The JAKV617F mutation is responsible for the majority of breakpoint cluster region (BCR)/Abelson (ABL)–negative myeloproliferative disorders. Ongoing clinical trials of Janus kinase 2 (JAK2) inhibitors in myeloproliferative disorder patients use small molecules targeting both wild-type and mutated JAK2. To selectively target malignant cells, we developed JAK2V617F-specific small interfering RNAs or short hairpin RNAs. Expression of these RNAs in cell lines or CD34+ cells from patients reduced JAK2V617F-driven autonomous cell proliferation. Mechanisms of inhibition involved selective JAK2V617F protein down-regulation, and consequently, decrease in signal transducer and activator of transcription 5 phosphorylation, cell-cycle progression, and cell survival. However, the addition of high concentrations of cytokines to cell lines or erythropoietin to patient cells greatly reduced growth inhibition. Similarly, the efficacy of a JAK2 small molecule inhibitor on cell line and patient cell proliferation dose dependently decreased with the addition of cytokines. Our results demonstrate that it is possible to specifically target JAK2V617F by RNA interference (RNAi) strategies. In addition, cytokines partially reverse the inhibition induced by both RNAi and small molecule approaches. This strongly suggests that patient cytokine levels in current JAK2 inhibitor clinical trials modulate the outcome of these therapies.
Introduction
Breakpoint cluster region (BCR)/Abelson (ABL)–negative classical myeloproliferative disorders (MPD) include essential thrombocythemia (ET), polycythemia vera (PV), and primary myelofibrosis (PMF).1 These clonal hematopoietic disorders originate from the transformation of a multipotent progenitor cell. They are characterized by overproduction of blood cells without obvious blockage in maturation. In PV, hyperplasia of the erythroid lineage predominates, whereas in ET or PMF, the megakaryocytic or both the megakaryocytic and the granulocytic lineages are prevalent, respectively. An essential feature of MPD is independence and/or hypersensitivity to growth factors leading to endogenous erythroid colony (EEC) or megakaryocytic colony formation. Hyperresponsiveness to erythropoietin (EPO), interleukin-3 (IL-3), stem cell factor (SCF), and granulocyte macrophage–colony stimulating factor (GM-CSF) has been demonstrated in PV.2,3 Similarly, megakaryocytic progenitor cells from ET and PMF are hypersensitive to thrombopoietin (TPO).4
In these disorders, we5 and others6,–8 have identified a unique and recurrent acquired mutation of the Janus kinase 2 (JAK2) protein corresponding to a valine-to-phenylalanine substitution (JAK2V617F) in the pseudokinase domain. The JAK2V617F mutation is present in a majority of PV patients and in approximately half of the patients suffering from PMF or ET.9 More recently, mutations in the thrombopoietin receptor (myeloproliferative leukemia [MPL])10 or other JAK2 mutations11 have been reported in a minority of PMF and ET or PV patients, respectively. The identification of the JAK2 pathway as the primary molecular lesion involved in the pathology of BCR/ABL-negative classical MPD combined with successful clinical uses of kinase inhibitors in chronic myeloid leukemia (CML)12 have rapidly promoted the development of therapeutic JAK2 inhibitors. Preclinical and clinical studies using several orally available small molecule inhibitors have recently been reported.13 These inhibitors are more or less specific for JAK2 among other kinases, but to date none is specific for the JAK2V617F mutation. Although the lack of specificity of the JAK2 inhibitors provides the advantage of extending their use to JAK2V617F-negative MPD and other pathologies involving the JAK2/signal transducer and activator of transcription (STAT) pathway, the fundamental role of JAK2 in hematopoiesis has raised concerns on the long-term efficacy and safety of these drugs. The location of the JAK2V617F mutation outside of the kinase domain presents a genuine challenge for the development of small molecule inhibitors targeting JAK2V617F. In contrast, mutation-specific inhibition of single point-mutated protein using transfected synthetic small interfering RNA (siRNA) duplexes or virally transduced short hairpin RNAs (shRNAs) has been reported with several genes, including p53R248W,14 TauV337M,15 or BRAFV599E.16 These RNAs selectively match to the endogenous target mRNA, leading to its degradation and the inhibition of the related protein expression in mammalian cells. A single-base mismatch between the siRNA and its target is believed to prevent mRNA degradation, thus allowing this type of inhibition to be highly specific to the targeted gene.
Using this strategy, we succeeded in developing siRNAs and shRNAs selectively suppressing JAK2V617F without affecting normal JAK2WT protein production. These RNAs abrogated JAK2V617F-dependent autonomous growth of cell lines and primary cells from MPD patients. However, as shRNA only induces a knockdown of JAK2V617F, high cytokine stimulation efficiently rescued cell proliferation, especially in cells displaying heterozygosity for the JAK2 mutation. This antagonizing action of cytokines was also demonstrated with a small molecule JAK2 inhibitor, suggesting a widespread cytokine-counteracting effect to all JAK2- or JAK2V617F-targeted therapies.
Methods
JAK2 inhibitor, antibodies, and cytokines
AZ960 is a potent and selective adenosine trisphosphate competitive inhibitor of JAK2 enzyme activity developed at AstraZeneca R&D, with an inhibition constant (Ki) of 0.00045 μM and a 50% inhibitory concentration (IC50) of less than 0.003 μM in a JAK2 enzyme assay.17 Stock solution was diluted in dimethylsulfoxide (DMSO; Sigma-Aldrich). Anti–phospho-STAT5 (Tyr694), anti-STAT5, anti–phospho-ERKp42-p44 (Thr202/Tyr204), and anti–Erkp42-p44 rabbit antibodies were purchased from Cell Signaling Technology. Anti–human JAK2 rabbit antibody and anti–human β-actin monoclonal antibody were from Santa Cruz Biotechnology. EPO and SCF were from Amgen, GM-CSF and IL-3 from Novartis Pharmaceuticals, and TPO from Kirin Brewery.
Cell culture
HEL and 293T cells were cultured in Dulbecco modified Eagle medium (Invitrogen), UKE-1 cells in Iscove modified Dulbecco medium (IMDM; Invitrogen), SET-2 and K562 cells in RPMI medium, and UT-7 cells in α-minimal essential medium (Invitrogen) with GM-CSF (10 ng/mL). All medium contained 10% fetal bovine serum, antibiotics (100 IU/mL penicillin, 50 μg/mL streptomycin), and 2 mM l-glutamine (Invitrogen). Medium for UKE-1 was also supplemented with 10% horse serum and 1 μM hydrocortisone.
Patient cells
Blood samples (phlebotomy) from PV patients and normal subjects were collected after informed consent was obtained in accordance with the Declaration of Helsinki. CD34+ cells were isolated from peripheral blood mononuclear cells by immunomagnetic selection (Automacs; Miltenyi Biotec), according to the manufacturer's recommendations. Cells were maintained in serum-free IMDM.
siRNA- and shRNA-containing lentivirus constructions
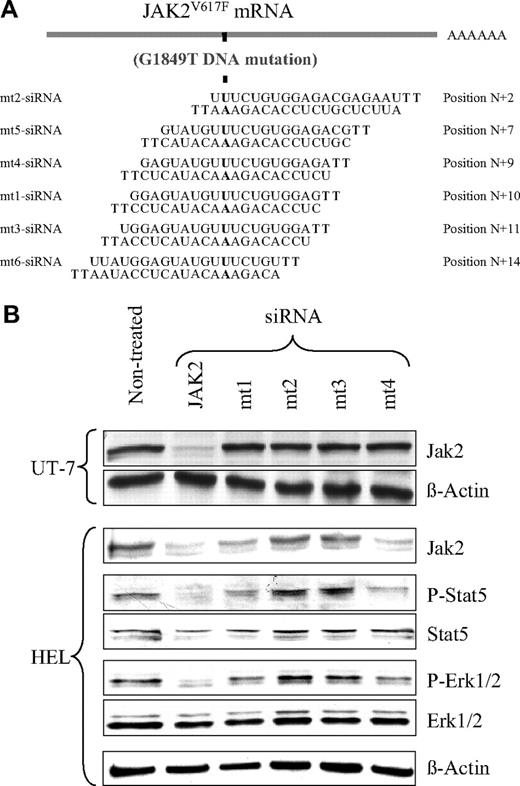
The 21-nucleotide siRNA duplexes with 2 3′-end overhang dT bases in the antisense strand, listed in Figure 1A, were designed using the siSearch-siRNA design website (http://sonnhammer.sbc.su.se/). Jak2 oligonucleotide short hairpin, designed from the functional mt1- and the mt4-siRNAs, and an irrelevant sequence (Scr, uaaucgaguguacgguuag, a sequence not matching any gene) were synthesized (Eurogentec) and inserted into the pBluescript plasmid under the control of the human H1 promoter. A single H1-shRNA-Jak2 or H1-shRNA-Scr cassette was then inserted into the HIV1-derived lentiviral vector (pRRLsin-PGK-eGFP-WPRE) upstream of the phospho glycerate kinase (PGK) promoter–green fluorescent protein (GFP) coding unit (Figure 2B), as already described.20
Selective inhibition of JAK2V617F protein expression and signaling by mt1- and mt4-siRNAs. (A) Sequences of the siRNAs designed to specifically knock down the JAK2V617F, but not the JAK2WT protein production. Mutated nucleotides are indicated in bold, and their positions are designated from the 5′ end of the Jak2V617F homologous sequence. HEL and UT-7 cells were transfected (siRNA) or not (nontreated) with the indicated siRNAs. HEL cells only express JAK2V617F, whereas UT-7 cells only express JAK2WT. (B) The JAK2 and β-actin (loading control) proteins were analyzed in UT-7 and HEL cells by Western blotting with the specific antibodies, 48 hours after treatment. Results show that the mt1- and mt4-siRNAs, but not the mt2- and mt3-siRNAs, were efficient in specifically knocking down JAK2V617F (only present in HEL), but not JAK2WT (only present in UT-7). The Jak2-siRNA (JAK2), used as a positive control, knocked down both JAK2WT and JAK2V617F. Western blotting also assessed the phosphorylation levels of the JAK2 downstream effectors STAT5 and ERK1/2 in HEL cells. Jak2-, mt1-, and mt4-siRNAs all caused a marked decrease in STAT5 and ERK1/2 phosphorylation without affecting their total protein level. Neither mt2- nor mt3-siRNA had an effect on JAK2 downstream signaling.
Selective inhibition of JAK2V617F protein expression and signaling by mt1- and mt4-siRNAs. (A) Sequences of the siRNAs designed to specifically knock down the JAK2V617F, but not the JAK2WT protein production. Mutated nucleotides are indicated in bold, and their positions are designated from the 5′ end of the Jak2V617F homologous sequence. HEL and UT-7 cells were transfected (siRNA) or not (nontreated) with the indicated siRNAs. HEL cells only express JAK2V617F, whereas UT-7 cells only express JAK2WT. (B) The JAK2 and β-actin (loading control) proteins were analyzed in UT-7 and HEL cells by Western blotting with the specific antibodies, 48 hours after treatment. Results show that the mt1- and mt4-siRNAs, but not the mt2- and mt3-siRNAs, were efficient in specifically knocking down JAK2V617F (only present in HEL), but not JAK2WT (only present in UT-7). The Jak2-siRNA (JAK2), used as a positive control, knocked down both JAK2WT and JAK2V617F. Western blotting also assessed the phosphorylation levels of the JAK2 downstream effectors STAT5 and ERK1/2 in HEL cells. Jak2-, mt1-, and mt4-siRNAs all caused a marked decrease in STAT5 and ERK1/2 phosphorylation without affecting their total protein level. Neither mt2- nor mt3-siRNA had an effect on JAK2 downstream signaling.
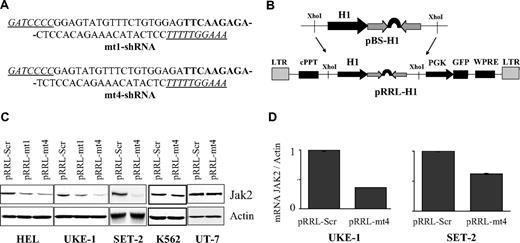
Specific and efficient Jak2V617F gene silencing by the shRNA-encoded pRRL-mt4 lentivirus. (A) Sequences of the mt1- and mt4-shRNAs. The 9-nucleotide–long loop is indicated in bold, and the BglII and HindIII sites are underlined. (B) Schematic representation of the shRNA shuttle (pBS-H1) and lentivirus (pRRL) vectors. The shRNA was directionally inserted into the BglII and HindIII sites of the pBS-H1 vector. The expression cassette comprising the shRNA under the control of the H1 promoter was excised from the shuttle vector as a XhoI fragment and inserted into the lentiviral vector. LTR indicates long terminal repeat; cPPT, central polypurine tract; WPRE, posttranscriptional cis-acting regulatory element of the woodchuck hepatitis virus.18,19 (C) JAK2 protein expression level in pRRL-mt1– or pRRL-mt4–infected HEL, UKE-1, SET-2, K562, and UT-7 cells. The cells were infected with the pRRL-Scr (control), pRRL-mt1, or pRRL-mt4 (both specific for Jak2V617F) virus, as indicated. Transduced cells were selected for GFP expression, and protein contents were analyzed by Western blotting. HEL and UKE-1 cells only express JAK2V617F, SET-2 expresses both JAK2V617F and JAK2WT, and K562 and UT-7 cells only express JAK2WT. Inhibition of JAK2V617F protein production in HEL and UKE-1 cells was observed after pRRL-mt1 infection and to a greater extent with pRRL-mt4 virus. Similarly, pRRL-mt4 infection decreased JAK2 content in SET-2 cells. As expected, pRRL-mt4 transduction had no effect on JAK2WT protein production in K562 and UT-7 cells. (D) Jak2V617F mRNA expression level, using allele-specific quantitative reverse transcription–PCR analysis, in UKE-1 and SET-2 cells transduced with pRRL-Scr or pRRL-mt4. Results represent ratios of Jak2V617F over actin mRNAs, normalized to cells infected with pRRL-Scr. The differences in RNA and protein suppression efficiencies may be explained by the fact that data originated from 2 separated experiments or that JAK2 protein and mRNA are regulated independently.
Specific and efficient Jak2V617F gene silencing by the shRNA-encoded pRRL-mt4 lentivirus. (A) Sequences of the mt1- and mt4-shRNAs. The 9-nucleotide–long loop is indicated in bold, and the BglII and HindIII sites are underlined. (B) Schematic representation of the shRNA shuttle (pBS-H1) and lentivirus (pRRL) vectors. The shRNA was directionally inserted into the BglII and HindIII sites of the pBS-H1 vector. The expression cassette comprising the shRNA under the control of the H1 promoter was excised from the shuttle vector as a XhoI fragment and inserted into the lentiviral vector. LTR indicates long terminal repeat; cPPT, central polypurine tract; WPRE, posttranscriptional cis-acting regulatory element of the woodchuck hepatitis virus.18,19 (C) JAK2 protein expression level in pRRL-mt1– or pRRL-mt4–infected HEL, UKE-1, SET-2, K562, and UT-7 cells. The cells were infected with the pRRL-Scr (control), pRRL-mt1, or pRRL-mt4 (both specific for Jak2V617F) virus, as indicated. Transduced cells were selected for GFP expression, and protein contents were analyzed by Western blotting. HEL and UKE-1 cells only express JAK2V617F, SET-2 expresses both JAK2V617F and JAK2WT, and K562 and UT-7 cells only express JAK2WT. Inhibition of JAK2V617F protein production in HEL and UKE-1 cells was observed after pRRL-mt1 infection and to a greater extent with pRRL-mt4 virus. Similarly, pRRL-mt4 infection decreased JAK2 content in SET-2 cells. As expected, pRRL-mt4 transduction had no effect on JAK2WT protein production in K562 and UT-7 cells. (D) Jak2V617F mRNA expression level, using allele-specific quantitative reverse transcription–PCR analysis, in UKE-1 and SET-2 cells transduced with pRRL-Scr or pRRL-mt4. Results represent ratios of Jak2V617F over actin mRNAs, normalized to cells infected with pRRL-Scr. The differences in RNA and protein suppression efficiencies may be explained by the fact that data originated from 2 separated experiments or that JAK2 protein and mRNA are regulated independently.
siRNA electroporation and shRNA-containing lentivirus production and cell infection
Cell lines (HEL and UT-7) were transfected with siRNAs using the Amaxa nucleofection technology (Amaxa). Cells were resuspended in the Amaxa nucleofector kit V optimization kit solution, following the manufacturer's instructions. Briefly, 0.1 mL of a 2 to 5 × 106 cell suspension was transfected with 1 μg siRNA and immediately transferred in 6-well plates containing 37°C prewarmed culture medium. For infection, lentiviral stocks were prepared by transient cotransfection of 3 plasmids (pRRL with the shRNA-Jak2 or shRNA-Scr sequence, the packaging plasmid pCMVΔR8.74, and the VSV-G protein envelope plasmid pMD.G) in 293T cells using Lipofectamine 2000 (Invitrogen), according to the manufacturer's instructions. The viral titer was determined by limiting dilutions in 293T cells. Cell lines (HEL, UKE-1, SET-2, UT7, and K562) or blood CD34+ cells were infected (multiplicity of infection 10) in the presence of 4 μg/mL polybrene (Sigma-Aldrich) with fresh virus-containing supernatants in either culture medium or serum-free IMDM containing growth factors (25 ng/mL SCF, 30 ng/mL IL-3, and 1 U/mL EPO for CD34+ cells), respectively. Infected cells were sorted based on GFP expression (FACSDiva; BD Biosciences) for further experiments.
Proliferation assay
Sorted transduced cells were washed and incubated in appropriate medium in the absence or presence of cytokines, as indicated. Viable cells were counted with Malassez chambers using the trypan blue exclusion assay.
Apoptosis assay
UKE-1 and SET-2 cells were either treated with AZ960 or infected with the shRNA-Scr- or shRNA-Jak2V617F–containing lentivirus, in the absence or presence of cytokines (30 ng/mL IL-3, 10 ng/mL TPO, or 10 ng/mL GM-CSF, accordingly), and examined 48 hours later for apoptosis. Annexin V–positive staining was examined by fluorescence-activated cell sorter analysis (Allophycocyanin Annexin V Staining Kit; BD Pharmingen), according to the manufacturer's instructions. Cell viability was determined by exclusion of the cell-impermeant nucleic acid 7-aminoactinomycin D dye.
Cell-cycle analysis
UKE-1 cells treated with AZ960 or infected with shRNA-Scr– or shRNA-Jak2V617F–containing lentivirus were cultured in the absence or presence of cytokines. After 48 hours, cells were incubated overnight in pH 7.6 buffer (1 mg/mL Tris Na citrate and 0.58 mg/mL NaCl) with 0.1% Igepal, 50 mg/mL RNase, and 50 mg/mL propidium iodide (Sigma-Aldrich). Cell samples were analyzed by FACSort cytometer (BD Biosciences).
Colony assays
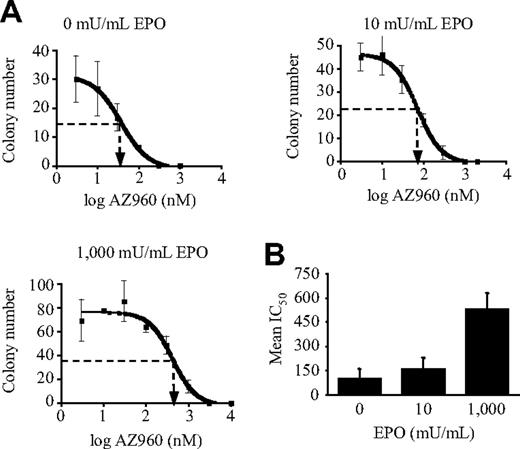
After infection with the lentiviruses, GFP-positive CD34+ cells from normal or PV patients were plated at 2 × 103 cells/mL in 35-mm-diameter dishes in 1% serum-free methylcellulose (StemCell Technologies) in triplicate in the presence of SCF (50 ng/mL), IL-3 (60 ng/mL), and in the absence or presence of different concentrations of EPO. Noninfected cells were treated with AZ960, as indicated. Cultures were incubated at 37°C in a humidified 5% CO2 atmosphere, and colonies were scored at day 14.
Western blot analysis
Whole-cell protein extracts were analyzed by Western blotting with adequate antibodies, as previously described,21 using peroxidase-conjugated AffinityPure anti–mouse and anti–rabbit goat IgG secondary antibody (Jackson ImmunoResearch Laboratories), chemiluminescence (Enhanced Chemiluminescence Western Blotting Detection Kit; Amersham), and Kodak Biomax films (Sigma-Aldrich). Intensities of bands were quantified using ImageJ software (National Institutes of Health).
Genotyping of colonies for the Jak21849G>T (JAK2617V>F) mutation
JAK2 mutational status of colonies was analyzed by using fluorescent competitive probes for quantitative real-time polymerase chain reaction (PCR) on an ABI 7500 (Applied Biosystems). The JAK21849G>T/total JAK2 Δ cycle thresholds were calculated as previously reported.22,23 At least 25 colonies per condition were analyzed.
Statistical analysis
Data were analyzed with the 2-tailed Student t test, and results are presented as mean (± SEM or SD).
Results
Development of Jak2V617F-specific siRNAs
The JAK2V617F gene differs from the wild-type (WT) sequence by a single nucleotide located at position G1849T. This difference provides the opportunity to develop specific siRNAs to knock down JAK2V617F without affecting WT JAK2 (JAK2WT). We designed 6 21-mer sequence siRNAs that included the mutated base at different positions (Figure 1A; mt1- to mt6-siRNAs). As a positive control, we used a previously described siRNA targeting the JAK2 gene in a region that does not include the mutation (Jak2-siRNA).5 To test the specificity of these siRNAs for the mutated versus the WT protein, we examined their effects on JAK2 protein expression in the HEL and the UT-7 cell lines (Figure 1B). The HEL cells are homozygous for JAK2V617F and carry an estimated 8 copies of the JAK2V617F gene per cell.24,25 In contrast, UT-7 cells are homozygous for the WT gene. Transfection of HEL cells with the mt1- and mt4-siRNAs, which include the mutation, resulted in a 55% (± 6%; n = 3) decrease in JAK2 protein expression in nonselected cell population compared with nontreated cells (Figure 1B). These siRNA also induced a reduction in JAK2 mRNA (data not shown). The 4 other Jak2V617F-siRNAs, mt2- and mt3-siRNAs (Figure 1B) and mt5- and mt6-siRNAs (results not shown), did not significantly modify the JAK2V617F protein level. None of the JAK2V617F-specific siRNAs was active against the JAK2WT protein in the UT-7 cells (Figure 1B). As expected, the nonspecific Jak2-siRNA control knocked down the JAK2V617F protein in HEL cells (80% ± 5% decrease; n = 3) as well as the JAK2WT protein in UT-7 cells (65% ± 2% decrease; n = 2). These results show that the 2 mt1- and mt4-siRNAs displaying the mutated base in central position (+9 and +10) can efficiently target the JAK2V617F protein without interfering with JAK2WT expression.
HEL cells display constitutive phosphorylation of the STAT5 and ERK1/2 proteins as a result of JAK2V617F activity (Figure 1B; nontreated). To determine whether the level of siRNA-dependent inhibition of JAK2V617F protein production was sufficient to reduce the kinase downstream signaling, we assessed the phosphorylation of these 2 known JAK2 effectors. The silencing of the JAK2V617F gene in HEL cells treated with the Jak2-, mt1-, and mt4-siRNAs caused a decrease in phospho-STAT5 and phospho-ERK1/2 without affecting their total protein levels (Figure 1B). Finally, mt2- and mt3-siRNAs, incapable of decreasing the JAK2 protein content, had no effect on the phosphorylation status of STAT5 and ERK1/2 (Figure 1B). These results demonstrate that knocking down the JAK2V617F protein by siRNAs is sufficient to suppress constitutive signaling induced by this protein.
Development of Jak2V617F-specific shRNAs
Based on Jak2-siRNA, mt1-siRNA, and mt4-siRNA sequences, we next developed 3 lentiviruses carrying shRNAs directed against the JAK2 gene (pRRL-Jak2) or specifically against the JAK2V617F gene (pRRL-mt1 and pRRL-mt4; Figure 2A-B). As a negative control, we developed a shRNA carrying a sequence unrelated to Jak2 (pRRL-Scr). We tested these shRNAs in HEL, UKE-1, and SET-2 cells that carry the mutated JAK2V617F gene and in the UT-7 and K562 cells that only harbor the WT gene. UKE-1 cells are homozygous for the mutation with solely 2 JAK2V617F genes.24 The SET-2 cells carry 5 JAK2 copies probably including 4 mutated JAK2V617F.25 All these cells were infected with the pRRL lentiviruses carrying the shRNAs, and transduced cells were sorted based on GFP expression. Western blot analysis revealed that either pRRL-mt1 or pRRL-mt4 reduced the expression of the JAK2V617F protein in HEL (54% or 77%, respectively) and UKE-1 (56% or 78%, respectively) cells (Figure 2C). The pRRL-mt4 displayed a better efficacy than the pRRL-mt1. Therefore, we used the pRRL-mt4 lentivirus for further experiments. In SET-2 cells, pRRL-mt4 decreased the JAK2 protein level by 80%, which may represent suppression of all JAK2V617F protein. As expected, this shRNA had no effect on JAK2 expression in UT-7 and K562 cells (Figure 2C). Consistent with protein expression inhibition, pRRL-mt4 was found to down-regulate Jak2V617F mRNA in UKE-1 and SET-2 cells (Figure 2D). Despite a perfect match with the initial Jak2-siRNA sequence, the pRRL-Jak2 was surprisingly inefficient in decreasing JAK2 protein in any of the cell lines tested (data not shown), indicating that efficiency of a siRNA sequence is not a guarantee for efficacy of a shRNA harboring the same sequence.
Effect of Jak2V617F-specific shRNA on cell line growth, influence of cytokine
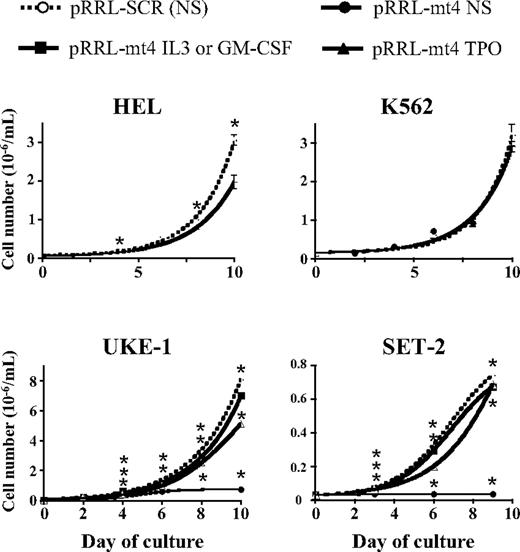
JAK2V617F is believed to support autonomous growth of HEL, UKE-1, and SET-2 cells. pRRL-Scr– and pRRL-mt4–transduced cells (GFP sorted) were assayed for proliferation in the absence of cytokines. As expected, there was no difference in the proliferation of the pRRL-mt4– compared with the control pRRL-Scr–transduced K562 cells (Figure 3). In contrast, growth of HEL and UKE-1 cells was reduced to different extents, whereas SET-2 cell growth was completely blocked (Figure 3). These results confirm that expression of JAK2V617F is involved in the autonomous growth of the 3 cell lines. However, pRRL-mt4 only partially reduced the proliferation rate of UKE-1 and HEL cells, suggesting that (1) other mechanisms and/or (2) residual JAK2V617F expression may be sufficient to sustain their proliferation in cytokine-free medium. In addition, JAK2V617F expression seems mandatory for cytokine-independent SET-2 cell growth.
Inhibition of autonomous cell line growth by Jak2V617F-shRNA. HEL, K562, UKE-1, and SET-2 cells were infected with the pRRL-mt4 or the control pRRL-Scr virus, selected for GFP expression, and grown in liquid culture in the absence or the presence of cytokines. Viable cells were counted every 2 or 3 days. Expression of the Jak2V617F-shRNA from the pRRL-mt4 virus inhibited the proliferation of the JAK2V617F-expressing HEL, UKE-1, and SET-2 cell lines, but had no effect on JAK2WT-expressing K562 cell growth. Results from typical experiments are represented and expressed as mean numbers (± SEM). *P ≤ .05 using the Student t test. Analysis of variance of linear models from 3 independent experiments using duplicate or triplicate counts shows that the shRNA significantly decreased cell numbers in all cell lines (P ≤ 106), except K562 (P = .7). IL-3 (30 ng/mL, UKE-1), GM-CSF (10 ng/mL, SET-2), and TPO (10 ng/mL, only for SET-2) significantly completely abolished the effect (n = 3). For TPO and UKE-1, the effect was only reduced.
Inhibition of autonomous cell line growth by Jak2V617F-shRNA. HEL, K562, UKE-1, and SET-2 cells were infected with the pRRL-mt4 or the control pRRL-Scr virus, selected for GFP expression, and grown in liquid culture in the absence or the presence of cytokines. Viable cells were counted every 2 or 3 days. Expression of the Jak2V617F-shRNA from the pRRL-mt4 virus inhibited the proliferation of the JAK2V617F-expressing HEL, UKE-1, and SET-2 cell lines, but had no effect on JAK2WT-expressing K562 cell growth. Results from typical experiments are represented and expressed as mean numbers (± SEM). *P ≤ .05 using the Student t test. Analysis of variance of linear models from 3 independent experiments using duplicate or triplicate counts shows that the shRNA significantly decreased cell numbers in all cell lines (P ≤ 106), except K562 (P = .7). IL-3 (30 ng/mL, UKE-1), GM-CSF (10 ng/mL, SET-2), and TPO (10 ng/mL, only for SET-2) significantly completely abolished the effect (n = 3). For TPO and UKE-1, the effect was only reduced.
PCR analysis revealed that UKE-1 and SET-2 cells both express the thrombopoietin receptor MPL and the receptor for GM-CSF (data not shown). In contrast, the α-chain of the IL-3 receptor was only detected in UKE-1 cells. This prompted us to test the effects of cytokine stimulation after knocking down the JAK2V617F protein. The addition of cytokines to the control pRRL-Src–infected SET-2 or UKE-1 cell culture did not influence autonomous cell proliferation (Figure 4A). In contrast, addition of either IL-3 (50% effective concentration [EC50] = 45 pg/mL) or TPO (EC50 = 43 pg/mL) tothe pRRL-mt4–infected UKE-1 cell culture resulted in a dose-dependent rescue of growth inhibition (Figures 3–4A). The restored proliferation was associated with an increase in STAT5 phosphorylation, suggesting that residual JAK2V617F expression was involved in mediating IL-3– and TPO-dependent growth of the pRRL-mt4–transduced UKE-1 cells (Figure 4B). Similarly, addition of high doses of GM-CSF (EC50 = 430 pg/mL) and TPO (EC50 = 70 pg/mL) resulted in almost complete rescue of pRRL-mt4–induced growth inhibition of SET-2 cells (Figure 4A). Interestingly, the doses necessary for half of these maximal rescues (EC50) were within normal serum physiologic ranges for TPO, but 10 times and 100 times the normal serum levels for IL-3 and GM-CSF, respectively.
Dose-dependent rescue by cytokines of Jak2V617F shRNA-induced cell growth inhibition. (A) UKE-1 and SET-2 cells were infected with the pRRL-mt4 or the control pRRL-Scr virus, selected for GFP expression, and plated in liquid culture in the absence or the presence of increasing concentrations of IL-3, TPO, or GM-CSF (expressed in nanograms per milliliter). Results [mean values (± SEM)] of 2 independent experiments from day 9 of culture are presented. *P ≤ .05. (B) Effect of IL-3 or TPO exposure on the phosphorylation levels of STAT5 in UKE-1 cells infected by the pRRL-mt4 or the control pRRL-Scr virus and cultured for 6 days with or without IL-3 (30 ng/mL) and TPO (10 ng/mL). Data indicate that rescue by cytokines could be mediated through signaling of the residual JAK2V617F protein, remaining after shRNA action, detected with this Western blot analysis.
Dose-dependent rescue by cytokines of Jak2V617F shRNA-induced cell growth inhibition. (A) UKE-1 and SET-2 cells were infected with the pRRL-mt4 or the control pRRL-Scr virus, selected for GFP expression, and plated in liquid culture in the absence or the presence of increasing concentrations of IL-3, TPO, or GM-CSF (expressed in nanograms per milliliter). Results [mean values (± SEM)] of 2 independent experiments from day 9 of culture are presented. *P ≤ .05. (B) Effect of IL-3 or TPO exposure on the phosphorylation levels of STAT5 in UKE-1 cells infected by the pRRL-mt4 or the control pRRL-Scr virus and cultured for 6 days with or without IL-3 (30 ng/mL) and TPO (10 ng/mL). Data indicate that rescue by cytokines could be mediated through signaling of the residual JAK2V617F protein, remaining after shRNA action, detected with this Western blot analysis.
Effect of Jak2V617F-specific shRNA and of the AZ960 JAK2 inhibitor on induction of apoptosis and cell cycle
AZ960 is a potent and selective inhibitor of the JAK2 kinase activity.17 This inhibitor blocked the autonomous proliferation of the UKE-1 and the SET-2 cells in a dose-dependent manner, with doses required for inhibition of half of cell growth (IC50) being 105 nM and 50 nM, respectively. The addition of IL-3 (10 ng/mL) to the UKE-1 or GM-CSF (10 ng/mL) to the SET-2 cells increased these IC50 by 2.9-fold and 5-fold, respectively (data not shown). We examined whether induction of apoptosis or cell-cycle arrest may be the mechanisms of growth inhibition induced by JAK2 inhibitors in these cell lines. Apoptosis of UKE-1 and SET-2 was determined 48 hours after either infection with pRRL-mt4 or treatment with AZ960, in the absence or presence of cytokines, as indicated. As shown in Figure 5A, both the shRNA and AZ960 induced an increase of apoptosis in UKE-1 and SET-2 cells cultured without cytokines. The percentages of annexin V–positive UKE-1 cells increased from 12% (± 4%) (pRRL-Scr) or 19% (± 4%; DMSO) in controls to 33% (± 8%) or 49% (± 8%) after treatment with pRRL-mt4 virus or AZ960, respectively (Figure 5A). Similarly, 14% (± 3%) or 21% (± 1%) of SET-2 cells treated with the pRRL-Scr virus or DMSO were annexin V labeled, and these percentages rose to 67% (± 4%) or 63% (± 7%) in the presence of the pRRL-mt4 virus or AZ960, respectively (Figure 5A). However, addition of cytokines (IL-3 or TPO for UKE-1 cells, and GM-CSF or TPO for SET-2 cells) consistently limited the number of apoptotic cells induced by pRRL-mt4 or AZ960 treatment, especially in UKE-1 cells (Figure 5A). This was significant for SET-2 or UKE-1, respectively, treated by pRRL-mt4 or AZ960 (P ≤ .05).
Induction of apoptosis and cell-cycle arrest by the JAK2V617F-specific shRNA and the anti-JAK2 inhibitor AZ960. (A) Apoptosis: pRRL-mt4 virus (left) and AZ960 (right) treatment increased the percentage of annexin V–positive cells in the absence of cytokine. SET-2 (top) and UKE-1 (bottom) cells were either cultured for 48 hours in the presence of 105 nM or 50 nM AZ960 (IC50), respectively, or transduced with pRRL-Scr (Scr) or pRRL-mt4 (mt4), in the absence or presence of cytokines. Cells were stained with annexin V–allophycocyanin and 7-aminoactinomycin D and analyzed by flow cytometry analysis. (B) Cell cycle: pRRL-mt4 virus (left) and AZ960 (right) treatment of UKE-1 cells decreased autonomous cell-cycle progression (S + G2/M phases, P ≤ .05). UKE-1 cell cycle was analyzed by propidium iodide staining, 48 hours after AZ960 and pRRL-mt4 virus treatment, or DMSO and pRRL-Scr virus treatment as controls, in the absence or presence of cytokines. The percentages of cells in the G0-G1, S, and G2-M phases are shown on each histogram. (A-B) The presence of cytokines blunted the increase in apoptotic or nondividing cells induced by AZ960 treatment or pRRL-mt4 transduction. Data are mean value (± SD) from 3 independent experiments. *P ≤ .05.
Induction of apoptosis and cell-cycle arrest by the JAK2V617F-specific shRNA and the anti-JAK2 inhibitor AZ960. (A) Apoptosis: pRRL-mt4 virus (left) and AZ960 (right) treatment increased the percentage of annexin V–positive cells in the absence of cytokine. SET-2 (top) and UKE-1 (bottom) cells were either cultured for 48 hours in the presence of 105 nM or 50 nM AZ960 (IC50), respectively, or transduced with pRRL-Scr (Scr) or pRRL-mt4 (mt4), in the absence or presence of cytokines. Cells were stained with annexin V–allophycocyanin and 7-aminoactinomycin D and analyzed by flow cytometry analysis. (B) Cell cycle: pRRL-mt4 virus (left) and AZ960 (right) treatment of UKE-1 cells decreased autonomous cell-cycle progression (S + G2/M phases, P ≤ .05). UKE-1 cell cycle was analyzed by propidium iodide staining, 48 hours after AZ960 and pRRL-mt4 virus treatment, or DMSO and pRRL-Scr virus treatment as controls, in the absence or presence of cytokines. The percentages of cells in the G0-G1, S, and G2-M phases are shown on each histogram. (A-B) The presence of cytokines blunted the increase in apoptotic or nondividing cells induced by AZ960 treatment or pRRL-mt4 transduction. Data are mean value (± SD) from 3 independent experiments. *P ≤ .05.
UKE-1 cell-cycle distribution in response to the shRNA and inhibitor was determined by propidium iodide staining. The percentage of UKE-1 cells in cycle (S + G2/M phases) showed a significant decrease from 35% (± 1%) to 27% (± 2%) or from 38% (± 2%) to 22% (± 6%) in cells treated for 48 hours with the Jak2V617F-shRNA or AZ960, respectively (P ≤ .05). Furthermore, the addition of IL-3 or TPO repeatedly (n = 3) partially or completely (IL-3 with Jak2V617F-shRNA) reversed inhibition of cell-cycle progression (Figure 5B).
These data show that induction of apoptosis and arrest of cell cycle in the G0/G1 phase are 2 mechanisms involved in the inhibition of JAK2V617F-dependent cell growth induced by both the Jak2V617F-shRNA and AZ960 inhibitor. The addition of cytokine counteracts both mechanisms of action.
Effect of Jak2V617F-specific shRNA on PV EEC and BFU-E growth
We analyzed hematopoietic progenitor growth from 4 typical PV patients encompassing different allele burden features. PV1 and PV3 were classical PV with a mixture of JAK2WT- and JAK2V617F-positive burst-forming unit–erythroid (BFU-E; 54% or 39% were JAK2V617F positive for PV1 or PV3, respectively), most being homozygous for the mutation. PV2 was clonal (100%) for homozygous JAK2V617F-positive BFU-E, and PV4 nearly clonal (88%) for heterozygous JAK2V617F-positive BFU-E (Figure 6A). In contrast to PV2 and PV4, a small percentage of EEC in PV1 and PV3 did not harbor the JAK2V617F mutation (Figure 6A).
Inhibition of erythroid colony formation from PV patients by the Jak2V617F-specific shRNA and rescue by EPO. (A) Percentage of Jak2V617F-positive BFU-E and EEC colonies isolated from 4 patient CD34+ cell cultures. Genomic DNA from individual colonies was analyzed for Jak2V617F or Jak2WT content by allele-specific PCR. Main zygosity of progenitor cells for the mutation is indicated (Hom., homozygous; Het., heterozygous; NS, no added EPO). (B) Western blot analysis of JAK2 and β-actin (loading control) protein expression levels from infected (GFP+) cells cultured 24 hours or 48 hours after infection in the presence of 1 U/mL EPO using 2 control, PV1, and PV3 patient CD34+ blood cells. Results show efficient Jak2V617F gene silencing in PV patient cells. (C) Western blot analysis of JAK2, phospho-STAT5, STAT5, and β-actin protein expression levels from infected cells (prepared as in B) using PV2 and PV4 patient cells. Results show efficient Jak2V617F gene silencing and a decrease in STAT5 phosphorylation in PV patient cells. (D) CD34+ cells from PV patients and 2 controls were infected with the pRRL-mt4 (■) or control pRRL-Scr (□) lentivirus, and GFP-positive cells were plated at 2 × 103 cells in methylcellulose semisolid culture medium in the presence of SCF, IL-3, and with (+) or without (−) EPO. The number of BFU-E–derived colonies from pRRL-mt4 or control pRRL-Scr lentivirus-infected cells was analyzed after 14 days of culture. (E) Number of BFU-E–derived colonies from pRRL-mt4 or control pRRL-Scr lentivirus-infected cells analyzed after 14 days of culture in the absence or the presence of 10 mU/mL, 100 mU/mL, or 1000 mU/mL EPO. (D-E) Results are expressed in mean numbers of colonies (± SD) per 2 × 103 plated cells from triplicate cultures of single experiments. **P ≤ .05 and ***P ≤ .005. They show efficient and selective inhibition of colony formation from PV patient cells by the Jak2V617F-specific shRNA. (E) The presence of high concentrations of EPO (100 and 1000 mU/mL) blocked the inhibition induced by the shRNA.
Inhibition of erythroid colony formation from PV patients by the Jak2V617F-specific shRNA and rescue by EPO. (A) Percentage of Jak2V617F-positive BFU-E and EEC colonies isolated from 4 patient CD34+ cell cultures. Genomic DNA from individual colonies was analyzed for Jak2V617F or Jak2WT content by allele-specific PCR. Main zygosity of progenitor cells for the mutation is indicated (Hom., homozygous; Het., heterozygous; NS, no added EPO). (B) Western blot analysis of JAK2 and β-actin (loading control) protein expression levels from infected (GFP+) cells cultured 24 hours or 48 hours after infection in the presence of 1 U/mL EPO using 2 control, PV1, and PV3 patient CD34+ blood cells. Results show efficient Jak2V617F gene silencing in PV patient cells. (C) Western blot analysis of JAK2, phospho-STAT5, STAT5, and β-actin protein expression levels from infected cells (prepared as in B) using PV2 and PV4 patient cells. Results show efficient Jak2V617F gene silencing and a decrease in STAT5 phosphorylation in PV patient cells. (D) CD34+ cells from PV patients and 2 controls were infected with the pRRL-mt4 (■) or control pRRL-Scr (□) lentivirus, and GFP-positive cells were plated at 2 × 103 cells in methylcellulose semisolid culture medium in the presence of SCF, IL-3, and with (+) or without (−) EPO. The number of BFU-E–derived colonies from pRRL-mt4 or control pRRL-Scr lentivirus-infected cells was analyzed after 14 days of culture. (E) Number of BFU-E–derived colonies from pRRL-mt4 or control pRRL-Scr lentivirus-infected cells analyzed after 14 days of culture in the absence or the presence of 10 mU/mL, 100 mU/mL, or 1000 mU/mL EPO. (D-E) Results are expressed in mean numbers of colonies (± SD) per 2 × 103 plated cells from triplicate cultures of single experiments. **P ≤ .05 and ***P ≤ .005. They show efficient and selective inhibition of colony formation from PV patient cells by the Jak2V617F-specific shRNA. (E) The presence of high concentrations of EPO (100 and 1000 mU/mL) blocked the inhibition induced by the shRNA.
Infected CD34+ cells were grown in liquid culture. Analysis of the CD34+-derived cells revealed efficient inhibition by the pRRL-mt4 virus of the following: (1) JAK2 protein expression (Figure 6B-C); (2) STAT5 phosphorylation (Figure 6C; PV2 and PV4); and (3) EEC growth (78% inhibition, P = .001 for PV1; 47% inhibition, P = .002 for PV2; 60% inhibition, P = .001 for PV3; and 80% inhibition, P = .001 for PV4) (Figure 6D-E).
We studied whether EPO could counteract the effects of the Jak2V617F shRNA in patients as observed with other cytokines in cell lines. In all patients, except PV2, who only had JAK2V617F homozygous erythroid progenitors, a high EPO concentration (1000 mU/mL) was able to counterbalance the effect of JAK2V617F inhibition on erythroid growth (Figure 6D-E). Similarly, an intermediate EPO concentration of 100 mU/mL (possibly found in PMF patient serum) rescued erythroid growth to the same extent as 1000 mU/mL EPO in PV3 and PV4 patients (Figure 6E). However, in these patients, a low EPO concentration (10 mU/mL; similar to normal EPO serum level) did not rescue the effects of the shRNA on erythroid progenitor growth (Figure 6E). In the 2 healthy patients, the shRNAs had no effect on the level of the JAK2 protein and on BFU-E growth in presence of 1 U/mL EPO (Figure 6B,D).
Effect of the JAK2 inhibitor AZ960 on PV EEC and BFU-E proliferation
Our results show that EPO stimulation antagonizes shRNA-mediated JAK2V617F inhibition. However, the level of JAK2V617F inhibition by shRNA is difficult to modulate, and the presence of the WT JAK2 gene may have skewed the results. Therefore, we used the JAK2 inhibitor AZ960, which is not specific for the mutation, to verify to what extent inhibition of PV BFU-E growth by JAK2 inhibition was antagonized by EPO stimulation. Methylcellulose assays showed that AZ960 inhibited EEC- and EPO-stimulated BFU-E colony formations derived from PV3 in a dose-dependent manner (Figure 7A). Similar results were found for PV1 and PV2 (data not shown). Interestingly, the IC50 increased with the dose of EPO, leading to mean fold increases in the IC50 for the PV1, PV2, and PV3 patients of 1.6 or 5.4 in the presence of 10 mU/mL or 1000 mU/mL EPO, respectively, compared with no added EPO (Figure 7B). In contrast to shRNA experiments (Figure 6E), high compound dosage (1 μM) strongly inhibited erythroid growth in the presence of 1000 mU/mL EPO, suggesting that cytokine rescue is dependent on the remaining JAK2 protein or activity.
Effect of EPO concentrations on the ability of the anti-JAK2 inhibitor AZ960 to inhibit erythroid colony formation from 3 PV patients. CD34+ cells from PV patients were plated (2 × 103 cells) in semisolid cultures in the presence of SCF, IL-3, and different concentrations of EPO and AZ960, as indicated. Colonies were counted after 14 days of incubation. (A) Typical results obtained from PV3 patient cells showing inhibition of colony formation by AZ960. (B) Mean IC50 (± SE) of AZ960 on colony formation with different concentrations of EPO, as indicated, obtained from PV1, PV2, and PV3 patient cells. IC50 were determined using Prism4 software analysis.
Effect of EPO concentrations on the ability of the anti-JAK2 inhibitor AZ960 to inhibit erythroid colony formation from 3 PV patients. CD34+ cells from PV patients were plated (2 × 103 cells) in semisolid cultures in the presence of SCF, IL-3, and different concentrations of EPO and AZ960, as indicated. Colonies were counted after 14 days of incubation. (A) Typical results obtained from PV3 patient cells showing inhibition of colony formation by AZ960. (B) Mean IC50 (± SE) of AZ960 on colony formation with different concentrations of EPO, as indicated, obtained from PV1, PV2, and PV3 patient cells. IC50 were determined using Prism4 software analysis.
In conclusion, our results show that a small molecule inhibitor abrogates malignant JAK2V617F-dependent proliferation of MPD progenitor cells and cell lines, but that cytokine stimulation is able to antagonize their efficacy, as also observed for shRNAs.
Discussion
In this study, we developed novel siRNA and shRNA sequences specifically targeting the JAK2V617F mutation, the main molecular lesion of classical MPD. Expression of these RNAi molecules in cell lines and primary cells induced partial inhibition of the JAK2V617F protein expression without affecting JAK2WT. Moreover, silencing of the JAK2V617F gene was sufficient to inhibit the following: (1) endogenous phosphorylation of the JAK2 downstream signaling molecules STAT5 and ERK1/2; (2) cell survival; (3) cell-cycle progression, and finally, JAK2V617F-dependent autonomous cell proliferation in JAK2V617F-expressing cell lines (HEL, UKE-1, or SET-2). In CD34+ cells from PV patients, expression of the Jak2V617F-shRNA resulted in reduction of STAT5 phosphorylation and EEC colony formation. Intriguingly, addition of IL-3, GM-CSF, or TPO to the UKE-1 and SET-2 cells, or EPO to the PV patient cells, dose dependently antagonized growth inhibition induced by the Jak2V617F-shRNA. The doses of cytokines necessary for this effect are physiologically relevant for TPO or EPO, that is, within normal or PMF patient serum levels, respectively. Finally, efficiency of a small JAK2 inhibitor molecule (targeting both the mutated and WT JAK2 activities) in suppressing EEC- andBFU-E–derived colony formation from PV patients decreased with increasing doses of EPO. In conclusion, our results show that JAK2V617F- or JAK2-targeted therapies can successfully block JAK2V617F-dependent cell growth, but that the presence of cytokines counteracts their effects.
The JAK2V617F mutation is expressed in approximately 95% of PV patients and half of the patients suffering from PMF or ET. In animal models, expression of this unique mutation in hematopoietic cells recapitulates features observed in these 3 types of classical MPD depending on the level and duration of expression of the mutated kinase.26,27 In line with this observation, heterozygous JAK2V617F progenitors are observed in ET patients, whereas homozygous JAK2V617F progenitors are mainly present in PV patients.28,29 With regard to the success of kinase inhibitors, notably imatimib, in the treatment of other MPD such as CML,12 JAK2 inhibitors have been developed for targeted therapy of JAK2-driven MPD. Preclinical studies in murine models combined with the preliminary outcomes in PMF clinical trials gave mitigated results.13,30 In particular, no change in allele burden, despite some clinical improvement, has been reported.30 However, none of the reported inhibitors have demonstrated any specificity for the JAK2 mutant.13 Given the fundamental role of JAK2 in normal hematopoiesis, demonstrated by in utero lethality of JAK2-deficient mice,31 the development of a selective JAK2V617F-targeted therapy could provide a clinical advantage in eliminating the malignant clone while preserving normal hematopoiesis. Such a preferential inhibition has been reported for BCR-ABL inhibitors in CML,32,33 but in contrast to BCR-ABL, JAK2V617F displays a single point mutation located outside of its kinase domain, making a chemistry-based mutation-specific inhibitory approach especially challenging. However, at the gene level, mutation-specific inhibition has been achieved using RNAi for proteins displaying a single amino acid substitution.14,–16 Thus, we developed siRNAi targeting the G-to-T JAK2 mutation at nucleotide 1849 to specifically knock down JAK2V617F.
Of 6 different siRNAs, 2 demonstrated high specificity for JAK2V617F, in that they decreased both the mRNA and corresponding protein production. As previously reported, centrally positioning the V617F mutation in the siRNA sequence, near the estimated cleavage site in the RNA-induced silencing complex (position 9, 10, or 11), was essential for efficacy.34 Specific inhibition of JAK2V617F protein expression was demonstrated in several cell lines and PV patient primary cells using shRNAs, including the 2 same sequences as the siRNAs. The shRNA was especially efficient in the heterozygous SET-2 cells compared with the homozygous HEL and UKE-1 cells. It is possible that, by competing for cytokine receptors, JAK2WT may suppress the residual JAK2V617F activity in the shRNA-transduced SET-2 cells, leading to complete inhibition of JAK2V617F-driven autonomous survival and proliferation.5
The addition of IL-3 or TPO to the UKE-1 cells and GM-CSF or TPO to the SET-2 cells partially or completely rescued the growth inhibition induced by the Jak2V617F-shRNA. Studies in JAK2-deficient animals have demonstrated that JAK2 plays a critical and nonredundant role in the function of these cytokines.31 Therefore, the effect of these cytokines in rescuing growth in the shRNA-transduced cells may result from cytokine-induced phosphorylation of JAK2WT and the nondegradated (residual) JAK2V617F in SET-2 and only of residual JAK2V617F in HEL and UKE-1 cells. A similar approach was taken with ex vivo samples from PV patients. Two patients, PV2 and PV4, were extremely informative, because both are almost clonal for Jak2V617F at the progenitor level. However, PV2 had only homozygous JAK2V617F-positive BFU-E, whereas PV4 had only heterozygous JAK2V617F-positive BFU-E. In both cases, the shRNAs inhibited EEC formation, but high EPO concentrations completely from PV4, but only partially for PV2, rescued BFU-E growth. Thus, as expected, specific Jak2V617F-shRNAs were proven to be more effective in homozygous clones in the presence of cytokines. It has to be underscored that inhibition of JAK2 protein was more than 50% in PV4 (heterozygous patient), in agreement with recent results showing that at the mRNA level the JAK2V617F/JAK2WT ratio may be more than one in heterozygous cells.27
A potential hypothetical limitation of JAK2V617F selective therapy resides in the occurrence of non-JAK2V617F–related molecular lesions in MPD pathogenesis. This may explain the rare JAK2V617F-negative EEC detected in PV1 and PV3 patients, confirming previous reports.11 It is noteworthy that HEL cell proliferation was refractory to full inhibition by small molecule JAK2 inhibitors24 and Jak2V617F-shRNAs, suggesting that endogenous JAK2 activation may not be the only mechanism involved in cytokine independency of this cell line.
Our results have consequences related to current anti-JAK2 clinical trials concerning dosage, safety, and mechanism of action. The JAK2V617F disease arises through progressive expansion of malignant mature cells with low cytokine requirement, leading to a related decrease in the cytokine levels. As a result, the number of normal mature clones with normal cytokine requirement decreases. For example in ET and PMF, TPO levels are inversely correlated with the platelet levels,4 and in PV, the excess of malignant erythroid cells leads to low EPO levels. In this low cytokine environment, JAK2 inhibitors should selectively affect the predominant malignant cell population. Then, as the cytokine levels should theoretically increase, as shown for Epo in PMF patients treated with the INCB018424 inhibitor,35 through reduction of the hematopoietic cell mass (red blood or megakaryocytic cells), this should allow re-emergence of normal clones and allele burden reduction, as shown recently in murine models.36 Our results predict that increase in cytokine levels during treatment will reduce JAK2 inhibitor efficiency as follows: (1) on malignant hematopoiesis, impairing efficacy on controlling disease progression, but also (2) on normal hematopoiesis, protecting the patients from developing anemia or cytopenia. At this stage, the long-lasting success of JAK2 inhibitor therapy will most likely depend on differences in sensitivity of malignant and normal cells to the inhibitor at normal or high cytokine levels in the case of hematopoiesis impairment. The lack of selectivity of small molecule inhibitors for the mutated protein suggests that disease might only be contained, but not eliminated. This is suggested by recent trials reporting no change in JAK2V617F allele burden.30 In this regard, this study offers a biologic explanation for this failure to eradicate the disease. In contrast, our results predict that JAK2V617F-specific inhibition by shRNA may be successful in progressively eradicating the disease. Indeed, protection of normal hematopoiesis should prevent cytokine levels from rising above normal, maintaining efficacy of shRNA on malignant cell population reduction, including heterozygous clones that would lose their proliferation advantage over the normal clones. In PV patients, we showed that high efficacy was still observed at normal EPO serum level (10 mU/mL). However, whether such a therapy may be efficient on JAK2V617F hematopoietic stem cells, whose proliferation and survival may not depend exclusively on JAK2, remains to be demonstrated. The same limitation has been described in imatimib treatment of BCR-ABL quiescent hematopoietic stem cells in CML.37
This work shows that Jak2V617F-RNAi therapy can be considered in the treatment of JAK2V617F-positive MPD and might present advantages over current small molecule JAK2 inhibitors with regard to safety and disease eradication. Despite recent progresses in shRNA delivery, more work remains to be performed before such therapy might be really practical in vivo. However, this study underscores the interest of selectively targeting JAK2V617F. Despite the challenge, this emphasizes potential value of small molecule inhibitors preferentially inhibiting JAK2V617F over JAK2WT activity to maximize suppression of the malignant clone burden.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Didier Pisani (CNRN, Nice, France) for biochemical expertise; Yann Lecluse (IGR, Villejuif, France) for cell-sorting experiments; Géraldine Jacob, Nicolas Soustelle, Victoria Raggueneau, and Céline Callens (Hôpital Dieu, Paris, France) for patient and control cell collections; and Dr Fielder (University Hospital Hamburg-Eppendorf, Hamburg, Germany) for providing the UKE-1 cells. We thank Dennis Huszar (AstraZeneca, Waltham, MA) for review of the manuscript and AstraZeneca R&D (Waltham, MA) for providing AZ960.
This work was supported by grants from Inserm, IGR, Institut National Contre le Cancer, and Cancéropôle Ile-de-France, and by special funding from La Ligue Nationale Contre le Cancer (labeled Team 2006). A.J. had fellowships from the Ministère de la Recherche et de la Technologie, Association pour la Recherche sur le Cancer, and Société Française d'Hématologie. C.M. is an Institut National du Cancer recipient.
Authorship
Contribution: A.J., C.M., and C.O. performed research and analyzed data; J.-L.V. designed experiments, performed research, analyzed data, and wrote the paper; J.-A.R. and N.C. provided patients and clinical data; L.J.-L. designed mt-siRNAs; and W.V. and A.G. designed experiments.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jean-Luc Villeval, Inserm, Unité 790, 39 rue Camille Desmoulins, Institut Gustave Roussy, PR1, 94805 Villejuif, France; e-mail: villeval@igr.fr.

![Figure 4. Dose-dependent rescue by cytokines of Jak2V617F shRNA-induced cell growth inhibition. (A) UKE-1 and SET-2 cells were infected with the pRRL-mt4 or the control pRRL-Scr virus, selected for GFP expression, and plated in liquid culture in the absence or the presence of increasing concentrations of IL-3, TPO, or GM-CSF (expressed in nanograms per milliliter). Results [mean values (± SEM)] of 2 independent experiments from day 9 of culture are presented. *P ≤ .05. (B) Effect of IL-3 or TPO exposure on the phosphorylation levels of STAT5 in UKE-1 cells infected by the pRRL-mt4 or the control pRRL-Scr virus and cultured for 6 days with or without IL-3 (30 ng/mL) and TPO (10 ng/mL). Data indicate that rescue by cytokines could be mediated through signaling of the residual JAK2V617F protein, remaining after shRNA action, detected with this Western blot analysis.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/114/9/10.1182_blood-2008-09-176875/5/m_zh89990941360004.jpeg?Expires=1769939355&Signature=yZ-E9-qIJXyoY-2ASsCzM6UbMbobB-6iK1Fa3Qe~pa8ntOwg8CLR-KtuvmpVJKykMdWq6dfykD3IYf1H74RTo5BjUbb9JI2L4yMAcGZlwMWJ4iguVwG1Dr-9rxEVkSOGqjQr-mqPbiVXi6np9YL2gaEpFdJ97p5mCiVEcQMyGUHi8hjUX~~I0ncw17UXCZwnTo2qu88xy2TUVnLu1u0O-M4CVS-rHgWx4NoMzQpJx3ZLRWzZN2lHxKYPstmMxqLE2FSfZFva26huV8d7RCHFRGeQDxX1wHU4cy5jyRYkPJ9vlMzq77fyQcX7Af7jdFHp7GwotLGxS37oLJvMZoaiNQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)