Abstract
Functional inactivation of self-reactive T lymphocytes contributes to the maintenance of immunologic self-tolerance. At the same time, tolerance induction limits immune responses against tumors expressing tolerizing self-antigens. Some cancer therapies include the adoptive transfer of tumor-reactive T lymphocytes into lymphopenic patients. Lymphopenia provides an activation signal to T lymphocytes, which undergo lymphopenia-induced proliferation (LIP), acquire effector functions, and reject tumors. However, it is so far unknown to which extent LIP may result in reversal of established antigen-specific CD8 T-cell tolerance. Here, we report that neonatally induced dominant CD8 T-cell tolerance remained stable under lymphopenic conditions also in the presence of systemic inflammation induced by Toll-like receptor ligands. However, when lymphopenic recipients were irradiated, the tolerant status was lost, because CD8 T cells acquired effector functions in an interleukin-15–dependent fashion and efficiently rejected tumors. In conclusion, we show that lymphopenia is not sufficient to break CD8 T-cell tolerance. Furthermore, we demonstrate that pretreatment regimens are crucial to circumvent this problem and to optimize adoptive T-cell therapy.
Introduction
The immune system is balancing protection of the person against the world of pathogens with the need for tolerance toward self-antigens. Several mechanisms operating in the thymus as well as in the periphery contribute to the deletion or inactivation of self-reactive lymphocytes, thereby controlling the potentially self-destructive capacity of the immune system.1,2 Although these tolerance mechanisms are beneficial in avoiding autoimmune diseases, they represent major hurdles in directing immune responses against tumors.3,4 Sophisticated immunization protocols as well as adoptive transfers of in vitro activated tumor-specific T cells are presently being used to overcome tolerance to tumor-associated antigens and to establish a potent cancer therapy.4-6 These strategies are frequently combined with either chemotherapy or total body irradiation.4-7 The treatments usually cause lymphopenia, which is associated with the increased availability of growth-promoting cytokines and self-peptide major histocompatibility complex complexes. These homeostatic factors trigger T-cell activation under lymphopenic conditions. Thus, T lymphocytes undergo lymphopenia-induced proliferation (LIP), develop a memory-like phenotype and acquire effector functions.8-10 As a consequence of LIP, T cells acquire the capacity to promote tissue destruction as shown for example for the pancreas and the eye or proposed for the case of acute or chronic graft-versus-host disease.11-18 It is, therefore, presently discussed whether the induction of lymphopenia may be a beneficial addition to cancer therapy to brake T-cell tolerance against tumors.3-8,13-19 We have previously shown that CD8 T cells are tolerized in the neonatal phase if their cognate antigen is expressed exclusively on keratinocytes.20 This type of tolerance induction is unique for the neonatal phase, because keratinocytes are not accessible for naive CD8 T cells in the adult anymore. Hence, a pool of tolerant CD8 T cells can be generated in the neonatal phase which persists until adulthood, remains tolerant, and is incapable of rejecting tumors expressing their cognate antigen.21 Although it was shown that homeostatic expansion can revert peptide-induced CD8 T-cell anergy and promote antitumor responses,22 it remained unclear whether this is also true for the remarkably robust form of peripheral self-tolerance that is induced in the neonate.
Here, we demonstrate that lymphopenia causes homeostatic expansion of neonatally tolerized CD8 T cells although antigen-specific tolerance remained intact even in the presence of systemic inflammation. However, LIP combined with irradiation, but not chemotherapy, reversed the tolerant status of CD8 T cells reliant on interleukin-15 (IL-15) and induced effective antitumor T-cell reactivity. These results could have important implications for tumor therapy protocols. They suggest that lymphopenia induced, for example, by chemotherapy may not result in reversal of tolerance but in homeostatic expansion of tolerant T cells. On the contrary, irradiation-induced lymphopenia may not only support transferred effector T cells but may also revert endogenous tolerant CD8 T cells.
Methods
Mice
Désiré-TCR (T-cell receptor) mice (Des-TCR mice, H2-Kk background) contain the transgene for a Kb-specific TCR.23 2.4KeratinIV-Kb mice (2.4KerIV-Kb, H2-Kd background) express the Kb molecule under control of the 2.4KeratinIV-promoter in keratinocytes of the skin.20 Des-TCR and 2.4KerIV-Kb transgenic mice were crossed to the recombination-activating gene 2-deficient (RAG2−/−) background21 and mated as single-transgenic mice or double-transgenic mice. C57BL/6N and CBA/N mice were obtained from Charles River Breeding Laboratories. CBA.Kb (CBK) mice were provided by A. L. Mellor (Medical College of Georgia). The IL-15−/− mice were purchased from Taconic and crossed with CBA/N mice to a H2-Kk background. As indicated, mice were sublethally irradiated with 3 or 6 Gy (x-ray source; Buchler; 0.701 Gy/min). All mice were kept under specific pathogen-free conditions at the central animal facility of the German Cancer Research Center. Animal care was approved by the regional regulating authorities.
Thymectomy
Fourteen-day-old mice were anesthetized with a mixture of ketamine (Fort Dodge Laboratories) and xylazine (Abbott Laboratories). A midline incision in the upper thoracic region exposed the thymic lobes. The thymus was then removed by suction. The mice were kept warm with a heat lamp and observed after surgery until they regained consciousness.
Tumor transplantation
The cell line P815 has been transfected with the genes encoding for the murine major histocompatibility complex class I molecule H-2Kb and the B7-1 gene21 and was cultured in DMEM containing 10% heat-inactivated fetal calf serum, 10mM HEPES, 2mM l-glutamine, 1mM sodium pyruvate, 0.05mM 2-mercaptoethanol, 100 U/mL penicillin, and 100 mg/mL streptomycin. P815.Kb.B7 cells were stably transfected with the murine IL-15 cDNA. IL-15 expression was verified by quantitative reverse transcription–polymerase chain reaction technology (supplementary Figure 1, available on the Blood website; see the Supplemental Materials link at the top of the online article). Cells of the respective clone (2 × 105) were injected subcutaneously 3 days after T-cell transfer, and tumor growth was followed. On large tumor burden the mice were killed. Tumor-free mice were continued to be followed for up to 4 weeks.
Antibodies, cytokines, and flow cytometry
Fluorochrome-conjugated and isotype-matched control monoclonal antibodies (mAbs) were obtained from BD Biosciences or R&D Systems. IL-15 and sIL-15Rα-Fc (R&D Systems) were coincubated for 20 minutes at 37°C before injection into mice.25 Each mouse received 1.5 μg of IL-15 and 7 μg of sIL-15Rα-Fc intraperitoneally 1 day after tumor injection. For the detection of interferon-γ (IFN-γ), an intracellular staining kit was used according to the manufacturer's instructions (BD Biosciences). To stimulate T cells before fixation, tissue-culture plates were coated with 4 μg/mL anti-CD3 mAb. Splenic T cells were cultured in the respective plates for 6 hours in complete RPMI 1640 (plus 10mM HEPES, 2mM l-glutamine, 1mM sodium pyruvate, 0.05mM 2-mercaptoethanol, 100 U/mL penicillin, and 100 mg/mL streptomycin) in the presence of brefeldin A. Flow cytometric analyses were done on a FACSCalibur with CellQuest software (BD Biosciences).
BrdU treatment
For bromodeoxyuridine (BrdU) incorporation studies, mice were given drinking water containing 0.8 mg/mL BrdU (Sigma-Aldrich); the water was changed daily. After surface staining cells were washed, resuspended in ice-cold 0.15M NaCl, and fixed by dropwise addition of cold 95% ethanol. Cells were then incubated for 30 minutes on ice, washed, and incubated with phosphate-buffered saline (PBS) containing 1% paraformaldehyde and 0.01% Tween 20 for 1 hour. Thereafter, cells were treated with 50 U of DNase I (Sigma-Aldrich) in 0.15M NaCl, 4.2mM MgCl2, pH 5, for 10 minutes. After washing, cells were stained with phycoerythrin-conjugated anti-BrdU mAb (BD Biosciences) and analyzed by flow cytometry.
T-cell in vitro proliferation
Splenocytes were cultured with the indicated stimuli (medium: RPMI with 10% fetal calf serum, 2mM l-glutamine, 100 U/mL penicillin/streptomycin, 1mM Na-pyruvate, and 0.1mM 2-mercaptoethanol). Anti-CD3 and anti-CD28 antibodies were obtained from BD Biosciences. After 72 hours, 0.5 μCi (0.0185 Bq) of [3H]thymidine was added 18 hours before termination of the culture, and the number of counts per minute (cpm) was measured in a β-counter. All cultures were set up in 96-well plates in triplicate wells.
Cytotoxic T lymphocyte–mediated cytotoxicity in vivo
Specific cytotoxic T lymphocyte activity in vivo was quantified against cells presenting H-2Kb complexes.23 Briefly, CBK spleen target cells from naive donor mice were labeled with a high carboxyfluorescein diacetate succinimidyl ester (CFSE) concentration. CBA splenocytes labeled with a low CFSE concentration were used as nonspecific target cells. The 2 target cell populations were mixed in equal ratio, and 20 × 106 cells in total were transferred intravenously into effector mice. Four hours later, the specific CTL activity was quantified in spleens of these mice. The ratio between CFSEhi and CFSElo cells recovered in nontransferred RAG2−/− mice indicated 0% specific kill activity.
Adoptive T-cell transfer
Single-cell suspensions of spleens were prepared from mice of the indicated strains. Splenocytes (2 × 106) containing 5% CD8 T cells were injected intravenously. In some experiments CD8 T cells were purified with the use of CD8-specific microbeads (Miltenyi Biotec) according to the manufacturer's instructions. The obtained purities ranged from 85% to 95%. For CFSE labeling, splenocytes were incubated with 5μM CFSE (Molecular Probes) in Dulbecco PBS (DPBS) for 15 minutes at 37°C. Cells were washed twice with ice-cold DPBS and were finally resuspended in DPBS before intravenous injection. Splenocytes or lymph node cells of 1 to 3 pooled recipient mice were analyzed at the indicated time points.
Toll-like receptor ligands
Lipopolysaccharide (LPS) and poly (I:C) were obtained from Sigma-Aldrich. Phosphorothioate-stabilized CpG (TCC ATG ACG TTC CTG ATG CT) oligonucleotides were synthesized by TIB Molbiol.
Statistical analysis
The Student t test with SigmaStat software (SPSS Inc) was used to test significant numerical differences between groups. P values of less than .05 were regarded as statistically significant.
Results
Comparable expansion of CD8 T cells in thymectomized Kb-tolerant and Kb-reactive mice
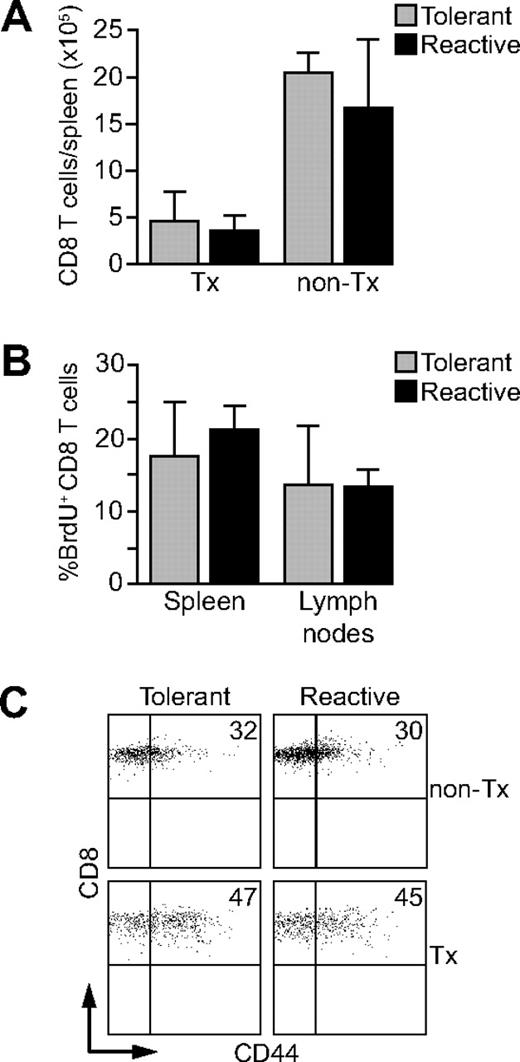
CD8 Des-TCR transgenic T cells23 are rendered tolerant in the neonatal phase if their cognate antigen H2-Kb is expressed selectively on keratinocytes.20 We have shown previously that adult Rag2−/− mice expressing the Des-TCR transgene on CD8 T cells and H2-Kb on keratinocytes (Des-TCR × 2.4KerIV-Kb.RAG2−/− mice) accumulate tolerant CD8 T cells thymectomized after the neonatal period.21 Tolerant CD8 T cells are similarly abundant in Des-TCR × 2.4KerIV-Kb.RAG2−/− mice compared with nontolerant CD8 T cells in Des-TCR.RAG2−/− mice (Figure 1A). Nevertheless, H2-Kb–dependent, CD8 T cell–mediated tumor rejection is strongly impaired in tolerant Des-TCR × 2.4KerIV-Kb.RAG2−/− mice, showing that functional rather than deletional tolerance is operative in our system. It is known that thymectomy causes lymphopenia.24 As expected, numbers of Des-TCR CD8 T cells were reduced by a factor of 3 in spleens of thymectomized Des-TCR.RAG2−/− mice compared with untreated controls (Figure 1A). Tolerant and nontolerant Des-TCR CD8 T cells are equally abundant in the spleen of thymectomized animals (Figure 1A21 ), indicating that thymectomy-induced lymphopenia is equally pronounced in both mouse lines. This suggested that functional differences between reactive and tolerant Des-TCR CD8 T cells do not affect their steady-state turnover in lymphopenic mice. This was confirmed by BrdU-based in vivo cell cycle analysis. As shown in Figure 1B, BrdU+ CD8 T cells were similarly abundant in thymectomized Des-TCR × 2.4KerIV-Kb.RAG2−/− mice (termed Kb-tolerant hereafter) and Des-TCR.RAG2−/− mice (termed Kb-reactive hereafter). Furthermore, the percentages of CD44hi CD8 T cells were elevated in both mouse lines compared with nonthymetomized controls (Figure 1C). These results show that Des-TCR CD8 T cells from Kb-tolerant and Kb-reactive mice are not differently affected in their steady-state proliferation and CD44 expression by thymectomy-induced lymphopenia.
Des-TCR CD8 T cells in Kb-tolerant and Kb-reactive mice do not differ in homeostatic expansion and CD44 expression. (A) The absolute number of CD8 T cells in spleens of adult nonthymectomized (non-Tx) and thymectomized (Tx) Des-TCR × 2.4KerIV-Kb.RAG2−/− (tolerant) and Des-TCR.RAG2−/− (reactive) mice were determined (at the age of 9 weeks; n = 5-9 mice/group; P < .01 for the respective nonthymectomized versus thymectomized mice). (B) Three-week-old thymectomized Des-TCR × 2.4KerIV-Kb.RAG2−/− (tolerant) and thymectomized Des-TCR.RAG2−/− (reactive) mice were given BrdU (0.8 mg/mL) into the drinking water for 10 days. Six weeks later, spleen and lymph node CD8 T cells were analyzed by flow cytometry for incorporation of BrdU. Shown are mean percentages ± SD of BrdU-positive CD8 T cells (n = 4). (C) Splenocytes from thymectomized (Tx) and from nonthymectomized (non-Tx) Des-TCR × 2.4KerIV-Kb.RAG2−/− (tolerant; percentage of CD44high CD8 T cells: 47.3% ± 13.8% versus 32.4% ± 3.6%, P < .05) and Des-TCR.RAG2−/− mice (reactive; percentage of CD44high CD8 T cells: 44.6% ± 12.9% versus 30.0% ± 4.2% P < .05) were analyzed by flow cytometry for CD44 expression after gating on Des-TCR CD8 T cells. Shown are relative log fluorescence intensities for CD8 and CD44. The numbers represent the mean percentages of CD44high CD8 T cells and were pooled from 4 independent experiments.
Des-TCR CD8 T cells in Kb-tolerant and Kb-reactive mice do not differ in homeostatic expansion and CD44 expression. (A) The absolute number of CD8 T cells in spleens of adult nonthymectomized (non-Tx) and thymectomized (Tx) Des-TCR × 2.4KerIV-Kb.RAG2−/− (tolerant) and Des-TCR.RAG2−/− (reactive) mice were determined (at the age of 9 weeks; n = 5-9 mice/group; P < .01 for the respective nonthymectomized versus thymectomized mice). (B) Three-week-old thymectomized Des-TCR × 2.4KerIV-Kb.RAG2−/− (tolerant) and thymectomized Des-TCR.RAG2−/− (reactive) mice were given BrdU (0.8 mg/mL) into the drinking water for 10 days. Six weeks later, spleen and lymph node CD8 T cells were analyzed by flow cytometry for incorporation of BrdU. Shown are mean percentages ± SD of BrdU-positive CD8 T cells (n = 4). (C) Splenocytes from thymectomized (Tx) and from nonthymectomized (non-Tx) Des-TCR × 2.4KerIV-Kb.RAG2−/− (tolerant; percentage of CD44high CD8 T cells: 47.3% ± 13.8% versus 32.4% ± 3.6%, P < .05) and Des-TCR.RAG2−/− mice (reactive; percentage of CD44high CD8 T cells: 44.6% ± 12.9% versus 30.0% ± 4.2% P < .05) were analyzed by flow cytometry for CD44 expression after gating on Des-TCR CD8 T cells. Shown are relative log fluorescence intensities for CD8 and CD44. The numbers represent the mean percentages of CD44high CD8 T cells and were pooled from 4 independent experiments.
CD8 T cells of Kb-tolerant and Kb-reactive mice undergo LIP
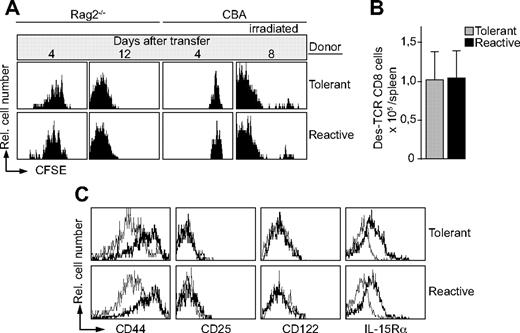
So far we analyzed resident CD8 T cells in the periphery of mice, which were rendered lymphopenic by thymectomy. However, these experiments did not allow a conclusion on the potential of tolerant and reactive Des-TCR CD8 T cells to undergo LIP. To address this issue we performed adoptive T-cell transfers. CD8 T cells of Kb-tolerant and Kb-reactive mice were labeled with CFSE and transferred intravenously into RAG2−/− mice. Similar proliferation rates were observed 4 days after transfer (Figure 2A). No proliferation was found after transfer into lymphoreplete CBA mice (Figure 2A). After 12 days all CD8 T cells adoptively transferred into RAG2−/− mice had lost the CFSE dye, and comparable numbers of CD8 T cells were present in spleens of the respective recipients (Figure 2B). Furthermore, similar proliferation rates were observed when CD8 T cells of Kb-tolerant and Kb-reactive mice were transferred into sublethally irradiated CBA mice (Figure 2A). Hence, irradiation-induced lymphopenia was sufficient to induce LIP of both types of T-cell populations. Consistent with their vigorous proliferation, tolerant and reactive CD8 T-cell populations isolated from RAG2−/− recipients showed similar expression patterns for several cell-surface molecules (Figure 2C). For example, CD44 and IL-15Rα were up-regulated after LIP, whereas no change in expression of CD25, CD122 (Figure 2C), CD69, CD8, and TCR (data not shown) was observed. In summary, lymphopenia induces rapid and extensive proliferation of the Kb-tolerant CD8 T-cell population to the same extent as observed for CD8 T cells of Kb-reactive mice.
Kb-tolerant Des-TCR CD8 T cells divide after transfer into lymphopenic mice. (A) CSFE-labeled splenocytes (2 × 106) from thymectomized Des-TCR × 2.4KerIV-Kb.RAG2−/− mice (tolerant) and thymectomized Des-TCR.RAG2−/− mice (reactive) were transferred intravenously into RAG2−/− mice, CBA mice, as well as irradiated CBA mice (6 Gy, 1 day before transfer). At the indicated time points, host splenocytes (n > 4 per time point) were analyzed by flow cytometry. Shown are relative log fluorescence intensities for CFSE after gating on Des-TCR CD8 cells. (B) The absolute number of Des-TCR CD8 T cells was determined 12 days after transfer into RAG2−/− mice. (C) The indicated surface marker expression level on Des-TCR CD8 T cells was determined by flow cytometry before (thin line) and 10 days after (bold line) transfer of 2 × 106 splenocytes from thymectomized Des-TCR × 2.4KerIV-Kb.RAG2−/− (tolerant) and thymectomized Des-TCR.RAG2−/− mice (reactive) into RAG2−/− mice. Specificity of antibody staining was verified with the respective isotype-matched control antibody. Shown are relative log fluorescence intensities for the indicted cell surface molecules. Representative data from 2 to 4 independent experiments are shown (5 to 8 mice per group in total).
Kb-tolerant Des-TCR CD8 T cells divide after transfer into lymphopenic mice. (A) CSFE-labeled splenocytes (2 × 106) from thymectomized Des-TCR × 2.4KerIV-Kb.RAG2−/− mice (tolerant) and thymectomized Des-TCR.RAG2−/− mice (reactive) were transferred intravenously into RAG2−/− mice, CBA mice, as well as irradiated CBA mice (6 Gy, 1 day before transfer). At the indicated time points, host splenocytes (n > 4 per time point) were analyzed by flow cytometry. Shown are relative log fluorescence intensities for CFSE after gating on Des-TCR CD8 cells. (B) The absolute number of Des-TCR CD8 T cells was determined 12 days after transfer into RAG2−/− mice. (C) The indicated surface marker expression level on Des-TCR CD8 T cells was determined by flow cytometry before (thin line) and 10 days after (bold line) transfer of 2 × 106 splenocytes from thymectomized Des-TCR × 2.4KerIV-Kb.RAG2−/− (tolerant) and thymectomized Des-TCR.RAG2−/− mice (reactive) into RAG2−/− mice. Specificity of antibody staining was verified with the respective isotype-matched control antibody. Shown are relative log fluorescence intensities for the indicted cell surface molecules. Representative data from 2 to 4 independent experiments are shown (5 to 8 mice per group in total).
LIP does not break tolerance
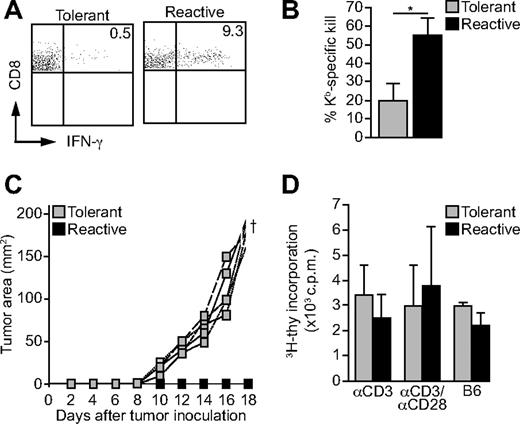
It is well established that CD8 T cells acquire effector functions as a consequence of LIP, among them the capacity to produce IFN-γ.8-10 Accordingly, T cells from Kb-reactive donor mice readily produced IFN-γ when isolated 7 to 10 days after transfer into RAG2−/− mice (Figure 3A). In contrast, only a very few cells of the tolerant CD8 T-cell population from Kb-tolerant donor mice showed IFN-γ production (Figure 3A). Furthermore, the cytolytic activity of tolerant CD8 T cells was strongly reduced in vivo when analyzed after LIP (Figure 3B). In addition, when RAG2−/− mice were challenged with 2 × 105 P815.Kb.B7 tumor cells and reconstituted with Kb-tolerant or Kb-reactive CD8 T cells 3 days beforehand, only those mice receiving tolerant CD8 T cells developed tumors (Figure 3C; Table 1). However, in vitro proliferation of Kb-tolerant and Kb-reactive CD8 T cells isolated from RAG2−/− recipients did not differ significantly (Figure 3D). Hence, the inability of Kb-tolerant CD8 T cells to gain effector functions under lymphopenic conditions remains uncoupled from their proliferative potential. In conclusion, lymphopenia promotes the expansion of tolerant CD8 T cells but not their gain of effector functions.
Tolerant Des-TCR CD8 T cells fail to acquire effector function after LIP. (A) Seven to 10 days after transfer of 2 × 106 splenocytes from thymectomized Des-TCR × 2.4KerIV-Kb.RAG2−/− (tolerant) and thymectomized Des-TCR.RAG2−/− (reactive) donor mice into RAG2−/− mice host splenocytes were restimulated in vitro for 6 hours with anti-CD3 antibody (4 μg/mL). Des-TCR CD8 T cells were analyzed for intracellular IFN-γ production by flow cytometry. Shown are relative log fluorescence intensities for CD8 and IFN-γ. Representative diagrams are depicted; numbers indicate mean percentages of IFN-γ–positive CD8 T cells (0.5% ± 0.7% versus 9.3% ± 7.6%; P < .01) and were pooled from 3 independent experiments (n = 8). (B) In vivo kill assay of Kb-tolerant and Kb-reactive Des-TCR CD8 T cells (2 × 106 splenocytes per mouse) analyzed 10 days after T-cell transfer into RAG2−/− mice. Splenocytes from CBA and CBK mice were used as targets (control = RAG2−/− mice without transfer). The data were pooled from 2 separate experiments (*P < .05; n = 8 per group). (C) Splenocytes (2 × 106) from Kb-tolerant and Kb-reactive donor mice were transferred intravenously into RAG2−/− mice, which were challenged subcutaneously with P815.Kb.B7 tumor cells 3 days later (see Table 1). Representative tumor growth curves of 4 individual mice per group are shown. Data are representative for 3 independent experiments. (D) Splenocytes (2 × 106) from tolerant and reactive donor mice (as above) were transferred intravenously into RAG2−/− mice. Ten days later recipient splenocytes were restimulated in vitro with αCD3 (4 μg/mL), αCD3, and αCD28 (2 and 4 μg/mL) or irradiated C57BL/6 splenocytes (B6), and 3H-thymidine incorporation was measured after 4 days of culture.
Tolerant Des-TCR CD8 T cells fail to acquire effector function after LIP. (A) Seven to 10 days after transfer of 2 × 106 splenocytes from thymectomized Des-TCR × 2.4KerIV-Kb.RAG2−/− (tolerant) and thymectomized Des-TCR.RAG2−/− (reactive) donor mice into RAG2−/− mice host splenocytes were restimulated in vitro for 6 hours with anti-CD3 antibody (4 μg/mL). Des-TCR CD8 T cells were analyzed for intracellular IFN-γ production by flow cytometry. Shown are relative log fluorescence intensities for CD8 and IFN-γ. Representative diagrams are depicted; numbers indicate mean percentages of IFN-γ–positive CD8 T cells (0.5% ± 0.7% versus 9.3% ± 7.6%; P < .01) and were pooled from 3 independent experiments (n = 8). (B) In vivo kill assay of Kb-tolerant and Kb-reactive Des-TCR CD8 T cells (2 × 106 splenocytes per mouse) analyzed 10 days after T-cell transfer into RAG2−/− mice. Splenocytes from CBA and CBK mice were used as targets (control = RAG2−/− mice without transfer). The data were pooled from 2 separate experiments (*P < .05; n = 8 per group). (C) Splenocytes (2 × 106) from Kb-tolerant and Kb-reactive donor mice were transferred intravenously into RAG2−/− mice, which were challenged subcutaneously with P815.Kb.B7 tumor cells 3 days later (see Table 1). Representative tumor growth curves of 4 individual mice per group are shown. Data are representative for 3 independent experiments. (D) Splenocytes (2 × 106) from tolerant and reactive donor mice (as above) were transferred intravenously into RAG2−/− mice. Ten days later recipient splenocytes were restimulated in vitro with αCD3 (4 μg/mL), αCD3, and αCD28 (2 and 4 μg/mL) or irradiated C57BL/6 splenocytes (B6), and 3H-thymidine incorporation was measured after 4 days of culture.
Homeostatic proliferation of Des-TCR CD8 T cells does not break tolerance
| Donor mouse . | Incidence of tumor growth (P815.Kb.B7) . | |
|---|---|---|
| 1 | No cell transfer | 14 of 14 |
| 2 | Des-TCR.RAG2−/− | 0 of 7 |
| 3 | Des-TCR.RAG2−/− (Tx) | 2 of 7 |
| 4 | Des-TCR × 2.4KerIV-Kb.RAG2−/− (Tx) | 14 of 15 |
| Donor mouse . | Incidence of tumor growth (P815.Kb.B7) . | |
|---|---|---|
| 1 | No cell transfer | 14 of 14 |
| 2 | Des-TCR.RAG2−/− | 0 of 7 |
| 3 | Des-TCR.RAG2−/− (Tx) | 2 of 7 |
| 4 | Des-TCR × 2.4KerIV-Kb.RAG2−/− (Tx) | 14 of 15 |
Thirty thousand MACS-purified Des-TCR CD8 T cells from reactive Des-TCR.RAG2−/−, thymectomized reactive Des-TCR.RAG2−/−, and thymectomized tolerant Des-TCR × 2.4KerIV-Kb.RAG2−/− donor mice were transferred intravenously into RAG2−/− mice. After 3 days the mice were challenged subcutaneously with 2 × 105 P815.Kb.B7 tumor cells and the mice were then analyzed for tumor growth. After 14 to 18 days, most tumor-bearing mice had to be sacrificed due to the size of the tumors. Tumor-free mice were continued to be observed. The summarized data are from 3 independent experiments.
LIP of CD8 T cells during systemic inflammation preserves or endangers tolerance dependent on the nature of the stimulatory milieu
It has been suggested that lymphopenia in combination with inflammatory signals induced by infections might cause T cell–mediated autoreactivity.13,14 Thus, we analyzed the effect of systemic inflammation on the CD8 T-cell population of Kb-tolerant mice in combination with LIP. For this, we first mimicked an acute infection by the application of a bolus dose of ligands for Toll-like receptor 3 (TLR3), TLR4, or TLR9. Up-regulation of CD80 on splenic dendritic cells and CD69 on all natural killer cells 18 hours after injection of CpG oligodeoxynucleotide, poly (I:C), or LPS into RAG2−/− mice confirmed the induction of a strong inflammatory response (data not shown). CD8 T cells of Kb-tolerant mice were subsequently transferred into treated RAG2−/− mice to induce LIP during the TLR stimulation. The expanding tolerant CD8 T-cell population failed to generate Kb-specific effector function because P815.Kb.B7 tumor grafts were accepted and because the number of CD8 T cells producing IFN-γ remained uniformly low (Table 2). Nearly all Des-TCR.RAG2−/− control mice rejected the tumor. Moreover, even multiple injections of CpG or the combination of CpG and LPS failed to break tolerance (Table 2). Thus, systemic inflammation induced by TLR ligands in combination with LIP did not revert T-cell tolerance.
Homeostatic proliferation of Des-TCR CD8 T cells during systemic inflammation does not break tolerance
| Donor T cells . | TLR ligand . | Incidence of tumor growth (P815.Kb.B7) . | Mean percent IFNγ-positive CD8 T cells ± SD . | |
|---|---|---|---|---|
| 1 | No cell transfer | CpG | 8 of 8* | |
| 2 | Tolerant Des-TCR CD8 | 9 of 9* and | ||
| 9 of 9† | 0.5 ± 0.4† | |||
| 3 | Tolerant Des-TCR CD8 | CpG | 8 of 8* and | |
| 8 of 9† | 0.6 ± 0.6† | |||
| 4 | Tolerant Des-TCR CD8 | 4× CpG | 5 of 5† | 1.1 ± 0.8† |
| 5 | Tolerant Des-TCR CD8 | Poly(I:C) | 10 of 10† | 0.7 ± 0.3† |
| 6 | Tolerant Des-TCR CD8 | LPS | 5 of 5† | 1.4 ± 0.4† |
| 7 | Tolerant Des-TCR CD8 | CpG/LPS | 5 of 5† | |
| 8 | Des-TCR.RAG2−/− (control mice) | 1 of 20 | 10.6 ± 7.8† | |
| Donor T cells . | TLR ligand . | Incidence of tumor growth (P815.Kb.B7) . | Mean percent IFNγ-positive CD8 T cells ± SD . | |
|---|---|---|---|---|
| 1 | No cell transfer | CpG | 8 of 8* | |
| 2 | Tolerant Des-TCR CD8 | 9 of 9* and | ||
| 9 of 9† | 0.5 ± 0.4† | |||
| 3 | Tolerant Des-TCR CD8 | CpG | 8 of 8* and | |
| 8 of 9† | 0.6 ± 0.6† | |||
| 4 | Tolerant Des-TCR CD8 | 4× CpG | 5 of 5† | 1.1 ± 0.8† |
| 5 | Tolerant Des-TCR CD8 | Poly(I:C) | 10 of 10† | 0.7 ± 0.3† |
| 6 | Tolerant Des-TCR CD8 | LPS | 5 of 5† | 1.4 ± 0.4† |
| 7 | Tolerant Des-TCR CD8 | CpG/LPS | 5 of 5† | |
| 8 | Des-TCR.RAG2−/− (control mice) | 1 of 20 | 10.6 ± 7.8† | |
As indicated, 120 μg of CpG-ODN was administered intraperitoneally 1 day before and 100 μg of poly(I:C) or 75 μg of LPS was administered intraperitoneally on the day of T-cell transfer. Group 4 received 120 μg of CpG on day −1 and 40 μg of CpG on days 2, 5, and 8. Group 7 received 40 μg of CpG on day −1 and 40 μg and LPS on day 0. Thirty thousand MACS-purified Des-TCR CD8 T cells (*) or
2 × 106 splenocytes (†) from thymectomized tolerant Des-TCR × 2.4KerIV-Kb.RAG2−/− donor mice were transferred intravenously into the respective RAG2−/− mice. After 3 days the mice were challenged subcutaneously with 2 × 105 P815.Kb.B7 tumor cells and tumor growth was followed. Upon large tumor burden the mice were sacrificed. Tumor-free mice were followed for up to 4 weeks. Des-TCR.RAG2−/− mice were used as a control for tumor rejection. For IFNγ staining, splenocytes were restimulated in vitro for 6 hours with anti-CD3 antibody (4 μg/mL) on day 18 after tumor injection. The pooled results were obtained in 3 independent experiments.
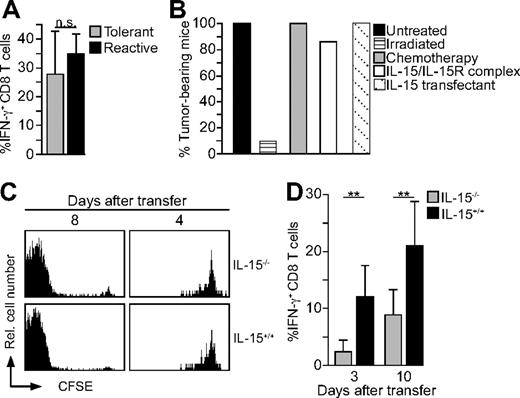
Irradiation of mice induces a strong response in many organs, including the liberation of cytokines and other inflammatory mediators, and thus might challenge tolerance if combined with LIP.26,27 Indeed, CD8 T cells of Kb-tolerant mice transferred into sublethally irradiated lymphopenic mice now produced IFN-γ to the same extent as control T cells of Kb-reactive mice did (Figure 4A). Furthermore, most sublethally irradiated RAG2−/− mice (3 Gy) receiving CD8 T cells of Kb-tolerant mice displayed no tumor growth even when they were continued to be observed for up to 4 weeks (Figure 4B; data not shown). In contrast, all nonirradiated recipients (Figure 4B) and all irradiated control RAG2−/− mice without T-cell transfer (data not shown) developed large tumor burden. Chemotherapy is often used to induce lymphopenia and improve adoptive T-cell therapy.4 However, treatment of RAG2−/− mice with melphalan (7.5 mg/kg intraperitoneally) instead of irradiation 2 days before adoptive transfer did not break tolerance of Kb-tolerant CD8 T cells (Figure 4B). Consequently, all treated mice developed tumors on challenge with P815.Kb.B7 tumor cells. Hence, irradiation but not chemotherapy allows the lymphopenia-associated activation of Kb-tolerant CD8 T cells.
CD8 T-cell tolerance is lost after transfer into irradiated lymphopenic mice dependent on the presence of IL-15. (A) Splenocytes (2 × 106) from thymectomized Des-TCR × 2.4KerIV-Kb.RAG2−/− mice (tolerant) and thymectomized Des-TCR.RAG2−/− mice (reactive) were injected intravenously into CBA mice irradiated with a dose of 6 Gy 1 day before transfer. Five days after transfer, recipient splenocytes were stimulated with anti-CD3 and analyzed by flow cytometry. Shown is the mean percentage of IFN-γ–producing CD8 T cells ± SD after gating on Des-TCR+ CD8 cells. Results are from 3 independent experiments which were summarized (n = 8). (B) Splenocytes (2 × 106) from thymectomized Des-TCR × 2.4KerIV-Kb.RAG2−/− mice (tolerant) were injected intravenously into RAG2−/− mice irradiated with a dose of 3 Gy 24 hours earlier. Nonirradiated control mice served as controls. P815.Kb.B7 cells were injected subcutaneously 3 days after T-cell transfer. The mice were monitored for up to 4 weeks for the occurrence of tumor growth. Data represent the respective percentage of tumor-bearing mice pooled from 3 independent experiments (n = 10). Alternatively, recipient RA2−/− mice were treated with melphalan (7.5 mg/kg intraperitoneally) 2 days before T-cell transfer, with IL-15/IL-15Rα complexes (1.5 μg of IL-15 and 7 μg of sIL-15Rα-Fc intraperitoneally 1 day after injection of tumor cells) or received P815.Kb.B7–IL-15 tumor cells (n = 7 for each group). (C) CSFE-labeled splenocytes (2 × 106) from thymectomized Des-TCR × 2.4KerIV-Kb.RAG2−/−mice (tolerant) were transferred intravenously into IL-15−/− or littermate IL-15+/+ mice, reisolated, and analyzed by flow cytometry (all H2-Kk background hosts were irradiated with 6 Gy 24 hours earlier, a minimum of 4 mice per time point). Shown are relative log fluorescence intensities for CFSE. (D) Similar experiments to panel A were analyzed on transfer of tolerant Des-TCR CD8 T cells into irradiated IL-15−/− or IL-15+/+ mice (6 Gy, H2-Kk) after 3 or 10 days (2 independent experiments and 7-9 mice per time point; **P < .01).
CD8 T-cell tolerance is lost after transfer into irradiated lymphopenic mice dependent on the presence of IL-15. (A) Splenocytes (2 × 106) from thymectomized Des-TCR × 2.4KerIV-Kb.RAG2−/− mice (tolerant) and thymectomized Des-TCR.RAG2−/− mice (reactive) were injected intravenously into CBA mice irradiated with a dose of 6 Gy 1 day before transfer. Five days after transfer, recipient splenocytes were stimulated with anti-CD3 and analyzed by flow cytometry. Shown is the mean percentage of IFN-γ–producing CD8 T cells ± SD after gating on Des-TCR+ CD8 cells. Results are from 3 independent experiments which were summarized (n = 8). (B) Splenocytes (2 × 106) from thymectomized Des-TCR × 2.4KerIV-Kb.RAG2−/− mice (tolerant) were injected intravenously into RAG2−/− mice irradiated with a dose of 3 Gy 24 hours earlier. Nonirradiated control mice served as controls. P815.Kb.B7 cells were injected subcutaneously 3 days after T-cell transfer. The mice were monitored for up to 4 weeks for the occurrence of tumor growth. Data represent the respective percentage of tumor-bearing mice pooled from 3 independent experiments (n = 10). Alternatively, recipient RA2−/− mice were treated with melphalan (7.5 mg/kg intraperitoneally) 2 days before T-cell transfer, with IL-15/IL-15Rα complexes (1.5 μg of IL-15 and 7 μg of sIL-15Rα-Fc intraperitoneally 1 day after injection of tumor cells) or received P815.Kb.B7–IL-15 tumor cells (n = 7 for each group). (C) CSFE-labeled splenocytes (2 × 106) from thymectomized Des-TCR × 2.4KerIV-Kb.RAG2−/−mice (tolerant) were transferred intravenously into IL-15−/− or littermate IL-15+/+ mice, reisolated, and analyzed by flow cytometry (all H2-Kk background hosts were irradiated with 6 Gy 24 hours earlier, a minimum of 4 mice per time point). Shown are relative log fluorescence intensities for CFSE. (D) Similar experiments to panel A were analyzed on transfer of tolerant Des-TCR CD8 T cells into irradiated IL-15−/− or IL-15+/+ mice (6 Gy, H2-Kk) after 3 or 10 days (2 independent experiments and 7-9 mice per time point; **P < .01).
Recombinant IL-15 has been reported to challenge CD8 T-cell tolerance in vitro.28 Therefore, we asked whether the irradiation-induced reversal of tolerance was IL-15 dependent. We first analyzed whether LIP of Kb-tolerant CD8 T cells is IL-15 dependent. As shown in Figure 4C, Kb-tolerant CD8 T cells proliferated equally well in irradiated IL-15−/− and IL-15+/+ mice. However, IFN-γ production was strongly impaired in Kb-tolerant CD8 T cells 3 and 10 days after transfer into IL-15−/− mice (Figure 4D). In contrast, Kb-tolerant CD8 T cells reisolated from IL-15+/+ mice produced IFN-γ at levels comparable to Figure 4A. To exclude a contribution of IL-15–producing donor cells to the reversal of tolerance, magnetic-activated cell sorting (MACS)–isolated CD8 T cells from lymphoreplete Des-TCR × 2.4KerIV-Kb.RAG2+/+ mice were transferred into irradiated IL-15+/+ and IL-15−/− mice. In these experiments, 12.5% plus or minus 1.3% CD8 T cells produced IFN-γ in irradiated IL-15+/+ mice but only 2.5% plus or minus 2.75% in irradiated IL-15−/− mice (P < .01). This strongly suggests that host- but not donor-derived IL-15 is required to induce IFN-γ production in adoptively transferred Des-TCR CD8 T cells. Next, we asked whether IL-15 could promote the loss of tolerance independent of irradiation. For this purpose, Kb-tolerant cells were transferred into nonirradiated Rag2−/− mice, challenged with P817.Kb.B7 and treated with IL-15/IL-15Rα complexes25 1 day after tumor cell injection. Because tumors grew out in all mice, we conclude that the transient oversupply of IL-15 is not sufficient to break tolerance of Kb-tolerant cells in nonirradiated Rag2−/− mice (Figure 4B). Even stable IL-15 production by P817.Kb.B7 tumor cells was not sufficient to induce tumor rejection by Kb-tolerant CD8 T cells in nonirradiated Rag2−/− mice (Figure 4B). These findings indicate that IL-15 is essential for the reversal of CD8 T-cell tolerance, but only in concert with as yet unknown factors, which are induced by irradiation.
Discussion
Loss of T lymphocytes because of infections, stress, or therapeutic manipulations may be compensated by homeostatic expansion of remaining or transferred T cells.8,9 Because proliferation of naive T cells under lymphopenic conditions results in T cells with effector functions, it is thought that LIP could increase the risk of autoimmune diseases13,14 and may be a beneficial addition to cancer therapy.4 However, our knowledge is rather limited how lymphopenia by itself or in combination with inflammatory stimuli could influence previously established antigen-specific CD8 T-cell tolerance. Therefore, we addressed these questions with the use of a transgenic mouse model of peripheral CD8 T-cell tolerance.20,21
As mentioned earlier LIP has been shown to revert CD8 T-cell anergy induced by intravenous injection of peptide to promote antitumor reactivity.22 In contrast, we show here that a tolerant CD8 T-cell population can expand to a similar extent as naive CD8 T cells under lymphopenic conditions (Figures 1–2) without gaining effector functions (Figure 3). We previously discussed that T cells may reach various levels of tolerance characterized by their capacity to be reactivated.29 Whereas peptide-induced anergy in the adult may easily be reverted by LIP, recognition of antigen on parenchymal cells in the neonate can generate a profound tolerance level, which enables CD8 T cells to actively expand under lymphopenic conditions without loosing their tolerant status.
It has been proposed that an inflammatory milieu and the loss or absence of regulatory CD4 T-cell function belong to the cofactors which can lead to autoimmunity during lymphopenia.13,14 We therefore tested whether stimulation of TLRs in the absence of regulatory CD4 T cells would revert tolerance of the CD8 T-cell population. However, neither single nor repetitive systemic application of TLR3, TLR4, or TLR9 ligands in combination with LIP induced a destructive T-cell response (Table 2). However, irradiation of lymphopenic recipients was sufficient to lead within the transferred tolerant CD8 T-cell population to the generation of IFN-γ–producing effector CD8 T cells effectively rejecting tumors (Figure 4). The CD8 T-cell population isolated from Kb-tolerant mice is characterized functionally by its capacity to tolerate Kb-positive skin and tumor transplants and to regulate naive Kb-specific CD8 T cells.21 Moreover, the tolerant CD8 T cells exhibit a specific gene expression profile, including up-regulation of granzyme B; transforming growth factor-β as well as transforming growth factor-β–induced genes and down-regulation of activation-related genes such as perforin21 (T.O., unpublished data, June 2008). However, we have not yet identified a cell surface marker to distinguish the individual tolerant CD8 T cell from naive or activated T cells possibly present in the tolerant T-cell population.20,21 Therefore, it is presently difficult to comment on the mechanism of the observed reversal of tolerance. There are multiple consequences of irradiation that may account for the reversal of tolerance, as for example changes in the availability of cytokines including IL-2, -7, and -15.4,26,27 IL-15 has been shown to break tolerance of CD8 T cells in an in vitro culture system.28 The rescued T cells were successfully used for adoptive cellular therapy thereafter.28 In our system, endogenous IL-15 is not necessary for the expansion and concomitant survival of tolerant CD8 T cells in vivo (Figure 4) but essential for the reversal of tolerance by LIP and irradiation (Figure 4). It is important to note that irradiation was not associated with a detectable increase of IL-15 mRNA levels in spleen and lymph nodes (data not shown). Thus, a possible enhancement of IL-15 bioavailability after irradiation might rather be due to a reduction of competing cells.4 In addition, reversal of CD8 T-cell tolerance requires as yet unknown irradiation-induced factors to synergize with IL-15. Together, our results suggest that the possible redirection of hyporesponsive CD8 T cells by LIP depends on their level of tolerance and a supportive IL-15–containing environment.
Furthermore, as discussed earlier, there are different levels of a tolerant status of a CD8 T cell.29 Yet, this holds true for the effector state of CD8 T cells.4 Here, we provide evidence that these divergent levels of T-cell reactivity are indistinguishable by cellular proliferation in vitro or in vivo. Therefore, it is conceivable that it may be complex to predict the therapeutic success or loss of an inhibited activity of a diverse T-cell population in the clinical situation.4 Thus, as evident by this study, there is the need to analyze multiple parameters of tumor-specific T cells.
Most often the immune system fails to control established tumor growth in the absence of therapeutic manipulation. Novel therapeutic strategies rely on the adoptive transfer of tumor-reactive lymphocytes.4-7 To generate high numbers of therapeutic T cells, tumor-reactive T cells are isolated from cancer patients to expand them in vitro. Alternatively, tumor-reactive TCR genes are introduced into blood T lymphocytes of patients with cancer with the help of retroviral vectors, and the modified T cells are subsequently reinfused.4-7 Lymphodepletion of the recipient is combined with adoptive cell transfer because T-cell manipulation in vitro and reinfusion during lymphopenic conditions is applied to overcome T-cell tolerance associated with tumor growth.4,5,7 On the basis of our results one might consider that either approach to generate therapeutic T cells and pretreatment of tumor-bearing patients might be associated with the expansion of tolerant, tumor-specific CD8 T cells. Depending on the conditions chosen, expanding tolerant CD8 T cells may not contribute to tumor rejection and might even block the function of potentially reactive CD8 T cells. Thus, in this respect chemotherapy may not lead to the reversal of T-cell tolerance. Yet, irradiation-induced lymphopenia may not only support transferred effector T cells but has the potential to also target remaining endogenous T cells with regulatory capacity.
In summary, we identify active division induced by lymphopenia as a novel functional potential of a tolerant CD8 T-cell population. Thus, CD8 T-cell tolerance induced by peripheral self-antigens may remain intact even in phases of lymphopenia and systemic, TLR-dependent inflammation. However, tolerance may be lost if LIP occurs in combination with distinct triggering events as shown for irradiation. Because, apparently, other cytokines are not able to compensate for the lack of IL-15 in this situation, we propose a central role for IL-15 in the observed challenge of CD8 T-cell tolerance in vivo.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the Deutsche Forschungsgemeinschaft (SFB 405 and TR36) and by research funding from the Sixth European Union Framework Programme for Reserach and Technological Development through the RISET Integrated Project.
This article reflects the views of its authors, and the European Commission is not liable for any use that may be made of the information contained herein.
Authorship
Contribution: T.O., M.P., and G.P. performed experiments and analyzed data; and T.O., G.J.H., B.A., and T.S. designed the research and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current address for T.S. is Charité, Campus Benjamin Franklin, Institute of Immunology, Hindenburgdamm 30, D-12200 Berlin, Germany.
Correspondence: Thilo Oelert, Division Molecular Immunology, German Cancer Research Center, Im Neuenheimer Feld 280, D-69120 Heidelberg, Germany; e-mail: t.oelert@dkfz.de.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal