In this issue of Blood, McGrath et al provide new insights into the influence of glycosylation on VWF proteolysis by ADAMTS13.1
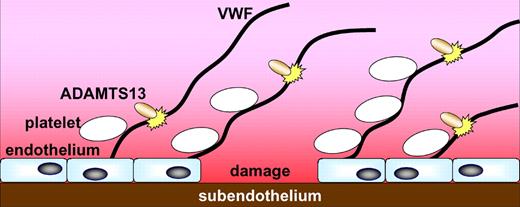
Von Willebrand factor (VWF) is synthesized in vascular endothelial cells and megakaryocytes and undergoes extensive processing prior to secretion. This processing includes the addition of carbohydrates (including the monosaccharide sialic acid) and polymerization into ultra-large (UL) multimers. These are stored in Weibel-Palade bodies in endothelial cells and α granules in platelets and are secreted in response to appropriate stimuli. Immediately on secretion, UL multimers are partially proteolyzed by the metalloprotease ADAMTS13 (a disintegrin and metalloprotease with thrombospondin repeats; see figure). This is an essential step in VWF biochemistry: the UL multimers are highly thrombogenic and their partial breakdown regulates their thrombotic potential. ADAMTS13 proteolysis gives rise to the heterogeneous distribution of VWF multimers in plasma. The fact that the length-distribution of VWF multimers in plasma appears to be remarkably similar between most of us indicates that proteolysis is tightly regulated. Factors that influence proteolysis may therefore be of physiologic and pathologic relevance.
At a site of vessel damage, endothelial cells release ultra-large VWF multimers; these recruit platelets to the site. ADAMTS13 proteolysis of VWF helps limit this process. McGrath et al show that sialic acid on VWF promotes cleavage by ADAMTS13; this may have physiologic and pathologic relevance.
At a site of vessel damage, endothelial cells release ultra-large VWF multimers; these recruit platelets to the site. ADAMTS13 proteolysis of VWF helps limit this process. McGrath et al show that sialic acid on VWF promotes cleavage by ADAMTS13; this may have physiologic and pathologic relevance.
The importance of VWF breakdown by ADAMTS13 is evidenced by the pathologies that arise when proteolysis is defective. Hyper-susceptibility of VWF to ADAMTS13 is found in a subset of type 2A von Willebrand disease (VWD); the excessive proteolysis leads to a loss of large (and sometimes intermediate) multimers from the plasma, with an associated loss of hemostatic function.2 In contrast, deficient ADAMTS13 activity is characteristic of thrombotic thrombocytopenic purpura, in which persistence of UL VWF multimers in the blood predisposes to the spontaneous formation of platelet-VWF aggregates in the microvasculature with potentially life-threatening consequences.3
VWD type 2A and thrombotic thrombocytopenic purpura represent extremes of defective ADAMTS13-mediated VWF breakdown; however, even within the normal range for proteolysis, pathologic states may arise depending on the biochemistry of each person. For example, marginally increased susceptibility of VWF to proteolysis has been found in association with the cysteine 1584 amino acid variant of the protein4 ; this variant is enriched in type 1 VWD,5 but not all people who have the variant have this bleeding disorder—other constitutive determinants must be involved.
McGrath et al reveal that terminal sialic acid residues on VWF glycans promote ADAMTS13-mediated proteolysis. VWF sialylation may therefore contribute to heterogeneity in ADAMTS13-mediated VWF cleavage between persons, and may be a determinant of VWF multimer composition. Through both of these possibilities, VWF sialylation may contribute toward heterogeneity in the hemostatic efficiency of VWF between persons.
It has previously been shown that the N-linked glycans of VWF possess ABO(H) blood group sugars and that these influence the rate of VWF breakdown by ADAMTS13. McGrath et al provide evidence that sialic acid plays a more important role than ABO(H) antigens in influencing proteolysis and it may actually be the degree of sialylation modulated by ABO blood group that alters proteolysis, not ABO blood group itself.
In addition to this newly discovered role of sialic acid in VWF proteolysis, the monosaccharide is known to play an important part in clearance of the protein from the plasma. In animal models, asialo-VWF is cleared much more rapidly than sialylated VWF and there is adequate evidence to support the involvement of the hepatic asialoglycoprotein receptor in this process.6,7 McGrath et al propose that sialic acid added to VWF within the cell of synthesis specifically enhances ADAMTS13 cleavage at the cell surface upon secretion, and that the sialic acid is gradually lost as VWF ages in the plasma, leading to clearance of the protein via the asialoglycoprotein receptor. If this model is correct, there appears to be a bittersweet relationship between sialic acid and VWF: the presence of the sugar on VWF is protective against clearance but permissive of cleavage by ADAMTS13, and the absence of the sugar on VWF decreases the chance of cleavage but increases the likelihood of clearance.
The findings of McGrath et al provide new insight into determinants that influence VWF proteolysis by ADAMTS13; such knowledge may contribute toward the understanding of VWF function in health and disease.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal