In this issue of Blood, Fauriat and colleagues provide a thorough study of the pattern and timing of NK-cell cytokine and chemokine secretion in response to target cell recognition. Most importantly, the authors demonstrate that CD56dim NK cells rather than CD56bright NK cells are the major cytokine and chemokine producer upon target recognition.1
Natural killer (NK) cells are heterogeneous and are divided into CD56bright and CD56dim subsets according to surface CD56 expression, where CD56dim NK cells constitute approximately 90% of human peripheral blood NK cells and display high cytotoxicity ex vivo.2 Broadly speaking, 2 major classes of stimuli are responsible for NK-cell activation: target cell recognition and cytokine stimulation.3 Upon exogenous stimulation with proinflammatory cytokines such as interleukin-12 (IL-12), IL-15, and IL-18, CD56bright NK cells secrete a greater amount of cytokines (eg, interferon gamma [IFN-γ] and tumor necrosis factor alpha [TNF-α]) relative to CD56dim NK cells.4 The current study by Fauriat et al demonstrates that, in response to target cell recognition, CD56dim NK cells rather than CD56bright NK cells are primarily responsible for production of cytokines and chemokines (see figure).
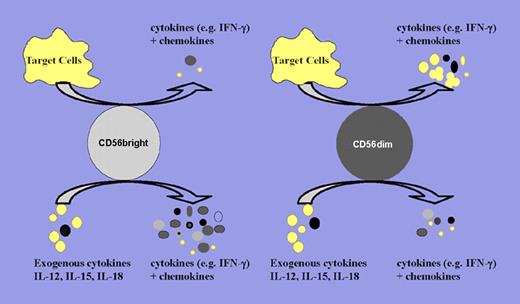
Schematic of CD56bright and CD56dim NK-cell cytokine and chemokine production upon target cell recognition and proinflammatory cytokine stimulation. Upon target cell recognition, CD56dim NK cells produce increased levels of cytokines and chemokines compared with CD56bright NK cells. In contrast, CD56bright NK cells are the major cytokine producers when stimulated by proinflammatory cytokines (eg, IL-12, IL-15, and IL-18) or combinations thereof. Larger dots denote cytokine secretions, and smaller dots denote chemokine secretions.
Schematic of CD56bright and CD56dim NK-cell cytokine and chemokine production upon target cell recognition and proinflammatory cytokine stimulation. Upon target cell recognition, CD56dim NK cells produce increased levels of cytokines and chemokines compared with CD56bright NK cells. In contrast, CD56bright NK cells are the major cytokine producers when stimulated by proinflammatory cytokines (eg, IL-12, IL-15, and IL-18) or combinations thereof. Larger dots denote cytokine secretions, and smaller dots denote chemokine secretions.
NK-cell recognition of target cells is based on specific receptor-ligand interactions, which trigger downstream signal transduction pathways and are ultimately integrated within the NK cell to determine its level of activity.5 Fauriat et al report that, upon stimulation by K562 target cells, NK cells produce cytokines such as IFN-γ and TNF-α, and chemokines MIP-1α, MIP-1β, RANTES, IL-8, MCP-1, and IP-10. By studying the temporal profile of cytokine and chemokine secretion and minimal receptor engagement requirements, the authors provide evidence that chemokine production by NK cells is an early feature of target cell recognition and requires fewer activating signals. However, TNF-α and IFN-γ production are more stringently controlled and require a greater need for receptor cooperation and result in delayed cytokine release upon target cell recognition. In addition, the authors demonstrate that exogenous cytokines (IL-12 plus IL-18) potentiate cytokine production by CD56dim NK cells in response to target cell recognition. Furthermore, individual NK cells show different responses depending on the number of activating ligands expressed by target cells, indicating a signaling threshold for NK-cell cytokine and chemokine production.
Some interesting questions are raised from the current study. First, the authors state that CD56bright and CD56dim subsets have similar expression levels of activating receptors. One question that remains is why these NK-cell subsets manifest different responses upon target cell recognition. Whether there is differential regulation of the signal transduction pathways downstream of these receptors in these NK subsets requires further study. Second, NK-cell responses to exogenous cytokines such as IL-12, IL-15, and IL-18 have been extensively studied. Do the intracellular signaling networks mediating NK-cell responses to target cells share similar mechanisms with the networks that mediate exogenous cytokine stimulation? Finally, it remains to be determined how these in vitro observations apply to in vivo microenvironments where both proinflammatory cytokines and target cells may coexist. Under these circumstances, future investigations will help clarify the division of labor among NK-cell subsets.
Although further studies are necessary to more fully understand NK-cell responses to target cell recognition, the work by Fauriat et al sheds light on the functional heterogeneity of human CD56bright and CD56dim NK-cell subsets. This study also advances our knowledge on how multiple NK cell–activating receptors contribute to cytokine and chemokine production of CD56dim NK cells upon target cell recognition. The authors show that CD56dim NK cells produce IFN-γ, rather than CD56bright NK cells, upon target cell recognition. Interestingly, less than 10% of CD56dim NK cells are IFN-γ–producing cells upon target cell recognition, while the majority of CD56bright NK cells produce IFN-γ upon cytokine IL-12 and IL-18 costimulation. A recent study by Yu et al identifies a functional intermediary between CD56bright and CD56dim NK-cell subsets, which is CD56dim phenotype and produces IFN-γ when stimulated by target cell (K562) recognition or cytokine costimulation with IL-12 and IL-18.6 This finding provides evidence that CD56bright NK cells might be the precursors of CD56dim IFN-γ–producing cells, which become fully functional in terms of cytokine production and cytotoxicity upon recognition and lysis of cellular targets, including tumor cells. However, further studies are necessary to investigate whether these observations are both developmentally and functionally related. These studies will help us to fully understand the mechanisms of immune differences between CD56bright and CD56dim NK-cell subsets.
Conflict-of-interest disclosure: The authors declare no competing financial interests. ■
Acknowledgment: We thank Dr Brian Becknell for critical reading and editing of the manuscript.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal