of c-Abl. Indeed, both LatB- and RGDfV-induced phosphorylation of c-Abl-Y412, as well as phosphorylation of Y245, were inhibited by STI-571 (Figure 3A-D).
These data demonstrate that LatB induces c-Abl nuclear translocation in HBMECs and that both LatB and RGDfV induce transient STI-571-inhibitable phosphorylation of c-Abl on Y412 and Y245. Taken together, this supports involvement of c-Abl in the apoptotic response of HBMECs to LatB and RGDfV.
STI-571 inhibits endothelial apoptosis induced by RGDfV and LatB
To investigate whether c-Abl was required in LatB- and RGDfV-induced HBMECs apoptosis, we examined whether STI-571 can inhibit apoptosis induced by LatB and RGDfV. Indeed, STI-571 inhibited PARP cleavage induced by LatB and by RGDfV in HBMECs by as much as 60% to 70% (Figure 4A). STI-571 also inhibited LatB- and RGDfV-induced TUNEL stain (Figure 4B-C), mitochondrial depolarization (Figure 4D-E), and caspase-8 activity and cleavage (Figure 4F-H), as well as LatB-induced annexin V staining of HBMECs in a 3D collagen drop model (Figure 4I). However, etoposide-induced PARP cleavage was not affected by STI-571, indicating that not all HBMEC apoptosis was inhibited by STI-571 (supplemental Figure 2A).
STI-571 decreases apoptosis induced by LatB and RGDfV in HBMECs. HBMECs (2 × 105 cells/well) were seeded and allowed to spread on VN-coated/HD-BSA–blocked 6-well plates in 0.4% BSA/RPMI 1640. Cells were then treated for 16 hours (I), 24 hours (A, D-H), or 48 hours (B-C) with vehicle, LatB (0.5μM) or RGDfV (5 μg/mL). Where indicated, STI-571 (5μM) was included 30 minutes before LatB or RGDfV. (A) Whole-cell lysates were resolved on 10% SDS-PAGE and analyzed by Western blotting for PARP cleavage. GAPDH served as loading control. (B-C) Apoptosis was assessed by flow cytometry using the Apo-Direct kit, measuring FITC-dUTP and PI content. Percentage of apoptotic cells in the 2 top quadrants is indicated. (B) Mean ± SEM of 3 independent experiments performed in triplicate as in panel C. P < .001 between presence or absence of STI-571 for each pair. (C) One representative experiment (percentage of apoptotic cells in the 2 top quadrants) is indicated. (D-E) Loss of mitochondrial membrane potential (ΔΨm) was measured by flow cytometry using JC-1 mitochondrial probe. (D) Representative flow cytometry plots. The values indicated represent the percentage of cells with depolarized mitochondrial membrane. The bars in panel E represent mean ± SEM percentage of cells with depolarized mitochondrial membrane potential from 2 experiments performed in triplicate. P < .001 between LatB with or without STI-571. P = .044 for RGDfV with or without STI-571. (F) Whole-cell lysates were analyzed for caspase-8 activity using the ApoTarget caspase-8/FLICE colorimetric protease assay. Bars represent mean ± SEM. P < .001 between LatB treatment with or without STI-571, P < .001 between RGDfV treatment with or without STI-571 (n = 4 replicates, shown is 1 of 2 experiments with similar results). (G-H) Whole- cell lysates were resolved on 12.5% SDS-PAGE and analyzed by Western blotting for caspase-8 cleavage. GAPDH served as loading control. (H) Mean densitometry of the cleaved caspase-8 p43/p41 fragment relative to GAPDH in 3 separate experiments. (I) HBMECs were cultured in 3D collagen as described in “Methods.” Cells were stained with Hoechst and annexin V–FITC, and photographed (original magnification ×400).
STI-571 decreases apoptosis induced by LatB and RGDfV in HBMECs. HBMECs (2 × 105 cells/well) were seeded and allowed to spread on VN-coated/HD-BSA–blocked 6-well plates in 0.4% BSA/RPMI 1640. Cells were then treated for 16 hours (I), 24 hours (A, D-H), or 48 hours (B-C) with vehicle, LatB (0.5μM) or RGDfV (5 μg/mL). Where indicated, STI-571 (5μM) was included 30 minutes before LatB or RGDfV. (A) Whole-cell lysates were resolved on 10% SDS-PAGE and analyzed by Western blotting for PARP cleavage. GAPDH served as loading control. (B-C) Apoptosis was assessed by flow cytometry using the Apo-Direct kit, measuring FITC-dUTP and PI content. Percentage of apoptotic cells in the 2 top quadrants is indicated. (B) Mean ± SEM of 3 independent experiments performed in triplicate as in panel C. P < .001 between presence or absence of STI-571 for each pair. (C) One representative experiment (percentage of apoptotic cells in the 2 top quadrants) is indicated. (D-E) Loss of mitochondrial membrane potential (ΔΨm) was measured by flow cytometry using JC-1 mitochondrial probe. (D) Representative flow cytometry plots. The values indicated represent the percentage of cells with depolarized mitochondrial membrane. The bars in panel E represent mean ± SEM percentage of cells with depolarized mitochondrial membrane potential from 2 experiments performed in triplicate. P < .001 between LatB with or without STI-571. P = .044 for RGDfV with or without STI-571. (F) Whole-cell lysates were analyzed for caspase-8 activity using the ApoTarget caspase-8/FLICE colorimetric protease assay. Bars represent mean ± SEM. P < .001 between LatB treatment with or without STI-571, P < .001 between RGDfV treatment with or without STI-571 (n = 4 replicates, shown is 1 of 2 experiments with similar results). (G-H) Whole- cell lysates were resolved on 12.5% SDS-PAGE and analyzed by Western blotting for caspase-8 cleavage. GAPDH served as loading control. (H) Mean densitometry of the cleaved caspase-8 p43/p41 fragment relative to GAPDH in 3 separate experiments. (I) HBMECs were cultured in 3D collagen as described in “Methods.” Cells were stained with Hoechst and annexin V–FITC, and photographed (original magnification ×400).
Despite inhibition of both LatB-induced c-Abl phosphorylation and the associated HBMEC apoptosis by STI-571 (Figures 3–4), LatB-induced disruption in cell shape proceeded unhampered in the presence of STI-571 (Figure 5). This indicated that, in HBMECs: (1) c-Abl functioned downstream of LatB-induced loss of cell spreading, (2) the LatB effect on c-Abl phosphorylation was independent of the change in cell shape, or (3) less likely, c-Abl was not the STI-571 target that was inhibited to block the LatB-induced apoptosis.
STI-571 does not alter the change in cell shape induced by LatB. HBMECs were seeded on VN blocked with HD-BSA and preincubated (2 hours) with STI-571 (5μM). LatB (0.5μM) or vehicle was added for the duration indicated, and cells were photographed (original magnification ×400).
STI-571 does not alter the change in cell shape induced by LatB. HBMECs were seeded on VN blocked with HD-BSA and preincubated (2 hours) with STI-571 (5μM). LatB (0.5μM) or vehicle was added for the duration indicated, and cells were photographed (original magnification ×400).
c-Abl is required in apoptosis induced by RGDfV and LatB
In addition to inhibition of Abl, STI-571 also inhibits the platelet-derived growth factor receptor (PDGFR) and the stem cell factor receptor, c-Kit. However, PDGFR and c-Kit are growth-stimulatory receptor tyrosine kinases not known to have proapoptotic effects, and are not likely to be the mediators of LatB- and RGDfV-induced HBMEC apoptosis. Therefore, we next examined whether up-regulating c-Abl would enhance LatB-induced apoptosis. Overexpressing wild-type c-Abl by itself did not induce HBMEC apoptosis, nor did it augment LatB- or RGDfV-induced apoptosis (supplemental Figure 3B). Transient overexpression of constitutively active v-Abl or Abl-PP (P242E/P249E, a constitutively active mutant with double proline mutation in the regulatory SH2-CD linker) also did not induce apoptosis nor accelerate apoptosis by LatB (supplemental Figure 3A-B). Densitometry shows that v-Abl even slightly protected HBMECs from LatB-induced apoptosis (supplemental Figure 3A), consistent with its reported oncogenic function.24 A possible explanation is that v-Abl and Abl-PP are both defective in nuclear translocation and localize to the cytoplasm,24,25 whereas a number of stimuli that induce c-Abl–mediated apoptosis require Abl nuclear localization.26-28
To definitively determine whether c-Abl mediates the LatB- and RGDfV-induced endothelial apoptosis, we down-regulated c-Abl using siRNA. We found that siRNA to c-Abl decreased apoptosis induced by LatB and by RGDfV compared with mock and nonspecific siRNA-negative controls as indicated by decreased TUNEL stain and decreased PARP cleavage (Figure 6A-C). c-Abl siRNA decreased c-Abl expression by more than 90% at 48 to 72 hours in HBMECs compared with nonspecific siRNA-negative control and mock transfection but did not down-regulate GAPDH (Figure 6C). Figure 6D shows that c-Abl-siRNA also mitigated LatB-induced activation of caspase-3 compared with scrambled siRNA. These data demonstrate that knockdown of c-Abl by siRNA mitigates endothelial apoptosis induced by LatB and RGDfV, indicating that c-Abl mediates at least part of the LatB- and RGDfV-induced apoptosis.
c-Abl is required in apoptosis induced by LatB and RGDfV. HBMECs (2 × 105 cells/well) seeded overnight on VN-coated/HD-BSA–blocked 6-well plates in 0.4% BSA/RPMI 1640 were transfected with nonspecific nonsilencing negative control siRNA, or c-Abl siRNA (100pM) for 5 hours or were mock-transfected (Lipofectamine 2000 without siRNA). LatB (0.5μM) or RGDfV (5 μg/mL) was added 30 hours after transfection. M indicates mock control; NC, nonspecific nonsilencing negative siRNA control; si, c-Abl siRNA. (A-B) Cells were collected 40 hours after LatB (0.5μM) or RGDfV (5 μg/mL) treatment (70 hours after transfection), and apoptosis was assessed by flow cytometry using the Apo-Direct kit, measuring FITC-dUTP and PI content. Percentage of apoptotic cells in the top quadrants is indicated. (A) Representative experiment of 3 with similar results. (B) Mean ± SEM of the 3 experiments. P < .001 between c-Abl siRNA and each of the controls (mock and nonspecific siRNA) in the presence of LatB- or RGDfV-treated groups. (C) Whole-cell lysates were collected after 24 hours of LatB (0.5μM) or RGDfV (5 μg/mL) treatment (54 hours after transfection) and analyzed by Western blotting for PARP cleavage and c-Abl (K-12 antibody). GAPDH served as loading control. (D) Whole-cell lysates were collected 24 hours after LatB (0.5μM) treatment (54 hours after transfection) and analyzed for caspase-3 activity using the ApoTarget caspase-3/CPP32 colorimetric protease assay. Bars represent mean ± SEM. P = .029 between nonspecific nonsilencing negative siRNA control with or without LatB. P = .18 between c-Abl-siRNA with or without LatB (n = 5 for each condition).
c-Abl is required in apoptosis induced by LatB and RGDfV. HBMECs (2 × 105 cells/well) seeded overnight on VN-coated/HD-BSA–blocked 6-well plates in 0.4% BSA/RPMI 1640 were transfected with nonspecific nonsilencing negative control siRNA, or c-Abl siRNA (100pM) for 5 hours or were mock-transfected (Lipofectamine 2000 without siRNA). LatB (0.5μM) or RGDfV (5 μg/mL) was added 30 hours after transfection. M indicates mock control; NC, nonspecific nonsilencing negative siRNA control; si, c-Abl siRNA. (A-B) Cells were collected 40 hours after LatB (0.5μM) or RGDfV (5 μg/mL) treatment (70 hours after transfection), and apoptosis was assessed by flow cytometry using the Apo-Direct kit, measuring FITC-dUTP and PI content. Percentage of apoptotic cells in the top quadrants is indicated. (A) Representative experiment of 3 with similar results. (B) Mean ± SEM of the 3 experiments. P < .001 between c-Abl siRNA and each of the controls (mock and nonspecific siRNA) in the presence of LatB- or RGDfV-treated groups. (C) Whole-cell lysates were collected after 24 hours of LatB (0.5μM) or RGDfV (5 μg/mL) treatment (54 hours after transfection) and analyzed by Western blotting for PARP cleavage and c-Abl (K-12 antibody). GAPDH served as loading control. (D) Whole-cell lysates were collected 24 hours after LatB (0.5μM) treatment (54 hours after transfection) and analyzed for caspase-3 activity using the ApoTarget caspase-3/CPP32 colorimetric protease assay. Bars represent mean ± SEM. P = .029 between nonspecific nonsilencing negative siRNA control with or without LatB. P = .18 between c-Abl-siRNA with or without LatB (n = 5 for each condition).
Taken together, our data demonstrate that endothelial apoptosis induced by integrin αvβ3/αvβ5 inhibition (RGDfV) and by LatB, which is associated with loss of cell spreading and disruption of F-actin, is at least partially mediated by c-Abl. Furthermore, STI-571 protected HBMECs from the apoptosis induced by LatB and by integrin αvβ3/αvβ5 inhibition.
Discussion
Attempts to differentiate between loss of integrin ligation and loss of cell spreading as a cause for apoptosis have been reported previously. Using micropatterned matrix-coated islands, Chen et al have shown that cell shape per se was a critical determinant of endothelial apoptosis.29 However, in their experiments, survival in endothelial cells that spread on VN was only mildly affected by change in cell shape compared with the robust apoptosis in cells that lost spreading on fibronectin or on collagen.29 This suggested that even small residual surface area of ligated integrins αvβ3/αvβ5 may be sufficient to overcome the proapoptotic effect of loss of endothelial cell spreading on VN. Therefore, it was interesting to find that disruption of cell spreading using the specific F-actin polymerization inhibitor LatB induced apoptosis in the HBMECs despite the cells remaining attached to the VN matrix (Figure 2). This LatB-induced apoptosis was not a general phenomenon because it only occurred in the 4 isolates of endothelial cells we tested, but not in 5 tumor cell lines we tested (Figure 2; supplemental Figure 1). Apoptosis after LatB was also not observed in the HT-29 colon cancer cell line, which undergoes apoptosis when detached from matrix but not when treated with LatB (1-5 μg/mL)30 nor in the M24Met melanoma cell line (Y. DeClerck, Childrens Hospital Los Angeles, written communication, May 24, 2009). Also interesting is that endothelial apoptosis occurred, although LatB-induced disruption of HBMECs shape was only transient and spreading was restored to baseline despite the continued presence of LatB. This suggested that the disruption of F-actin dynamics and transient change in cell shape induced by LatB were sufficient to trigger an irreversible signal to apoptosis, which continued unabated despite the return to normal cell shape and integrin αvβ3/αvβ5 engagement. This may not be surprising considering that actin serves as a hub for many integrin-induced signaling cascades. However, although changes in the dynamic state of F-actin in yeast and in mammalian cells can trigger apoptosis31 and although LatB is regarded as a specific inhibitor of F-actin polymerization,32 we cannot rule out that the apoptosis triggered in HBMECs by LatB was a direct effect of LatB that is independent of the transient change in the actin cytoskeleton.
Abl, a ubiquitously expressed nonreceptor tyrosine kinase, is oncogenic in chronic myelogenous leukemia and some acute leukemias with chromosomal translocation that forms the cytoplasmic fusion protein, BCR-ABL1. Therefore, it is not surprising that inhibitors of Abl, such as imatinib, are effective agents in these leukemias. Although wild-type cytoplasmic c-Abl participates in the stimulatory response to growth factors,25,33,34 the function of c-Abl is diverse and nuclear c-Abl can negatively regulate cell proliferation35 and can mediate apoptosis in response to γ-irradiation- and chemotherapy-induced DNA damage,26,36 serum starvation,37 and inhibition of the 26S proteosome.38 In addition, c-Abl undergoes dynamic nuclear-cytoplasmic shuttling and reciprocally participates in remodeling of F-actin.13,39 In fibroblasts, transient c-Abl activation occurs after integrin-mediated attachment to fibronectin, with decreased activation after detachment from matrix.12,14,40 However, the effect of integrin-mediated attachment on c-Abl activation varies depending on the cells studied.38,41 Our data demonstrate that both inhibition of F-actin by LatB as well as inhibition of integrin αvβ3/αvβ5 by the cyclic peptide RGDfV in HBMECs caused phosphorylation of endogenous c-Abl on both Y412 (catalytic domain) and Y245 (SH2-domain-linker). Because sequential phosphorylation of Y412 and Y245 is considered necessary for full activation of c-Abl,42 this suggests that c-Abl was activated by LatB and by RGDfV in HBMECs. Phosphorylation began within 5 minutes of exposing HBMECs to LatB and by 30 minutes for RGDfV (Figure 3), reminiscent of the different kinetics of disruption of cell shape observed with the 2 agents (Figure 1). For comparison, in 10T½ fibroblasts, maximal activation of c-Abl is observed within minutes of seeding the cells on a fibronectin matrix and engaging α5 integrins, and is absent in cells in suspension.40 Interestingly, regardless of whether the HBMECs respread (LatB) or detached from the matrix (RGDfV), c-Abl phosphorylation was only transient. The initial phosphorylation and its subsequent decline in the presence of LatB suggests that, in HBMECs, c-Abl activation may be induced by the loss of cell spreading and the dephosphorylation may be linked to the respreading of the cells and recovery of F-actin structure. However, this does not explain the dephosphorylation at later time points in RGDfV-treated HBMECs when they detach from the matrix, which is more reminiscent of the decreased phosphorylation and decreased activity of c-Abl in fibroblasts in suspension (Figure 3).12,14,40,43 One explanation could be that the direct effect of LatB and the integrin-mediated effect of RGDfV on F-actin result in different effects on F-actin dynamics, as suggested by the phalloidin stain (Figure 1C), resulting in a different effect on c-Abl. It is also possible that c-Abl phosphorylation was transient because of downstream activation of phosphatases, which may serve in negative feedback regulation to limit duration of c-Abl activation, as previously suggested,44 or that the phosphorylated c-Abl undergoes cleavage and subsequent degradation38,45,46 whereas the nonphosphorylated c-Abl remains unchanged.
Our data demonstrate that endothelial apoptosis induced by inhibition of integrins αvβ3/αvβ5 (RGDfV) and by LatB was mediated by c-Abl because apoptosis resulting from each was blocked by STI-571 as well as by siRNA to c-Abl. Different from our data, in fibroblasts there is decreased activation of c-Abl on detachment from a fibronectin matrix and requirement for integrin-mediated cell adhesion to induce Abl activation.40,43 Interestingly, c-Abl also mediates the adhesion-induced sensitivity of cells to apoptosis by chemotherapy, which is a highly cell-specific event.41 Contributing to the heterogeneity of LatB effect in cells of different origin, our data also show that HBMEC apoptosis induced by LatB, which was mediated by c-Abl, was not a universal effect because tumor cell lines we and others examined did not exhibit LatB-induced apoptosis.30 Further specificity of the role of c-Abl in HBMEC apoptosis is suggested because, whereas LatB-induced apoptosis in HBMECs was inhibited by STI-571 and siRNA to c-Abl, etoposide-induced PARP cleavage was not inhibited by STI-571, and etoposide- and vincristine-induced caspase-8 cleavage was also not inhibited by siRNA to c-Abl (supplemental Figure 2).
Relevant to cell adhesion, c-Abl has reciprocal regulatory interactions with F-actin that are mediated through the F-actin binding domain in the C-terminus of c-Abl.12,14,23 Thus, it is possible that c-Abl may act either downstream of changes in F-actin and/or upstream of them, or both. In Figure 5, the LatB-induced proapoptotic effect of c-Abl seemed to be exerted downstream of disruption of F-actin because inhibition of c-Abl by STI-571 and siRNA did not prevent the change in cell shape and the disruption of F-actin. However, our data do not rule out a possibility that the c-Abl–mediated induction of apoptosis by LatB was independent of the effect of LatB on F-actin and cell shape or that there is a dual effect with c-Abl functioning both upstream and downstream of F-actin.
Yan et al recently showed that overexpression of wild-type c-Abl in hTERT- and SV40 large T-immortalized endothelial cells enhanced basic fibroblast growth factor (bFGF)–induced (but not VEGF-induced) proliferation, in vivo tube formation, Matrigel plug vascularization, and subcutaneous MDA-MB-231/endothelial coxenograft tumor growth.47 This was reversed in cells overexpressing the K290R kinase-dead c-Abl, suggesting that c-Abl mediates the proangiogenic effects of bFGF on endothelial cells.47 This is different from our findings, in which endogenous c-Abl mediated a proapoptotic effect on endothelial cells, and its inhibition protected the endothelial cells from apoptosis. The difference may be related to our reliance on endogenous c-Abl and the use of native untransfected endothelial cells without induced immortalization, or differences in the stimuli used: bFGF in the investigation by Yan et al47 compared with inhibition of integrin ligation and F-actin dynamics in our work. Both differences may be related to the intracellular localization of Abl in the different scenarios and its nuclear-cytosolic shuttling, where preferential cytosolic localization would be associated with bFGF-induced increased proliferation, but nuclear localization would favor apoptosis.26-28,35 In our HBMECs, endogenous c-Abl, which is already abundant in the nucleus of resting cells, further increased its nuclear localization early after LatB addition (Figure 3E). However, LatB did not seem to increase nuclear localization of c-Abl in the 2 LatB-resistant tumor cell lines we examined (Figure 2G-H; supplemental Figure 1), possibly explaining their resistance to LatB-induced apoptosis. It is also possible that failure to increase LatB-induced apoptosis in HBMECs overexpressing the constitutively active mutants of Abl (v-Abl and Abl-PP) may be the result of their reported defective nuclear translocation and preferential cytoplasmic localization.24,25 Further work will be needed to decipher the complex relationship and molecular ordering between LatB-induced F-actin disruption and cell shape change, c-Abl transient phosphorylation, c-Abl nuclear-cytoplasmic shuttling, and apoptosis in the HBMECs.
STI-571, an inhibitor of BCR-ABL1, c-Abl, PDGFR, and c-Kit, is the first in a family of efficient targeted therapies for chronic myelogenous leukemia. Use of imatinib and its analog dasatinib (which also inhibits Src family kinases) is also being examined in a number of other cancers. STI-571 has been shown to have antiangiogenic effect on endothelial cells from bone marrow, mediated by inhibition of PDGFRα receptors that are expressed on bone microvascular endothelial cells.48 In mouse tumor models, imatinib also exhibited antiangiogenic effects, but these were through inhibition of pericyte coverage and inhibition of PDGFR-dependent angiogenic growth factor secretion from carcinoma-associated fibroblasts rather than direct effect on endothelial cells.49 On the other hand, a protective effect of STI-571 was seen in vivo in a mouse model of Niemann-Pick type C, where STI-571 increased survival of cerebellar cells by inhibiting c-Abl.50 In our study, STI-571 protected brain microvascular endothelial cells from the apoptotic effect of RGDfV by suppression of the ability of c-Abl to mediate apoptosis. These findings underscore the potential heterogeneity of imatinib's effects, which may stem from its multiple targets and its different functions in different cells and different settings. This complex biology will need to be addressed when developing new combination therapies that include imatinib or dasatinib, and will require testing each combination in the relevant cancer and organ.
In conclusion, we have demonstrated that transient disruption of F-actin by latrunculin B and inhibition of integrins αvβ3/αvβ5 by RGDfV are associated with transient c-Abl phosphorylation and nuclear localization in HBMECs, and that c-Abl in turn mediates the ensuing endothelial apoptosis (Figure 7). Taken together, these data provide evidence for a mechanistic role for c-Abl in endothelial apoptosis induced by LatB and by inhibition of integrins αvβ3/αvβ5.
Model summary of integrins, actin, c-Abl, and apoptosis. Our data support a model in which under baseline conditions, integrin signaling supports polymerization of F-actin and F-actin maintains c-Abl in its inactive state, thus permitting survival (left). Inhibition of integrin αvβ3/αvβ5 by RGDfV or treatment with LatB both results in decreased F-actin, which then relieves the inhibition from c-Abl, allowing c-Abl to mediate apoptosis (right). Inhibition of the activated c-Abl using STI-571 (right) can inhibit the apoptosis despite the continued disruption of F-actin.
Model summary of integrins, actin, c-Abl, and apoptosis. Our data support a model in which under baseline conditions, integrin signaling supports polymerization of F-actin and F-actin maintains c-Abl in its inactive state, thus permitting survival (left). Inhibition of integrin αvβ3/αvβ5 by RGDfV or treatment with LatB both results in decreased F-actin, which then relieves the inhibition from c-Abl, allowing c-Abl to mediate apoptosis (right). Inhibition of the activated c-Abl using STI-571 (right) can inhibit the apoptosis despite the continued disruption of F-actin.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Mr Jason Scott for expert technical assistance, Ms Kelly Engell for advice on immunofluorescence microscopy, and Dr Daneila Barila (Rome, Italy) for kindly providing Abl plasmids.
This work was supported by the National Institutes of Health (grants CA98568 and CA112177) and in part by Grayson's Gift, the Nautica Malibu Triathlon Fund, the Bogart Pediatric Cancer Research Program, and the T. J. Martell Foundation for Leukemia, Cancer, and AIDS Research.
National Institutes of Health
Authorship
Contribution: J.X. planned and performed experiments, analyzed and interpreted data, and cowrote, edited, and reviewed the manuscript; M.M., X.R., and O.T.C. performed experiments, analyzed and interpreted data, and reviewed the manuscript; and A.E.-E. designed the research, planned experiments, analyzed and interpreted data, and cowrote, edited, and reviewed the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Anat Erdreich-Epstein, Childrens Hospital Los Angeles, 4650 Sunset Blvd, Mailstop #57, Los Angeles, CA 90027; e-mail: epstein@usc.edu.