In this issue of Blood, Lapalombella and colleagues present evidence that the immunomodulatory drug lenalidomide (Revlimid) may repair and reverse the humeral defect in CLL.1
Clinical response rates have improved significantly for chronic lymphocytic leukemia (CLL) patients treated with chemo-immunotherapy, but recurrent disease is common and chemotherapy may select for drug-resistant leukemic subclones and amplify immune suppression. The clinical efficacy of immunotherapy in CLL has been demonstrated after allogeneic stem cell transplantation (with all its potential toxicity) where long-term disease control has been achieved by exploiting a graft-versus-leukemia effect.2 Expanding immunotherapeutic treatment options should aid identification of potentially curative therapies for CLL and other malignancies. Cellular and humoral immune defects impair immunotherapeutic approaches. CLL cells are dysfunctional antigen-presenting cells (APCs) and can actively suppress T-cell cytoskeletal signaling pathways and function.3 Repair and reversal of autologous immune dysfunction in CLL should reprogram patients' immune system to elicit antitumor responses.
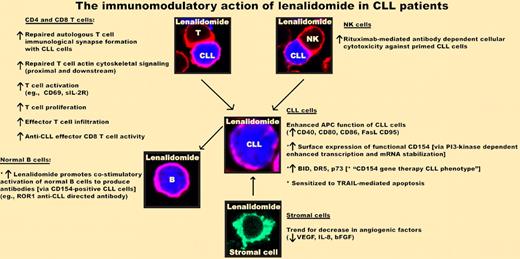
Lenalidomide (Revlimid) repairs and reverses cellular and humoral immune dysfunction in CLL. This agent enhances autologous T-cell immunologic synapse formation and functional activity (repairs impaired T-cell actin cytoskeletal signaling). NK-mediated antibody-dependent cellular cytotoxicity of rituximab-treated CLL cells is enhanced by preincubation of NK cells with lenalidomide before exposure to rituximab-exposed CLL cells. Lenalidomide enhances APC function of CLL cells (up-regulation of costimulatory molecules). Exposure of drug increases functional surface CD154 expression on CLL cells, inducing a CD154 gene therapy activation phenotype. Importantly, lenalidomide-induced CD154-positive CLL cells promote antibody (IgM and IgG) production by normal B cells. The immunomodulatory effect of lenalidomide on stromal cells, nurse-like cells, and angiogenic status remains to be fully elucidated.
Lenalidomide (Revlimid) repairs and reverses cellular and humoral immune dysfunction in CLL. This agent enhances autologous T-cell immunologic synapse formation and functional activity (repairs impaired T-cell actin cytoskeletal signaling). NK-mediated antibody-dependent cellular cytotoxicity of rituximab-treated CLL cells is enhanced by preincubation of NK cells with lenalidomide before exposure to rituximab-exposed CLL cells. Lenalidomide enhances APC function of CLL cells (up-regulation of costimulatory molecules). Exposure of drug increases functional surface CD154 expression on CLL cells, inducing a CD154 gene therapy activation phenotype. Importantly, lenalidomide-induced CD154-positive CLL cells promote antibody (IgM and IgG) production by normal B cells. The immunomodulatory effect of lenalidomide on stromal cells, nurse-like cells, and angiogenic status remains to be fully elucidated.
The most promising immunotherapy in CLL has been using adenovirus-delivered CD154 gene therapy.4,5 Transduction of CD154 into patient CLL cells ex vivo, followed by systemic reintroduction, induces CD40 activation of both transduced and nontransduced CLL cells, which stimulate autologous leukemia-specific T cells, reduce leukemia cell counts, and induce development of antibodies to the CLL tumor-specific antigen ROR1. CD154-ligand treatment also induces expression of death receptors CD95 and DR5, and p73 that can render CLL cells sensitive to TRAIL-mediated apoptosis and fludarabine-based therapies.6 The impaired immune response in CLL patients likely explains why CD154 gene therapy has failed to achieve more durable clinical responses.
The article by Lapalombella et al identifies lenalidomide (Revlimid; Celgene) as a pharmacologic agent that can mimic a CD154 gene therapy activation phenotype in CLL and reverse humoral immune tolerance. Lenalidomide is clinically effective as a single agent in relapsed or refractory CLL,7 but no direct in vitro proapoptotic effect has been observed using primary CLL cells. The exact mechanisms of action of lenalidomide in hematologic malignancies are not fully defined but include activation of cellular and innate immunity, and blocking angiogenic and stromal cell activity.
Lapalombella and colleagues demonstrate that lenalidomide exposure induces CD154 transmembrane protein expression on primary CLL cells. Mechanistic data show that the drug enhances CD154 mRNA gene transcription via NFATc1/NF-κB complex binding to the CD154 promoter and also through downstream mRNA stabilization, both of which are dependent on the PI3-kinase pathway. This study also presents elegant functional coculture data showing that lenalidomide-induced surface expression of CD154 on CLL cells promotes immunoglobulin production by normal B cells and increases BID, DR5, and p73 expression as seen with CD154 gene therapy. In vivo data show that patients receiving lenalidomide exhibit the CD154 gene therapy CLL phenotype, and in one patient, development of polyclonal hypergammaglobulinemia with increasing titers of the CLL-specific antibody ROR1. It would have been very informative to strengthen the in vivo patient data showing that lenalidomide can reverse humoral tumor tolerance. The authors suggest that the reason only a single case report identified recovery of immunoglobulins and development of ROR1 tumor-specific antibodies was that all the other patients received prior rituximab, which depletes normal B cells as well as malignant cells.
CLL-induced T-cell dysfunction can be reversible with lenalidomide.3 This agent can repair autologous T-cell recognition and formation of the immunologic synapse with CLL cells leading to enhanced cytolytic killing. Pretreatment of autologous natural killer (NK) cells with lenalidomide enhances rituximab-mediated antibody-dependent cellular cytotoxicity of CLL8 at clinically relevant lenalidomide concentrations (0.5μM) that repair and reverse the humoral and cellular immune defects. This relatively low pharmacologic dose might help minimize toxicity associated with the drug-induced tumor flare reaction. The ability of lenalidomide to reverse and repair both T-cell and humoral immune dysfunction provides strong evidence that a major mode of action of this agent in CLL is immunomodulatory (see figure). The combined results of rigorous correlative science functional studies suggest that the timing of lenalidomide should be carefully designed to ensure that immunosuppressive chemotherapeutic therapies and rituximab do not block the immunomodulatory action of this exciting new agent in CLL. Further work in CLL and cross-fertilization of results using lenalidomide in other B-cell malignancies should maximize the clinical application of this agent. Lenalidomide's immunotherapeutic activity suggests that it should be considered in combination with vaccination in clinical trials of immunotherapy, especially in light of the current findings that this drug may reverse the humoral immune defect in CLL.
Conflict-of-interest disclosure: J.G.G. has received honoraria from Celgene for work on advisory boards. A.G.R. declares no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal