The functional development of tumor-specific CD4+ T cells has a critical impact on the outcome of antitumor immune responses. Adoptive immunotherapy involving tumor-specific CD4+ T cells has shown encouraging clinical benefits in some cancer patients. To mount an effective antitumor immunity, it is desirable to elicit activated type 1 T helper cells. Here, we report that type 1 T helper cell–like effector cells that arose in tumor-bearing hosts progressively expressed programmed death 1 during tumor growth. The programmed death 1hi effector cells displayed a dysfunctional phenotype, characterized by selective down-regulation of interleukin-7 receptor, heightened apoptosis, and poor antitumor efficacy. This tumor-driven aberrant T-cell response could be prevented by a single dose of the widely used chemotherapy agent cyclophosphamide. We show that chemotherapy conditioned the host environment, creating a transient window for optimal effector differentiation for adoptively transferred CD4+ T cells. This robust effector differentiation, which was antigen-driven and mechanistically dependent on an intact host response to type I interferon, gave rise to activated polyfunctional T helper cells with high interleukin-7 receptor, rapid clonal expansion, and potent antitumor activity against established B-cell lymphomas. We hypothesize that prevention of tumor-induced effector cell dysfunction is a major mechanism contributing to the efficacy of combined chemoimmunotherapy.
Introduction
CD4+ T helper (Th) cells are critically important in activating a broad spectrum of tumor-reactive immune cells, including cytotoxic T lymphocytes (CTLs), eosinophils, natural killer cells, macrophages, and B cells.1,2 CD4+ T-cell help is of particular importance to ensure the survival and proper function of CTLs that serve as the primary tumor-killing effectors in majority of current cancer immunotherapies.3 In addition, accumulating evidence has demonstrated that CD4+ T cells also can act as direct antitumor effectors with potent tumoricidal activities.4,5 Recent anecdotal success in treating a patient with metastatic melanoma with the use of autologous CD4+ T cells has brought these cells to prominence in cancer immunotherapy.6
As a phenotypically and functionally diverse population, CD4+ T cells of different lineages have different effects on tumor growth. Th1 and Th2 effector cells can mediate tumor rejection by different mechanisms.4 The role of the newly identified proinflammatory Th17 cells in antitumor immunity is controversial and may be context dependent.7,–9 Foxp3-expressing T regulatory (Treg) cells have been shown to inhibit the antitumor function of a variety of immune cells, including Th1 cells, CTLs, natural killer cells, and tumor-infiltrating dendritic cells (reviewed in Zou10 ). Although Treg-mediated immunosuppression is unequivocally involved in promoting tumor immune evasion, it is increasingly recognized that tumors may use multiple mechanisms simultaneously or sequentially to evade immune attacks. Emerging evidence suggests that the programmed death 1 (PD-1) signaling pathway, acting in concert with Tregs, significantly contributes to tumor-specific immune tolerance.11,,–14
PD-1, a member of the immunoglobulin superfamily, which includes CD28/CTLA-4, has been implicated in induction and maintenance of peripheral T-cell tolerance to self-antigens through interaction with its 2 ligands (PD-L1 and PD-L2).15 In CD8+ T cells, PD-1 has been shown to mediate CTL functional exhaustion during chronic viral infections.16 The exhausted state was reversible with anti-PD-1 or anti-PD-L1 monoclonal antibody blockade, as manifested by restored CTL effector functions and clearance of virus.16 PD-1 signaling blockade in various animal tumor models also resulted in enhanced antitumor immunity,17,–19 suggesting that the PD-1/PD-L1 pathway may operate similarly in both chronic infection and cancer.20 The existence of PD-1–expressing exhausted CD8+ T cells during tumor progression recently has been established.21 However, the role of PD-1 in regulating CD4+ T-cell response in the tumor context remains unclear.
The functional development of CD4+ T cells in tumor-bearing hosts critically influences the outcome of cancer immunotherapy or host antitumor immune responses. We and others22,–24 have shown that tumor-specific CD4+ effector T cells and Treg cells develop in parallel during tumor progression. Treg cells dominantly suppress the effector T cells, rendering the overall pool of tumor-specific CD4+ T cells anergic.22 In the current study, we used a well-established mouse model of B-cell lymphoma to show that adoptively transferred tumor-specific CD4+ T cells express increasing levels of PD-1 during tumor progression, with down-regulation of interleukin-7 receptor (IL-7R) and heightened apoptosis, consistent with a dysfunctional phenotype. We further show that this defective antitumor CD4+ T-cell response cannot be corrected by simply ablating PD-1 expression on T cells but can be prevented by a single dose of the chemotherapeutic agent cyclophosphamide (Cy).
Methods
Mice
BALB/c mice (Thy1.2+/+) of 4 to 6 weeks of age were purchased from the National Cancer Institute. T-cell receptor (TCR) transgenic mice on a BALB/c background expressing an αβTCR specific for amino acids 110 to 120 from influenza hemagglutinin (HA) presented by major histocompatibility complex (MHC) class II molecule IAd were originally generated in the laboratory of Dr H. von Boehmer (Harvard Medical School). DO11.10 mice expressing TCRs specific for amino acids 323 to 339 from chicken ovalbumin (OVA) presented by MHC class II molecule IAd were purchased from Taconic and crossed to a Thy1.1+/+ background. The Foxp3GFP mice25 generated in the laboratory of Dr Alexander Rudensky (Memorial Sloan-Kettering Cancer Center) were inbred 9 generations onto the BALB/c background in the laboratory of Dr Hyam I. Levitsky (The Johns Hopkins University School of Medicine). Both Thy1.1+/+ HA-TCR Tg mice and BALB/c-Foxp3GFP mice were generous gifts from Dr Hyam I. Levitsky. The PD-1KO mice on a BALB/c background (PD-1-KO-N12[BALB/c]), generated in the laboratory of Dr Tasuku Honjo,26 were purchased from RIKEN BioResource Center. HA-TCR Tg mice were crossed with PD-1KO mice or Foxp3GFP mice to generate the respective double transgenic mice. The interferon-α (IFN-α)/βRKO mice were originally backcrossed from C57BL/6 background to BALB/c background in the laboratory of Dr Daniel A. Portnoy (University of California). All mice were housed under specific pathogen–free conditions by Laboratory Animal Services of the Medical College of Georgia. All animal experiments were approved by the Institutional Animal Care and Use Committee of the Medical College of Georgia.
Antibodies and reagents
The following fluorochrome-conjugated antibodies were used for flow cytometry: anti–mouse CD4 peridinin chlorophyll protein and allophycocyanin (APC), PD1-phycoerythrin (PE; clone J43 and RPM1-40), CD127-PE, IL-2–PE, tumor necrosis factor-α (TNF-α)–PE, B7.1-PE, B7.2-PE, I-A/I-E, IFN-γ–APC, annexin V–APC, Foxp3-APC, CD11b-APC, CD11c–fluorescein isothiocyanate, and control immunoglobulin G monoclonal antibodies were purchased from eBioscience. Thy1.1-peridinin chlorophyll protein and cytokine intracellular staining kits were purchased from BD Biosciences. Chloromethyfluorescein diacetate succinimidyl ester (CFSE) was purchased from Invitrogen. Cy was purchased from Sigma-Aldrich, dissolved in phosphate-buffered saline (PBS), and filtered through a 0.22-μm membrane each time before intraperitoneal administration to mice at 150 mg/kg. A recombinant vaccinia virus encoding HA from the 1934 PR8 strain of influenza (vacHA) was previously described.27 For immunization, mice were intraperitoneally inoculated with 1 × 107 plaque-forming units of vacHA suspended in 0.1 mL of Hanks balanced salt solution.
Tumor cells and adoptive transfer
The generation and maintenance of the wild-type murine B-cell lymphoma cell line A20 (A20WT) and its derivative A20-HA was described previously.27 A20-OVA tumor cell line was established by transfecting A20 with pcDNA3 containing membrane-bound OVA cDNA. Tumor cells (1 × 106) were injected into each recipient via tail vein. For adoptive transfer, spleens and lymph nodes from specified Tg mice were harvested, homogenized, lysed by ACK lysing buffer (Invitrogen) to remove red blood cells, and resuspended in PBS buffer. Cells were enriched for CD4+ T cells by magnetic-activated cell sorting (Miltenyi Biotec) and labeled with 2μM CFSE. The percentage of lymphocytes positive for CD4 and the clonotypic TCR (monoclonal antibody 6.5 for HA-specific CD4+ T cells, KJ1-26 for OVA-specific CD4+ T cells) was determined by flow cytometry. A total of approximately 2.5 to 3 × 106 CD4+TCR+ T cells were injected intravenously into each recipient.
Flow cytometric analysis
Spleen samples were mechanically dissociated to obtain a single-cell suspension. Tail blood samples were collected directly into ACK lysing buffer. Cells were depleted of erythrocytes with ACK lysing buffer and resuspended in PBS. Staining of surface antigens was performed in staining buffer on ice for 20 minutes. Annexin V staining was performed by use of the provided binding buffer (eBioscience). Intracellular Foxp3 staining was performed following the manufacturer's instructions (eBioscience). For cytokine intracellular staining, CD4+ cells were purified from splenocytes by magnetic-activated cell sorting with the use of the CD4+ isolation kit. A total of 0.5 × 106 purified CD4+ T cells were incubated with 1.5 × 106 fresh splenocytes from syngenic mice in the presence of the cognate HA peptide and Golgistop for 5 hours at 37°C. Cells were harvested and surface stained, followed by cytokine staining with the intracellular staining kit from BD Biosciences. Flow cytometry was performed on a FACSCalibur (BD Biosciences). All data were analyzed with FlowJo software (version 7.0; TreeStar Inc).
Statistical analysis
Data were analyzed with Prism 4.0 (GraphPad Software, Inc). The statistical significance of the results was determined with the Student t test. Data for tumor survival were analyzed with a log-rank test. P values less than .05 were considered statistically significant.
Results
Tumor-specific CD4+ T cells express increasing levels of PD-1 and Foxp3 during tumor progression
There is now ample evidence that both tumor-specific effector CD4+ T cells and tumor-activated Tregs develop concomitantly in tumor-bearing hosts.22,–24 The features of the CD4+ effector T cells that develop in the tumor context, however, have not been fully addressed. As shown in the schema in Figure 1, a small number of CFSE-labeled, influenza HA-specific CD4+ T cells were transferred into mice with established systemic B-cell lymphomas expressing HA as a surrogate tumor antigen (A20-HA), and the changes in activation status and phenotype of the donor T cells were monitored at different time points. We examined the expression of Foxp3, the hallmark of Treg cells, and PD-1, a coinhibitory receptor associated with T-cell dysfunction,16 on transferred CD4+ T cells. Both markers were prominently induced in the donor cells that divided as the result of antigen encounter in vivo. By day 4 after adoptive transfer, a sizable fraction (37%) of the donor cells had divided. Foxp3+ cells began emerge in these divided cells, whereas approximately 30% of the divided cells already up-regulated PD-1 expression (Figure 1A top row). By day 7, there were further cell divisions, and Foxp3 expression was more evident in the highly divided cells (Figure 1A middle row). By day 15, in mice that showed signs of ascites and displayed extensive tumor burden in multiple organs upon dissection, up to 90% of the donor cells had divided extensively (> 5 divisions), and the proportions of Foxp3+ and PD-1hi cells both increased markedly (Figure 1A bottom row). Costaining of Foxp3 and PD-1 further illustrated the incremental increase in expression of both molecules in the divided donor cells (Figure 1B).
Antigen-specific CD4+ T cells express increasing levels of Foxp3 and PD-1 during tumor progression. The timeline of the procedure is outlined. A total of approximately 2.5 to 3.0 × 106 HA-specific naive CD4+ T cells (Thy1.1+) were labeled with CFSE and adoptively transferred into BALB/c recipients with established A20-HA tumors. (A) At the indicated time points, spleen cells were subjected to fluorescence-activated cell-sorting (FACS) analysis to evaluate the frequency and cell-division status of the donor CD4+ T cells (Thy1.1 vs CFSE). Numbers represent percentage of donor CD4+ T cells in total spleen cells. Foxp3 and PD-1 expression profiles relative to cell division of the gated donor CD4+ T cells are shown. Numbers indicate the percentage of cells in the corresponding quadrant. (B) Costaining of PD-1 and Foxp3 is shown for divided donor CD4+ T cells. Numbers represent the percentage of each subpopulation in divided donor CD4+ T cells. Plots shown are representative of 2 independent experiments, with 4 to 6 mice per group. (C) OVA-specific CD4+ T cells up-regulate the expression of Foxp3 and PD-1 during tumor progression. Identical experiments were conducted with the use of OVA-specific CD4+ T cells purified from DO11.10 Tg mice and A20-OVA tumors. Tumor-free mice receiving T-cell transfer were included as control. At 15 days after T-cell transfer, spleen cells were subjected to analyses as described in panel A. Plots shown are representative of 2 independent experiments with 5 mice per group.
Antigen-specific CD4+ T cells express increasing levels of Foxp3 and PD-1 during tumor progression. The timeline of the procedure is outlined. A total of approximately 2.5 to 3.0 × 106 HA-specific naive CD4+ T cells (Thy1.1+) were labeled with CFSE and adoptively transferred into BALB/c recipients with established A20-HA tumors. (A) At the indicated time points, spleen cells were subjected to fluorescence-activated cell-sorting (FACS) analysis to evaluate the frequency and cell-division status of the donor CD4+ T cells (Thy1.1 vs CFSE). Numbers represent percentage of donor CD4+ T cells in total spleen cells. Foxp3 and PD-1 expression profiles relative to cell division of the gated donor CD4+ T cells are shown. Numbers indicate the percentage of cells in the corresponding quadrant. (B) Costaining of PD-1 and Foxp3 is shown for divided donor CD4+ T cells. Numbers represent the percentage of each subpopulation in divided donor CD4+ T cells. Plots shown are representative of 2 independent experiments, with 4 to 6 mice per group. (C) OVA-specific CD4+ T cells up-regulate the expression of Foxp3 and PD-1 during tumor progression. Identical experiments were conducted with the use of OVA-specific CD4+ T cells purified from DO11.10 Tg mice and A20-OVA tumors. Tumor-free mice receiving T-cell transfer were included as control. At 15 days after T-cell transfer, spleen cells were subjected to analyses as described in panel A. Plots shown are representative of 2 independent experiments with 5 mice per group.
We confirmed the aforementioned experiments by using CD4+ T cells specific for a different antigen, OVA, and A20 tumors expressing OVA (A20-OVA). Similar results were obtained (Figure 1C), indicating that the phenomenon is not peculiar to a specific antigen or T-cell receptor. These data suggested that the effector cells that arise during tumor progression may be driven toward a dysfunctional state.
Cy preconditioning facilitates the generation of activated polyfunctional CD4+ effectors and inhibits aberrant CD4+ T-cell response
Recent studies (reviewed in Zitvogel et al28 ) indicated that chemotherapy can significantly enhance the efficacy of certain forms of cancer immunotherapy. We asked whether Cy, a widely used chemotherapeutic drug, could rescue aberrant CD4+ differentiation in tumor-bearing mice. Tumor-bearing mice were pretreated with Cy (150 mg/kg) or PBS control 24 hours before receiving tumor-specific CD4+ T cells. Figure 2 shows that 7 days after T-cell transfer, a few of the donor CD4+ T cells in control tumor-bearing mice (without Cy) had undergone a small amount of cell division but without any significant net increase in cell percentage and total cell number (Figure 2A-B). In contrast, in tumor-bearing mice pretreated with Cy, transferred cells underwent vigorous cell division and substantial clonal expansion. Even after correcting for the altered cellularity in Cy-treated mice, the extensive cell proliferation still led to markedly increased accumulation of the tumor-specific donor cells in the spleens (Figure 2B) and lymph nodes (data not shown).
Cy conditioning of the tumor-bearing mice prevents aberrant CD4+ T-cell differentiation and generates activated polyfunctional effector cells. The timeline of the procedure is outlined. On day 17 after tumor inoculation, some mice were treated with 150 mg/kg Cy before receiving T-cell transfer the next day. (A) At 7 days after T-cell transfer, spleen cells were examined for donor cell division status. Numbers indicate the percentage of the donor cells in spleen. (B) Absolute number of donor CD4+ T cells recovered from spleen (total splenocyte count × percent donor CD4+ T cells). Data are from 2 independent experiments and are shown as mean ± SD of at least 4 mice per group. (C) Expression profiles of Foxp3 and PD-1 on donor CD4+ T cells. Numbers indicate the percentage of cells in the corresponding quadrant. (D) Cytokine intracellular staining on divided donor CD4+ T cells. Purified CD4+ T cells were stimulated with HA peptide–pulsed fresh splenocytes for 5 hours before intracellular staining for IL-2, IFN-γ, and TNF-α. Plots shown are gated on the divided donor CD4+ T cells. Numbers represent the percentage of cells in each quadrant in divided donor CD4+ T cells. (E) Annexin V and CD127 (IL-7R) expression profiles on donor CD4+ T cells. Solid curve in each histogram represents divided donor cells, and shaded curve represents undivided donor cells. Numbers indicate percent of the gated population for divided donor cells. Results shown are representative of 3 independent experiments.
Cy conditioning of the tumor-bearing mice prevents aberrant CD4+ T-cell differentiation and generates activated polyfunctional effector cells. The timeline of the procedure is outlined. On day 17 after tumor inoculation, some mice were treated with 150 mg/kg Cy before receiving T-cell transfer the next day. (A) At 7 days after T-cell transfer, spleen cells were examined for donor cell division status. Numbers indicate the percentage of the donor cells in spleen. (B) Absolute number of donor CD4+ T cells recovered from spleen (total splenocyte count × percent donor CD4+ T cells). Data are from 2 independent experiments and are shown as mean ± SD of at least 4 mice per group. (C) Expression profiles of Foxp3 and PD-1 on donor CD4+ T cells. Numbers indicate the percentage of cells in the corresponding quadrant. (D) Cytokine intracellular staining on divided donor CD4+ T cells. Purified CD4+ T cells were stimulated with HA peptide–pulsed fresh splenocytes for 5 hours before intracellular staining for IL-2, IFN-γ, and TNF-α. Plots shown are gated on the divided donor CD4+ T cells. Numbers represent the percentage of cells in each quadrant in divided donor CD4+ T cells. (E) Annexin V and CD127 (IL-7R) expression profiles on donor CD4+ T cells. Solid curve in each histogram represents divided donor cells, and shaded curve represents undivided donor cells. Numbers indicate percent of the gated population for divided donor cells. Results shown are representative of 3 independent experiments.
Consistent with the results shown in Figure 1, the divided donor CD4+ T cells in tumor-bearing mice without Cy up-regulated the expression of Foxp3 and PD-1, whereas Cy pretreatment markedly reduced expression of these molecules in the divided donor cells (Figure 2C). Most notably, a sizable fraction (one-third) of the divided donor cells from Cy-conditioned mice concurrently produced multiple proinflammatory cytokines (IFN-γ, TNF-α, and IL-2) upon restimulation with the cognate peptide (Figure 2D), resembling the so-called polyfunctional effector cells that have been shown to be more efficacious in controlling viral infections and tumor growth.29,30 In contrast, the divided cells from mice without Cy pretreatment showed no cytokine production and were essentially unresponsive to restimulation.
In the preceding studies, we noted that the donor cells in tumor-bearing mice without Cy showed some degree of cell division, but their absolute numbers did not increase correspondingly. To test whether this failure to accumulate was attributable to heightened cell death, we examined annexin V level on the antigen-experienced donor cells. Divided donor cells in tumor-bearing mice without Cy had increased level of annexin V, indicative of apoptosis, which was notably reduced by pretreatment with Cy (Figure 2E left). Moreover, the divided donor cells in tumor-bearing mice without Cy significantly down-modulated CD127 (Figure 2E right), the α-subunit of the high-affinity receptor for IL-7, a cytokine shown to play a pivotal role in promoting cell survival and the transition of CD4+ effectors to memory cells.31 In contrast, most of the divided donor cells in Cy-treated mice retained high-level expression of CD127. Taken together, these data demonstrate that modulation of the lymphoma-bearing hosts with Cy effectively prevents aberrant CD4+ T-cell response and promotes the generation of activated effector T cells.
Cy preconditioning prevents the emergence of PD-1hi CD4+ effector cells that selectively down-regulate IL-7R and undergo high rates of apoptosis
Our previous study22 demonstrated that the tumor-specific CD4+ T cells after adoptive transfer into tumor-bearing hosts comprised both Treg cells and Th1-like effector cells. We next asked whether the Th1-like effector cells and Treg populations were differentially affected by Cy pretreatment. Following the same experimental protocol depicted in Figure 2, we transferred CD4+ T cells derived from (HA-TCR × Foxp3GFP) double transgenic mice into mice with established A20HA tumors, with or without Cy preconditioning. This system allowed us to readily discriminate Treg (GFP+) and non-Treg (GFP−) cells for the evaluation of cell apoptosis.
Figure 3A shows that CD4+ cells in mice without Cy conditioning developed into a Foxp3− (non-Treg) population, many of which expressed high levels of PD-1, and a population of Foxp3+ Treg cells (many of which also expressed PD-1). In contrast, in mice preconditioned with Cy, there were few Foxp3+ cells, and the large majority of Foxp3− cells did not up-regulate PD-1. Focusing on the non-Treg cells (GFP−) generated under these 2 conditions, we found that the PD-1hi donor cells in non-Cy–treated mice selectively down-regulated IL-7R and were high in annexin V, whereas the PD-1low cells in the same pool of donor cells remained IL-7Rhi and Annexin Vlow (Figure 3B). In contrast, the majority of the donor cells in Cy-treated mice retained IL-7R expression and showed little sign of apoptosis.
PD-1hi effector cells that develop in tumor-bearing mice selectively down-regulate IL-7R and undergo apoptosis. Following the same experimental procedure depicted in Figure 2, we transferred Thy1.1+ CD4+ T cells derived from HA-TCR Foxp3GFP double transgenic mice into BALB/c mice with established A20-HA tumors, with or without Cy preconditioning of the hosts. Donor cells were recovered 7 days after transfer and analyzed by FACS. (A) Expression of PD-1 and Foxp3(GFP) on donor CD4+ T cells. Costaining of PD-1 and Foxp3(GFP) on donor CD4+ T cells before transfer is included for comparison. Numbers represent the percentage of cells in each quadrant in donor CD4+ T cells. (B) Representative expression profiles of CD127 and annexin V on non-Treg donor cells. The Foxp3(GFP)-negative cells in each sample are gated and evaluated for expression of PD-1 vs CD127 and PD-1 vs annexin V. Numbers represent the percentage of cells in each quadrant in the Foxp3− donor CD4+ T cells. The results of all samples are presented as percent of CD127hi or percent of annexin Vhi cells in Foxp3− donor CD4+ T cells and shown in scatter plots. Horizontal bars represent mean values. (C) PD-1–deficient CD4+ T cells have accelerated cell divisions. CFSE-labeled, PD-1–sufficient (PD-1WT) or PD-1–deficient (PD-1KO) HA-specific CD4+ T cells (Thy1.1+) were transferred into BALB/c mice with A20-HA tumors established 10 days earlier. At 7 days after T-cell transfer, spleen cells were collected, enumerated, and subjected to FACS analysis. Cell division status is reflected by the CFSE profile gated on Thy1.1+ donor CD4+ T cells. The numbers in each histogram represent the percentage of highly divided (> 5 divisions), divided (1-5 divisions), and undivided donor cells. (D) PD-1–deficient CD4+ T cells fail to accumulate in mice. The bar graph shows the absolute number of donor CD4+ T cells recovered from spleen (total splenocyte count × percent donor CD4+ T cells). Data are from 2 independent experiments and shown as mean ± SD of 4 to 5 mice per group. (E) Representative expression profiles of PD-1 and Foxp3 relative to cell division of the donor CD4+ T cells. Numbers in Foxp3 vs CFSE dot plots represent the percentage of Foxp3+ cells in the highly divided donor cells (gated). The results of all samples are summarized in bar graph. Data are shown as mean ± SD of 4 mice per group. (F) Cytokine intracellular staining for recovered donor CD4+ T cells. Purified donor CD4+ T cells were stimulated with HA peptide-pulsed fresh splenocytes for 5 hours before intracellular staining for IFN-γ and TNF-α. Plots shown are gated on the divided donor CD4+ T cells. The numbers represent the percentage of cells in each quad in divided donor CD4+ T cells. Results shown are representative of 2 independent experiments with similar results.
PD-1hi effector cells that develop in tumor-bearing mice selectively down-regulate IL-7R and undergo apoptosis. Following the same experimental procedure depicted in Figure 2, we transferred Thy1.1+ CD4+ T cells derived from HA-TCR Foxp3GFP double transgenic mice into BALB/c mice with established A20-HA tumors, with or without Cy preconditioning of the hosts. Donor cells were recovered 7 days after transfer and analyzed by FACS. (A) Expression of PD-1 and Foxp3(GFP) on donor CD4+ T cells. Costaining of PD-1 and Foxp3(GFP) on donor CD4+ T cells before transfer is included for comparison. Numbers represent the percentage of cells in each quadrant in donor CD4+ T cells. (B) Representative expression profiles of CD127 and annexin V on non-Treg donor cells. The Foxp3(GFP)-negative cells in each sample are gated and evaluated for expression of PD-1 vs CD127 and PD-1 vs annexin V. Numbers represent the percentage of cells in each quadrant in the Foxp3− donor CD4+ T cells. The results of all samples are presented as percent of CD127hi or percent of annexin Vhi cells in Foxp3− donor CD4+ T cells and shown in scatter plots. Horizontal bars represent mean values. (C) PD-1–deficient CD4+ T cells have accelerated cell divisions. CFSE-labeled, PD-1–sufficient (PD-1WT) or PD-1–deficient (PD-1KO) HA-specific CD4+ T cells (Thy1.1+) were transferred into BALB/c mice with A20-HA tumors established 10 days earlier. At 7 days after T-cell transfer, spleen cells were collected, enumerated, and subjected to FACS analysis. Cell division status is reflected by the CFSE profile gated on Thy1.1+ donor CD4+ T cells. The numbers in each histogram represent the percentage of highly divided (> 5 divisions), divided (1-5 divisions), and undivided donor cells. (D) PD-1–deficient CD4+ T cells fail to accumulate in mice. The bar graph shows the absolute number of donor CD4+ T cells recovered from spleen (total splenocyte count × percent donor CD4+ T cells). Data are from 2 independent experiments and shown as mean ± SD of 4 to 5 mice per group. (E) Representative expression profiles of PD-1 and Foxp3 relative to cell division of the donor CD4+ T cells. Numbers in Foxp3 vs CFSE dot plots represent the percentage of Foxp3+ cells in the highly divided donor cells (gated). The results of all samples are summarized in bar graph. Data are shown as mean ± SD of 4 mice per group. (F) Cytokine intracellular staining for recovered donor CD4+ T cells. Purified donor CD4+ T cells were stimulated with HA peptide-pulsed fresh splenocytes for 5 hours before intracellular staining for IFN-γ and TNF-α. Plots shown are gated on the divided donor CD4+ T cells. The numbers represent the percentage of cells in each quad in divided donor CD4+ T cells. Results shown are representative of 2 independent experiments with similar results.
Genetic ablation of PD-1 in tumor-specific CD4+ T cells does not restore effector function in these cells
The aforementioned results demonstrated that PD-1 expression on tumor-specific CD4+ T cells is associated with a dysfunctional phenotype. This finding prompted us to test whether disruption of PD-1 signaling would correct the defects in tumor-specific CD4+ T cells. The administration of blocking monoclonal antibodies specific for PD-1 (J43) led to a direct reduction in tumor burden and selective reduction of tumor-specific CD4+ T cells that would otherwise primarily become Tregs and PD-1hi effector cells (supplemental Figure 1, available on the Blood website; see the Supplemental Materials link at the top of the online article). Because the antibody had multiple effects on both tumor and T cells, this made it difficult to interpret data by the use of a blocking-antibody approach. Therefore, we took a more definitive approach to address this issue by using TCR-Tg CD4+ T cells with a genetic deficiency in PD-1.
To this end, either PD-1–deficient (PD-1KO) or the control PD-1–sufficient (PD-1WT) HA-specific CD4+ T cells were transferred into tumor-bearing mice. At 7 days after T-cell transfer, the proportion of divided cells was greater in the PD-1–deficient donor cells than that in the control PD-1–sufficient donor cells (62.8% ± 6.8% vs 46.0% ± 6.1%). However, overall the total donor cell numbers did not vary significantly in spleens (Figure 3C-D) or in lymph nodes (data not shown), suggesting that PD-1 ablation by itself did not rescue tumor-specific CD4+ T cells from apoptosis. Notably, highly divided cells (> 5 cell divisions) were predominant in PD-1–deficient donor cells but not in the control cells. However, these highly divided PD-1KO CD4+ T cells contained a greater fraction of Foxp3+ Tregs (55.4% ± 9.7%) than their PD-1WT counterparts (28.7% ± 11.9%; Figure 3E) and were still defective in cytokine productions (Figure 3F). Thus, taken together, these data demonstrate that mere ablation of PD-1 does not restore an activated effector phenotype in tumor-specific CD4+ T cells and suggest that additional strategies would be needed to reverse the tolerogenic milieu of the tumor-bearing host.
Tumor relapse is associated with loss of the activated effector phenotype in tumor-specific CD4+ T cells
Therefore, we extended our analysis of mechanisms underlying antitumor response after Cy plus CD4+ adoptive transfer. We next conducted survival experiments to test the hypothesis that the activated polyfunctional CD4+ effector cells elicited by the combinatory therapy were associated with survival benefit. Following the experimental procedure depicted in Figure 2, we treated or did not treat tumor-bearing mice with CD4+ T-cell adoptive transfer only, or Cy only, or the combination of Cy and CD4+ T-cell transfer. As shown in Figure 4A, CD4+ T-cell adoptive transfer alone only marginally delayed tumor growth compared with no treatment group (median survival 30.5 days vs 26 days, respectively; P = .042). Cy treatment alone significantly delayed tumor growth (median survival, 55.5 days), which is consistent with a direct cytotoxic effect of Cy against A20HA tumors (a B-cell lymphoma). Combination of Cy plus adoptive transfer significantly prolonged survival (median survival, 72 days).
Tumor relapse after chemoimmunotherapy is associated with reacquisition of Foxp3 and PD-1 in CD4+ effector cells. Following the experimental procedure outlined in Figure 2, we monitored mice for survival. (A) The Kaplan-Meier plot depicts overall survival. Results shown are pooled data from 2 independent experiments. The number of mice in each group is shown. (B) Correlation of tumor relapses with up-regulation of PD-1 and Foxp3 in tumor-specific donor CD4+ T cells. At different time points, tail blood samples or spleen cells from the mice treated with combination of Cy and CD4+ T-cell transfer were collected and subjected to FACS analysis. Plots of PD-1 vs Foxp3 are gated on divided donor CD4+ T cells. Plots shown are representative blood sample staining. Numbers indicate the percentage of cells in each quadrant. (C) The results of all samples are presented as percent of PD-1hi cells or percent of Foxp3+ cells in divided donor cells and summarized in scatter plots. Horizontal bars represent mean values.
Tumor relapse after chemoimmunotherapy is associated with reacquisition of Foxp3 and PD-1 in CD4+ effector cells. Following the experimental procedure outlined in Figure 2, we monitored mice for survival. (A) The Kaplan-Meier plot depicts overall survival. Results shown are pooled data from 2 independent experiments. The number of mice in each group is shown. (B) Correlation of tumor relapses with up-regulation of PD-1 and Foxp3 in tumor-specific donor CD4+ T cells. At different time points, tail blood samples or spleen cells from the mice treated with combination of Cy and CD4+ T-cell transfer were collected and subjected to FACS analysis. Plots of PD-1 vs Foxp3 are gated on divided donor CD4+ T cells. Plots shown are representative blood sample staining. Numbers indicate the percentage of cells in each quadrant. (C) The results of all samples are presented as percent of PD-1hi cells or percent of Foxp3+ cells in divided donor cells and summarized in scatter plots. Horizontal bars represent mean values.
The occurrence of tumor relapse in most of the mice that received the combination treatment implied that the therapeutic effect of the CD4+ effector cells did not lead to complete tumor destruction. To monitor the immune status of the tumor-specific CD4+ effector cells during the process of tumor relapse, we assessed Foxp3 and PD-1 expression over time on donor CD4+ T cells from mice that received the combinatory treatment. Mouse tail blood samples were collected at different time points, and spleen cells were harvested when mice were euthanized at experimental end points. As shown in Figure 4B, by day 32 (2 weeks after T-cell transfer) when all the mice were undergoing remission, the divided donor cells in all examined mice expressed low levels of Foxp3 and PD-1, representing an activated effector phenotype. Around day 50, some mice (6 of 8) began show signs of illness. Blood samples from these symptomatic mice displayed elevated expression of Foxp3 and PD-1 in the donor CD4+ T cells (Figure 4B-C). These mice were confirmed to have visible tumors in multiple organs upon dissection, and the donor CD4+ T cells in their spleens had the same expression pattern of Foxp3 and PD-1 as the blood samples (data not shown). The donor cells from the 2 mice that remained asymptomatic throughout the study maintained an activated effector phenotype. Thus, the eventual relapse of disease was associated with loss of the activated effector phenotype achieved after Cy pretreatment.
The host response to type I IFN is critical for the beneficial effect of Cy on donor CD4+ T cells
Type I IFNs (ie, IFN-α and IFN-β) have been shown in other settings to play an important role in mediating the immune-enhancing effects of Cy pretreatment.32,33 To test the hypothesis that type I IFNs drove CD4+ T-cell differentiation in a postchemotherapy environment, adoptive transfer experiments were conducted in recipient mice with or without a genetic ablation of IFN-α/β receptor (IFN-α/βRKO). Without Cy conditioning, the proportion (Figure 5A) and the accumulation (Figure 5B) of divided donor cells in the spleens were comparable in tumor-bearing wild-type and IFN-α/βRKO recipients. However, after Cy conditioning, the robust response of the donor cells usually seen in the wild-type tumor-bearing mice was lost in IFN-α/βRKO mice. Because the donor cells were IFN-α/β receptor sufficient, our data strongly suggest that Cy-induced type I IFNs play a critical role in modulating the host cells, most likely dendritic cells,34,35 that are essential for the clonal expansion and differentiation of the donor cells. Consistent with this hypothesis, we found that Cy treatment induced up-regulation of costimulatory molecules B7.1 and B7.2, as well as MHC class II levels, on CD11b+CD11c+ myeloid/conventional dendritic cells (supplemental Figure 2).
The host response to type I IFN after Cy conditioning is required for enhancing adoptive immunotherapy. Following the same experimental protocol as shown in Figure 2, we treated tumor-bearing wild-type mice or IFN-α/βRKO mice with or without Cy 1 day before adoptive transfer of Tg CD4+ T cells. At 7 days after T-cell transfer, donor cell division status was examined by FACS (A). Numbers in each plot represent the percentage of each individual subset in the donor population. (B) Enumerated number of divided donor cells in spleen (total splenocyte count × percent CFSElow donor cells in spleen). Data are shown as mean ± SD. Data shown are pooled from 2 independent experiments with 5 mice per group.
The host response to type I IFN after Cy conditioning is required for enhancing adoptive immunotherapy. Following the same experimental protocol as shown in Figure 2, we treated tumor-bearing wild-type mice or IFN-α/βRKO mice with or without Cy 1 day before adoptive transfer of Tg CD4+ T cells. At 7 days after T-cell transfer, donor cell division status was examined by FACS (A). Numbers in each plot represent the percentage of each individual subset in the donor population. (B) Enumerated number of divided donor cells in spleen (total splenocyte count × percent CFSElow donor cells in spleen). Data are shown as mean ± SD. Data shown are pooled from 2 independent experiments with 5 mice per group.
The receptive milieu of the postchemotherapy host environment is labile
Consistent with previous reports,34,36 Cy-induced lymphodepletion reached a nadir in spleen 2 days after treatment, followed by repopulation to pretreatment level around day 10 (Figure 6A). To determine the window of time capable of supporting robust effector differentiation in the postchemotherapy environment, we delayed the transfer of CD4+ T cells relative to Cy preconditioning for varying times, as shown in the schema for Figure 6B. The magnitude of donor cell expansion decreased as the interval between Cy treatment and T-cell transfer increased (Figure 6B-C), suggesting a decline in antigen availability and/or loss of favorable proinflammatory cytokines and costimulatory signals in the immune milieu. Because T-cell transfer was delayed, the fraction of divided donor cells declined, and the relative proportion of Foxp3+ cells increased. Thus, the optimal window for adoptive transfer occurred soon after Cy preconditioning.
The duration of favorable immune-stimulatory environment created by chemotherapy is transient. (A) Kinetics of cellular restoration in mice spleens after Cy treatment. BALB/c mice were treated or not treated with 150 mg/kg Cy. Spleen cell numbers were enumerated at the indicated time points. Results are shown as mean ± SD of 3 mice each group. (B) Transient duration of favorable immune-stimulatory environment in postchemotherapy hosts. The schema delineates the timeline of the experimental procedure. All tumor-bearing mice were treated with Cy on the same day but received T-cell transfer at different time points. To normalize for the expansions of donor cells that were transferred at different time, a group of tumor-free mice (No tumor, −Cy) were given the same amount of donor cells as the Cy-treated tumor-bearing mice (A20HA, +Cy). Mice in each group were always analyzed 7 days after T-cell transfer. At the time of analysis, mouse spleen cells were prepared and analyzed by FACS. The profiles of cell division (Thy1.1 staining), PD-1, and Foxp3 expression of the donor CD4+ T cells are shown as dot plots. Numbers represent the percentage of each individual subset in the donor population. (C) Summary of the results shown in panel B. To determine the fold expansion of donor cells, the absolute numbers of donor cells (calculated as total splenocyte count × percent donor cells in spleen) in Cy-treated tumor-bearing mice were divided by the average number of donor cells in no Cy-treated tumor-free mice. Results are shown as mean ± SD of 3 mice each group.
The duration of favorable immune-stimulatory environment created by chemotherapy is transient. (A) Kinetics of cellular restoration in mice spleens after Cy treatment. BALB/c mice were treated or not treated with 150 mg/kg Cy. Spleen cell numbers were enumerated at the indicated time points. Results are shown as mean ± SD of 3 mice each group. (B) Transient duration of favorable immune-stimulatory environment in postchemotherapy hosts. The schema delineates the timeline of the experimental procedure. All tumor-bearing mice were treated with Cy on the same day but received T-cell transfer at different time points. To normalize for the expansions of donor cells that were transferred at different time, a group of tumor-free mice (No tumor, −Cy) were given the same amount of donor cells as the Cy-treated tumor-bearing mice (A20HA, +Cy). Mice in each group were always analyzed 7 days after T-cell transfer. At the time of analysis, mouse spleen cells were prepared and analyzed by FACS. The profiles of cell division (Thy1.1 staining), PD-1, and Foxp3 expression of the donor CD4+ T cells are shown as dot plots. Numbers represent the percentage of each individual subset in the donor population. (C) Summary of the results shown in panel B. To determine the fold expansion of donor cells, the absolute numbers of donor cells (calculated as total splenocyte count × percent donor cells in spleen) in Cy-treated tumor-bearing mice were divided by the average number of donor cells in no Cy-treated tumor-free mice. Results are shown as mean ± SD of 3 mice each group.
Cy-induced tumor antigen release and host immune milieu change are both required for optimal effector differentiation
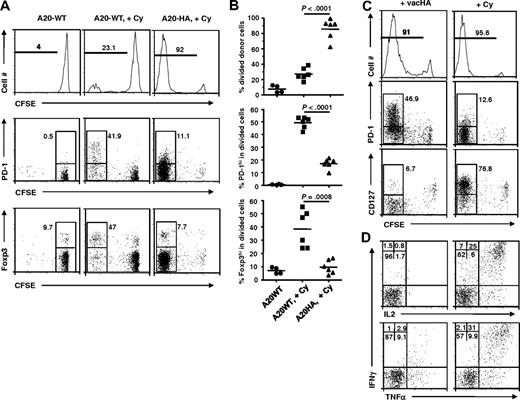
Cy is directly cytotoxic to a variety of tumor cells, including A20 B-cell lymphomas, resulting in a wave of tumor antigen release. In our system, it was not clear whether this antigen release was itself the main driver of CD4+ T-cell expansion, whether the change in host immune milieu was the driver, or whether both were required together. To address this issue, we tested the effect of Cy treatment in the absence of cognate antigen. HA-specific CD4+ T cells were transferred into mice bearing established wild-type A20 tumors without the target HA antigen (A20WT), with or without Cy preconditioning. Without Cy treatment, HA-specific CD4+ T cells did not undergo appreciable cell division in mice with antigen-negative tumors (Figure 7A left). The addition of Cy induced a small amount of antigen-independent cell division, consistent with lymphopenia-induced homeostatic expansion (Figure 7A middle). In comparison, donor cells in mice with antigen-positive A20HA tumors treated with Cy underwent massive expansion (Figure 7A right). Notably, the few divided donor cells in Cy-treated mice without antigen had relatively greater percentages of Foxp3+ and PD-1hi cells compared with Cy-treated mice with antigen-expressing tumor (Figure 7A-B middle and bottom). These results indicate that the immune-enhancing effects of Cy on donor CD4+ T cells required cognate antigen and was not simply a nonspecific effect of lymphopenia.
Cy-induced tumor antigen release and host immune milieu change both contribute to optimal effector differentiation of the donor cells. Following the experimental procedure depicted in Figure 2, we treated or did not treat mice with established A20-WT tumors with Cy 1 day before receiving HA-specific CD4+ T-cell transfer. For comparison, a third group of mice with A20-HA tumors were treated with Cy followed by adoptive CD4+ T-cell transfer. (A) Profiles of donor cell division, PD-1 and Foxp3 expression. At 7 days after T-cell transfer, mouse spleen cells were subjected to FACS analysis. The histograms are gated on the donor CD4+ T cells. Numbers in histograms represent the percentage of divided donor cells. The expression profiles of PD-1 vs CFSE and Foxp3 vs CFSE of the donor CD4+ T cells are shown in dot plots. Numbers in plots represent the percentage of PD-1hi or Foxp3+ cells in the divided donor cells (gated) or undivided donor cells for the group with A20-WT tumors but without Cy treatment. Data shown are representative of 2 independent experiments with similar results. (B) Summary of the results shown in panel A. Horizontal bars represent mean values. (C) Phenotype comparison of donor CD4+ T cells in response to vaccinia virus immunization and Cy-conditioning in tumor-bearing mice. Mice with established A20-HA tumors were either immunized with vacHA, or treated with Cy, on day 17 after tumor inoculation, followed by adoptive CD4+ T-cell transfer the next day. At 7 days after T-cell transfer, mouse spleen cells were subjected to the same FACS analyses as in panel A. (D) Cytokine intracellular staining for donor CD4+ T cells from vacHA immunized vs Cy-conditioned A20-HA–bearing mice. Purified CD4+ T cells were stimulated with HA peptide-pulsed fresh splenocytes for 5 hours before ICS. Plots shown are gated on the divided donor CD4+ T cells. The percentage of cells in each quadrant is shown. Results shown in panels C and D are representative of 2 independent experiments with 5 mice per group.
Cy-induced tumor antigen release and host immune milieu change both contribute to optimal effector differentiation of the donor cells. Following the experimental procedure depicted in Figure 2, we treated or did not treat mice with established A20-WT tumors with Cy 1 day before receiving HA-specific CD4+ T-cell transfer. For comparison, a third group of mice with A20-HA tumors were treated with Cy followed by adoptive CD4+ T-cell transfer. (A) Profiles of donor cell division, PD-1 and Foxp3 expression. At 7 days after T-cell transfer, mouse spleen cells were subjected to FACS analysis. The histograms are gated on the donor CD4+ T cells. Numbers in histograms represent the percentage of divided donor cells. The expression profiles of PD-1 vs CFSE and Foxp3 vs CFSE of the donor CD4+ T cells are shown in dot plots. Numbers in plots represent the percentage of PD-1hi or Foxp3+ cells in the divided donor cells (gated) or undivided donor cells for the group with A20-WT tumors but without Cy treatment. Data shown are representative of 2 independent experiments with similar results. (B) Summary of the results shown in panel A. Horizontal bars represent mean values. (C) Phenotype comparison of donor CD4+ T cells in response to vaccinia virus immunization and Cy-conditioning in tumor-bearing mice. Mice with established A20-HA tumors were either immunized with vacHA, or treated with Cy, on day 17 after tumor inoculation, followed by adoptive CD4+ T-cell transfer the next day. At 7 days after T-cell transfer, mouse spleen cells were subjected to the same FACS analyses as in panel A. (D) Cytokine intracellular staining for donor CD4+ T cells from vacHA immunized vs Cy-conditioned A20-HA–bearing mice. Purified CD4+ T cells were stimulated with HA peptide-pulsed fresh splenocytes for 5 hours before ICS. Plots shown are gated on the divided donor CD4+ T cells. The percentage of cells in each quadrant is shown. Results shown in panels C and D are representative of 2 independent experiments with 5 mice per group.
The preceding study showed that antigen was required, but it did not show whether antigen alone was sufficient (ie, whether the beneficial effect of Cy was solely attributable to enhanced antigen release). If the main immunomodulatory function of Cy was to release tumor antigens, we reasoned that other forms of immunogenic antigen provision might have similar stimulatory effect. To test this, we asked whether Cy treatment could be replaced by immunization of tumor-bearing mice with vaccinia virus expressing the HA target antigen (vacHA), a potent noncytotoxic vaccine. Figure 7C shows that donor cell division in vacHA immunized tumor-bearing mice was extensive and comparable with that in Cy-treated tumor-bearing mice. However, in stark contrast to Cy-treated mice, the divided donor cells in vacHA immunized mice expressed high levels of PD-1 and substantially down-regulated IL-7R, suggestive of aberrant differentiation. In contrast, the divided donor cells in Cy-conditioned mice displayed little PD-1 expression and abundant IL-7R. Consistent with aberrant effector differentiation, the divided cells in vacHA-treated mice were defective in antigen-induced cytokine production, whereas the Cy-treated mice showed large numbers of polyfunctional effector cells (Figure 7D). Thus, we conclude that simply providing antigen to mice with established tumors, even via a highly immunogenic vaccine, is insufficient to drive the robust polyfunctional effector cell differentiation seen with Cy. Collectively, the aforementioned results strongly suggest that it is the synergistic combination of Cy-induced antigen release and Cy-induced host immune milieu change, which together are required to drive a robust, productive effector cell differentiation.
Discussion
In the present study, we provided evidence that tumor-specific CD4+ T cells in lymphoma-bearing mice were driven almost entirely toward aberrant differentiation by the prevailing immunosuppressive milieu, resulting in a preponderance of defective effector cells and suppressive Tregs. However, the same starting population of cells could be redirected into robust effector differentiation by a single injection of the widely used chemotherapy drug Cy. The beneficial effect of Cy on adoptive immunotherapy has long been recognized,37,38 but the underlying molecular mechanisms are still not well understood. Our data now show that one of the key limiting factors in antitumor CD4+ T-cell response is the failure to develop robust effector differentiation, and that Cy treatment can effectively prevent this tumor-driven aberrant CD4+ T-cell differentiation and restore an activated, polyfunctional phenotype. Mechanistically, we found that Cy-induced type I IFNs played a critical role in modulating the host environment to favor a robust CD4+ T-cell response.
The authors of some recent studies11,12,39,40 suggested that Treg-mediated suppression and PD-1–mediated T-cell dysfunction are 2 independent yet synergistic mechanisms primarily governing peripheral immune tolerance. Notably, 2 reports41,42 demonstrated a nonoverlapping expression pattern of Foxp3 and PD-1 on tumor-infiltrating CD4+ T cells from lymphoma patients. Our study is consistent with these reports in that Treg and effector cells are both present in tumor environment, although we clearly show that both populations significantly up-regulated PD-1 during tumor progression (Figure 1B). We suspect that PD-1 is a contributor but not the sole cause of tumor-specific CD4+ T-cell dysfunction because simply making the HA-specific T cells deficient in PD-1 expression does not correct the defects in effector differentiation (Figure 3C-F). We predict that the tolerogenic milieu in tumor-bearing hosts is multifactoral and includes the influence of Tregs as well as other mechanisms. Cy conditioning can comprehensively mitigate these tolerogenic mechanisms, including inhibitory PD-1/PD-L1 pathway and Treg suppression, and create an immune milieu receptive to productive CD4+ T-cell differentiation. The key point shown by our studies is that CD4+ T cells have the option for either aberrant or productive effector differentiation in tumor-bearing hosts, and that productive differentiation can be strongly promoted by pretreatment with Cy.
One of the most striking effects of Cy treatment was to support extensive effector cell division without loss of IL-7R (CD127). It has been shown that IL-7 plays pivotal roles in T-cell lymphopoiesis, peripheral homeostasis, and memory cell survival.43 IL-7R is constitutively expressed on naive T cells and down-regulated upon T-cell activation. Reexpression of IL-7R on a subset of effector cells enables them to differentiate into memory T cells.31,44 Administration of exogenous recombinant IL-7 drives enhanced T-cell response to tumors.45 In our system, the sustained IL-7R expression on a large number of effector cells generated in Cy-conditioned tumor-bearing mice suggests a robust proliferative and survival advantage and may imply a highly efficient effector-to-memory transition. This feature appeared to be particular to Cy preconditioning of the tumor-bearing host because vaccinia virus–primed CD4+ T cells, normally deemed as potent effector cells, did not maintain IL-7R expression in hosts with established tumors (Figure 7C).
In conclusion, the current study demonstrates that the default differentiation pathway of antitumor CD4+ T cells is profoundly defective in tumor-bearing hosts. Not only may these cells become suppressive Tregs, but even those that appear to retain an effector phenotype are functionally impaired, fail to expand effectively, and are prone to apoptosis. Strikingly, however, we show that a single dose of cytotoxic chemotherapy can effectively reverse this aberrant CD4+ T-cell response and promote vigorous clonal expansion and beneficial effector differentiation. These findings illustrate the importance of disrupting the immunosuppressive and tolerogenic milieu of the tumor-bearing host to promote effective CD4+ T-cell immune responses and provide a robust, clinically applicable mechanism for doing so.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Dr Hyam I. Levitsky (The Johns Hopkins University School of Medicine) and Dr Tasuku Honjo (Kyoto University) for transgenic mice; Dr Yukai He for insightful discussion; Drs. Phillip Chandler and Deyan Hou for reagents; and Judy Gregory and Doris McCool for able technical assistance.
This work was supported by Medical College of Georgia Special Funding Initiative (to G.Z.) and the National Institutes of Health (grants R01 CA72669 and P01 AI056299 to B.R.B.; grant R01 CA096651 to D.H.M.).
National Institutes of Health
Authorship
Contribution: Z.-C.D. performed research and analyzed results; B.R.B., A.L.M., and D.H.M. contributed reagents, discussed results, and edited the paper; and G.Z. designed and performed research, analyzed results, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Gang Zhou, Cancer Immunotherapy Program, Medical College of Georgia Cancer Center, Medical College of Georgia, 1120 15th St, CN-4140, Augusta, GA 30912; e-mail: GZHOU@mcg.edu.







This feature is available to Subscribers Only
Sign In or Create an Account Close Modal