Abstract
Delayed engraftment or graft failure is one of the major complications after cord blood transplantation (CBT). To investigate factors impacting engraftment, we conducted a retrospective analysis of adult patients who underwent reduced-intensity CBT at our institute, in which preparative regimens mainly consisted of fludarabine, melphalan, and total body irradiation with graft-versus-host (GVH) disease prophylaxis using single calcineurin inhibitors. Among 152 evaluable patients, the cumulative incidence of neutrophil engraftment was 89%. High total nucleated cell and CD34+ cell dose were associated with the faster speed and higher probability of engraftment. In addition, the degree of human leukocyte antigen (HLA) mismatch in the GVH direction was inversely associated with engraftment kinetics, whereas no statistically significant association was observed with the degree of HLA mismatch in the host-versus-graft direction. Similarly, the number of HLA class I antigens mismatched in the GVH direction, but not in the host-versus-graft direction, showed a negative correlation with engraftment kinetics. HLA disparity did not have significant impact on the development of GVH disease or survival. This result indicates the significant role of HLA disparity in the GVH direction in the successful engraftment, raising the novel mechanism responsible for graft failure in CBT.
Introduction
Recent studies have demonstrated cord blood transplantation (CBT) as a safe and feasible alternative to bone marrow (BM) or peripheral blood (PB) stem cell transplantation (SCT) in adults when no suitable related donor is available.1-4 The incidence and severity of acute graft-versus-host disease (GVHD) after CBT have been low compared with those after unrelated donor BM transplantation,1-4 permitting use of a mismatched unit as a graft. The use of CBT has also been increasing because of the potential advantage of rapid availability and the lower risk to donors. The development of reduced-intensity (RI) conditioning regimens for transplantation, which results in less toxicity and depends largely on graft-versus-tumor effects rather than high-dose therapy to eliminate malignant cells, has been shown to allow elderly patients to undergo allogeneic transplantation.5,6 We and other groups have reported the feasibility of RI-CBT for adult patients with advanced hematologic diseases.7-12
Despite the obvious advantage of CBT, high treatment-related toxicity has been observed, which precludes the application of CBT as a primary graft source. One of the major complications of CBT is delayed engraftment or graft failure. Thus far, several factors have been found to impact engraftment, including total nucleated cell (TNC) dose, CD34+ cell dose, and human leukocyte antigen (HLA) disparity.13-15 Here, we report the results of a retrospective analysis of 163 adult patients who underwent RI-CBT at our institute, which revealed, for the first time, the importance of HLA disparity in the graft-versus-host (GVH) direction, adding a new viable factor in choosing cord blood (CB) units as transplant-able grafts.
Methods
Study patients
This study included adult patients with hematologic malignancies who underwent RI-CBT as their first allogeneic SCT at Toranomon Hospital between January 2002 and December 2006 consecutively. Twenty-nine patients who had active serious infection or showed an Eastern Cooperative Oncology Group performance status of 3 or 4 before transplantation were not eligible for this study because of differences in transplantation procedures or supportive care resulting from serious organ dysfunction and active infection. Then, the remaining 163 consecutive patients were reviewed. All patients had diseases that were incurable with conventional treatments, lacked suitable sibling or unrelated donors, and were considered inappropriate for conventional allo-SCT as they were older than 50 years and/or had organ dysfunction (often attributable to previous intense chemotherapy and/or radiotherapy). Characteristics of the 163 patients are summarized in Table 1.
Patient and cord blood characteristics
| Variable . | Value . |
|---|---|
| No. of patients | 163 |
| Median age, y (range) | 55 (17-79) |
| Sex: male/female, no. of patients | 98/65 |
| Primary diseases, no. of patients | |
| Acute lymphoblastic leukemia | 20 |
| Acute myeloid leukemia | 63 |
| Chronic myelogenous leukemia | 5 |
| Myelodysplastic syndrome | 12 |
| Malignant lymphoma | 39 |
| Adult T-cell leukemia/lymphoma | 18 |
| Multiple myeloma | 2 |
| Others | 4 |
| Risk of underlying disease, no. of patients: standard/high | 32/131 |
| Preparative regimens, no. of patients | |
| Flu + Mel + TBI 2-8 Gy | 135 |
| Flu + BU + TBI 4-8 Gy | 18 |
| Flu + Mel | 6 |
| Flu + BU | 4 |
| Median no. of infused nucleated cells, 107/kg (range) | 2.68 (1.82-4.83) |
| Median no. of infused CD34+ cells, 105/kg (range) | 0.76 (0.05-4.40) |
| Blood-type mismatch, no. of patients: match/mismatch | 47/116 |
| HLA antigen mismatch, no. of patients | |
| 0 | 3 |
| 1 | 24 |
| 2 | 136 |
| GVHD prophylaxis, no. of patients | |
| Cyclosporine A alone | 73 |
| Tacrolimus alone | 90 |
| Variable . | Value . |
|---|---|
| No. of patients | 163 |
| Median age, y (range) | 55 (17-79) |
| Sex: male/female, no. of patients | 98/65 |
| Primary diseases, no. of patients | |
| Acute lymphoblastic leukemia | 20 |
| Acute myeloid leukemia | 63 |
| Chronic myelogenous leukemia | 5 |
| Myelodysplastic syndrome | 12 |
| Malignant lymphoma | 39 |
| Adult T-cell leukemia/lymphoma | 18 |
| Multiple myeloma | 2 |
| Others | 4 |
| Risk of underlying disease, no. of patients: standard/high | 32/131 |
| Preparative regimens, no. of patients | |
| Flu + Mel + TBI 2-8 Gy | 135 |
| Flu + BU + TBI 4-8 Gy | 18 |
| Flu + Mel | 6 |
| Flu + BU | 4 |
| Median no. of infused nucleated cells, 107/kg (range) | 2.68 (1.82-4.83) |
| Median no. of infused CD34+ cells, 105/kg (range) | 0.76 (0.05-4.40) |
| Blood-type mismatch, no. of patients: match/mismatch | 47/116 |
| HLA antigen mismatch, no. of patients | |
| 0 | 3 |
| 1 | 24 |
| 2 | 136 |
| GVHD prophylaxis, no. of patients | |
| Cyclosporine A alone | 73 |
| Tacrolimus alone | 90 |
Flu indicates fludarabine; Mel, melphalan; TBI, total body irradiation; and BU, busulfan.
For disease status, those with hematologic malignancies in the first or second complete remission at the time of transplantation, those in the chronic phase or accelerated phase of chronic myeloid leukemia, and those with refractory anemia of myelodysplastic syndrome were defined as being at standard risk (n = 32), whereas those in other situations were defined as being at high risk (n = 131). All patients received a single CB unit. All patients provided written informed consent in accordance with the Declaration of Helsinki, and the study was conducted in accordance with the requirements of the Institutional Review Board of Toranomon Hospital.
Donor selection
CB units were obtained from the Japanese Cord Blood Bank Network. All CB samples, as well as the patient's blood samples, were serologically typed for HLA-A, -B and -DR antigens before transplantation. Alleles at the HLA-A, -B, and -DRB1 loci were identified by high-resolution DNA typing in 107 pairs because HLA typing of alleles was not routinely performed in Japanese CB banks. In 127 pairs, HLA-A and -B antigens were identified by serologic typing and HLA-DRB1 alleles were determined by high-resolution DNA typing. CB grafts had at most 2 mismatches for HLA-A, -B, and -DR antigens and had a cryopreserved cell dose of at least 1.8 × 107 nucleated cells per kg of recipient body weight. Mismatch was counted separately in the GVH and host-versus-graft (HVG) direction, respectively. HLA mismatch in the GVH direction was defined when the recipient's antigens or alleles were not shared by the donor, whereas HLA mismatch in the HVG direction was defined when the donor's antigens or alleles were not shared by the recipient.
Transplantation procedures
Pretransplantation conditioning regimens varied and were determined by each attending physician according to the patient's disease, disease status, and history of prior therapy. All patients received purine analog-based preparative regimens. The majority of patients (n = 119) received preparative regimens consisting of fludarabine 125 mg/m2, melphalan 80 mg/m2, and 4 Gy total body irradiation (TBI). Patients in relatively poor performance status were conditioned with busulfan to avoid severe gastrointestinal tract toxicity induced by the use of melphalan. GVHD prophylaxis was carried out using a continuous infusion of cyclosporine A 3 mg/kg or tacrolimus 0.03 mg/kg from day −1 until the patients could tolerate oral administration.
Supportive care
All patients were treated in reverse isolation in laminar airflow-equipped rooms and received trimethoprim/sulfamethoxazole for Pneumocystis jirovecii prophylaxis. Fluoroquinolone, azole, and acyclovir were administered to prevent bacterial, fungal, and herpes virus infection, respectively. Cytomegalovirus pp65 antigenemia was monitored weekly. Hemoglobin and platelet counts were maintained at more than 7 g/dL and at 10 × 109/L, respectively. Granulocyte colony-stimulating factor was administered intravenously from day 1 until neutrophil recovery became durable.
Definition of engraftment, GVHD, and survival
Date of engraftment was defined as the first of 3 consecutive days when the neutrophil counts exceeded 0.5 × 109/L. Patients who did not achieve this criterion at any time after transplantation were considered as primary graft failure. Chimerism was assessed using fluorescent in situ hybridization in sex-mismatched donor-recipient pairs. In sex-matched pairs, polymerase chain reaction for variable numbers of tandem repeats was used with donor cells detected at a sensitivity of 10%. Acute and chronic GVHD was diagnosed and graded according to standard criteria.16,17 Overall survival was calculated from the day of transplantation until death from any cause or last follow-up. Event-free survival was defined as the duration of survival after transplantation without disease progression, relapse, graft failure, or death. Final follow-up was conducted in December 2007, with a median follow-up of surviving patients being 29.0 months (range, 3.7-58.9 months).
Statistical methods
Cumulative incidence of neutrophil engraftment was calculated using the Gray method, treating death before engraftment or second transplantation as competing events.18 Similarly, in the analysis of GVHD, death resulting from other causes or relapse leading to early withdrawal of immune suppression was considered competing risk. The probabilities of survival were estimated using the Kaplan-Meier method. Multivariate analysis was performed using the proportional hazards model. P values < .05 were considered statistically significant.
Results
Engraftment
Eleven of the 163 patients reviewed were not evaluable for the analyses of donor engraftment resulting from early death (before 28 days after transplantation) from disease progression (n = 1), infection (n = 6), and multiple organ failure (n = 4). Of 152 evaluable patients, 135 patients achieved neutrophil engraftment. The cumulative incidence of engraftment at day 60 was 89%, and the median time to engraftment was 20 days (range, 11-55 days). Chimerism analyses were performed in 125 of 135 patients who achieved engraftment using either PB or BM samples at the time of neutrophil recovery. All patients except for one who had residual leukemic cells in PB at the time of engraftment showed complete donor chimerism (> 90%). The median length of time required to donor chimerism was 22 days (range, 11-55 days).
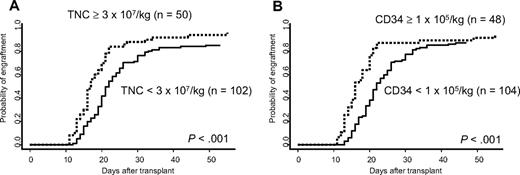
Age, recipient sex, risk of underlying disease, blood type mismatch, and GVHD prophylaxis did not affect engraftment kinetics (data not shown). TNC more than or equal to 3 × 107/kg was associated with a significantly higher probability of engraftment (P < .001), with the median time to engraftment of 16.5 days (range, 11-55 days) compared with 21 days (range, 12-49 days) for those who received less than 3 × 107/kg (Figure 1A). Similarly, CD34+ cell dose more than or equal to 105/kg was associated with a significantly faster engraftment (P < .001) than those who received less than 105/kg (Figure 1B).
Cumulative incidence of neutrophil engraftment. (A) Effect of TNC dose. (B) Effect of CD34+ cell dose.
Cumulative incidence of neutrophil engraftment. (A) Effect of TNC dose. (B) Effect of CD34+ cell dose.
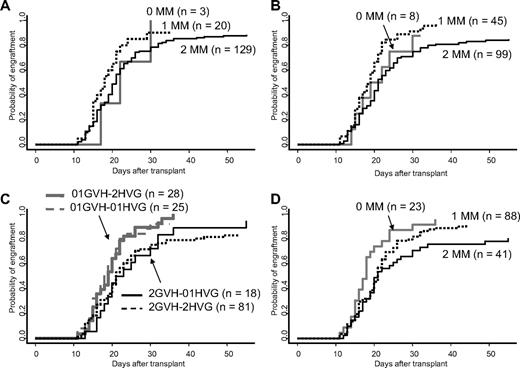
The cumulative incidence of engraftment and the time to engraftment according to the degree of HLA mismatch are shown in Table 2. Patients who had 0 and 1 antigen mismatch with the grafts were combined, considering the small number of patients in 0 mismatch group and comparable rate of engraftment and time to neutrophil recovery between 0 and 1 antigen-mismatched group (Figure 2A-B), and were compared with those of 2 antigens mismatched. Although patients with 0 or 1 antigen mismatch showed a trend toward superior engraftment kinetics compared with patients with 2 antigens mismatched, the differences did not reach statistical significance (Figure 2A; Table 2). We further analyzed the influence of HLA disparity on engraftment in both the HVG and GVH direction. In the HVG direction, the cumulative incidence of engraftment at day 60 was 93% in 0 or 1 antigen mismatch and 87% in 2 antigens mismatched (P = .4, Table 2). In the GVH direction, however, the cumulative incidence of engraftment was 96% in 0 or 1 antigen mismatch and 85% in 2 antigens mismatched (P < .001, Figure 2B; Table 2), demonstrating that HLA antigen disparity in the GVH direction was significantly associated with engraftment kinetics. As shown in Figure 2C, HLA antigen disparity in the HVG direction did not contribute to engraftment kinetics in patients with 0 or 1 antigen mismatch in the GVH direction, as was also observed in those with 2 antigens mismatched in the GVH direction. Although the number of patients in each group was small, patients with 0 or 1 mismatch in the GVH direction but 2 mismatches in the HVG direction (n = 28) showed a trend toward superior engraftment kinetics compared with patients with 0 or 1 mismatch in the HVG direction but 2 mismatches in the GVH direction (n = 18; P = .07). This finding may indicate that HLA disparity in the GVH direction plays a greater role in engraftment than that in the HVG direction.
Univariate analyses of engraftment kinetics according to HLA disparity
| No. of HLA mismatches . | Neutrophil engraftment . | ||||
|---|---|---|---|---|---|
| n . | Cumulative incidence, % . | Median day . | Range . | P . | |
| HLA-A, -B, -DR (antigen) | .09 | ||||
| 0 + 1 | 23 | 91 | 17 | 11-30 | |
| 2 | 129 | 89 | 20 | 11-55 | |
| HLA-A, -B, -DR (antigen, HVG) | .4 | ||||
| 0 + 1 | 43 | 93 | 19 | 11-55 | |
| 2 | 109 | 87 | 20 | 11-49 | |
| HLA-A, -B, -DR (antigen, GVH) | < .001 | ||||
| 0 + 1 | 53 | 96 | 19 | 11-36 | |
| 2 | 99 | 85 | 20 | 11-55 | |
| HLA-A, -B (class I antigen) | .1 | ||||
| 0 | 13 | 92 | 17 | 12-30 | |
| 1 | 86 | 91 | 20 | 11-44 | |
| 2 | 53 | 85 | 20 | 11-55 | |
| HLA-A, -B (class I antigen, HVG) | .4 | ||||
| 0 | 22 | 96 | 18 | 12-36 | |
| 1 | 86 | 89 | 20 | 11-55 | |
| 2 | 44 | 84 | 20 | 11-49 | |
| HLA-A, -B (class I antigen, GVH) | .006 | ||||
| 0 | 23 | 95 | 17.5 | 11-36 | |
| 1 | 88 | 91 | 20.5 | 11-44 | |
| 2 | 41 | 81 | 20 | 12-55 | |
| HLA-A (antigen) | .7 | ||||
| 0 | 87 | 89 | 19 | 11-44 | |
| 1 + 2 | 65 | 89 | 20 | 11-55 | |
| HLA-A (antigen, HVG) | .8 | ||||
| 0 | 96 | 89 | 20 | 11-55 | |
| 1 + 2 | 56 | 89 | 20 | 11-49 | |
| HLA-A (antigen, GVH) | .2 | ||||
| 0 | 103 | 90 | 19 | 11-44 | |
| 1 + 2 | 49 | 86 | 20 | 13-55 | |
| HLA-B (antigen) | .07 | ||||
| 0 | 36 | 94 | 19 | 12-34 | |
| 1 + 2 | 116 | 87 | 20 | 11-55 | |
| HLA-B (antigen, HVG) | .06 | ||||
| 0 | 45 | 95 | 19 | 12-36 | |
| 1 + 2 | 107 | 86 | 20 | 11-55 | |
| HLA-B (antigen, GVH) | .04 | ||||
| 0 | 42 | 95 | 18.5 | 11-36 | |
| 1 + 2 | 110 | 86 | 20 | 11-55 | |
| HLA-DR (antigen) | .4 | ||||
| 0 | 70 | 87 | 20 | 11-55 | |
| 1 + 2 | 82 | 90 | 19.5 | 11-44 | |
| HLA-DR (antigen, HVG) | .7 | ||||
| 0 | 76 | 88 | 20 | 11-55 | |
| 1 + 2 | 76 | 89 | 20 | 11-44 | |
| HLA-DR (antigen, GVH) | .8 | ||||
| 0 | 83 | 88 | 20 | 11-55 | |
| 1 + 2 | 69 | 90 | 20 | 11-44 | |
| HLA-A, -B (antigen), -DR (allele) | .5 | ||||
| 0 + 1 | 13 | 92 | 18 | 14-30 | |
| 2 | 63 | 84 | 20 | 11-47 | |
| 3 + 4 | 44 | 86 | 20 | 11-49 | |
| HLA-A, -B (antigen, HVG), -DR (allele, HVG) | .2 | ||||
| 0 + 1 | 25 | 96 | 18 | 11-32 | |
| 2 | 54 | 80 | 20 | 11-44 | |
| 3 + 4 | 41 | 90 | 20 | 11-49 | |
| HLA-A, -B (antigen, GVH), -DR (allele, GVH) | .05 | ||||
| 0 + 1 | 26 | 96 | 18 | 11-36 | |
| 2 | 57 | 84 | 19.5 | 11-49 | |
| 3 + 4 | 37 | 84 | 20 | 11-34 | |
| HLA-A, -B, -DR (allele) | .4 | ||||
| 0 + 1 | 10 | 90 | 18 | 14-30 | |
| 2 | 36 | 86 | 20 | 11-44 | |
| 3 + 4 + 5 | 56 | 84 | 19 | 11-49 | |
| HLA-A, -B, -DR (allele, HVG) | .3 | ||||
| 0 + 1 | 19 | 94 | 19 | 11-32 | |
| 2 | 34 | 79 | 20 | 13-44 | |
| 3 + 4 + 5 | 49 | 86 | 21 | 11-49 | |
| HLA-A, -B, -DR (allele, GVH) | .05 | ||||
| 0 + 1 | 16 | 94 | 17 | 11-30 | |
| 2 | 40 | 88 | 20 | 11-44 | |
| 3 + 4 + 5 | 46 | 80 | 20 | 11-49 | |
| No. of HLA mismatches . | Neutrophil engraftment . | ||||
|---|---|---|---|---|---|
| n . | Cumulative incidence, % . | Median day . | Range . | P . | |
| HLA-A, -B, -DR (antigen) | .09 | ||||
| 0 + 1 | 23 | 91 | 17 | 11-30 | |
| 2 | 129 | 89 | 20 | 11-55 | |
| HLA-A, -B, -DR (antigen, HVG) | .4 | ||||
| 0 + 1 | 43 | 93 | 19 | 11-55 | |
| 2 | 109 | 87 | 20 | 11-49 | |
| HLA-A, -B, -DR (antigen, GVH) | < .001 | ||||
| 0 + 1 | 53 | 96 | 19 | 11-36 | |
| 2 | 99 | 85 | 20 | 11-55 | |
| HLA-A, -B (class I antigen) | .1 | ||||
| 0 | 13 | 92 | 17 | 12-30 | |
| 1 | 86 | 91 | 20 | 11-44 | |
| 2 | 53 | 85 | 20 | 11-55 | |
| HLA-A, -B (class I antigen, HVG) | .4 | ||||
| 0 | 22 | 96 | 18 | 12-36 | |
| 1 | 86 | 89 | 20 | 11-55 | |
| 2 | 44 | 84 | 20 | 11-49 | |
| HLA-A, -B (class I antigen, GVH) | .006 | ||||
| 0 | 23 | 95 | 17.5 | 11-36 | |
| 1 | 88 | 91 | 20.5 | 11-44 | |
| 2 | 41 | 81 | 20 | 12-55 | |
| HLA-A (antigen) | .7 | ||||
| 0 | 87 | 89 | 19 | 11-44 | |
| 1 + 2 | 65 | 89 | 20 | 11-55 | |
| HLA-A (antigen, HVG) | .8 | ||||
| 0 | 96 | 89 | 20 | 11-55 | |
| 1 + 2 | 56 | 89 | 20 | 11-49 | |
| HLA-A (antigen, GVH) | .2 | ||||
| 0 | 103 | 90 | 19 | 11-44 | |
| 1 + 2 | 49 | 86 | 20 | 13-55 | |
| HLA-B (antigen) | .07 | ||||
| 0 | 36 | 94 | 19 | 12-34 | |
| 1 + 2 | 116 | 87 | 20 | 11-55 | |
| HLA-B (antigen, HVG) | .06 | ||||
| 0 | 45 | 95 | 19 | 12-36 | |
| 1 + 2 | 107 | 86 | 20 | 11-55 | |
| HLA-B (antigen, GVH) | .04 | ||||
| 0 | 42 | 95 | 18.5 | 11-36 | |
| 1 + 2 | 110 | 86 | 20 | 11-55 | |
| HLA-DR (antigen) | .4 | ||||
| 0 | 70 | 87 | 20 | 11-55 | |
| 1 + 2 | 82 | 90 | 19.5 | 11-44 | |
| HLA-DR (antigen, HVG) | .7 | ||||
| 0 | 76 | 88 | 20 | 11-55 | |
| 1 + 2 | 76 | 89 | 20 | 11-44 | |
| HLA-DR (antigen, GVH) | .8 | ||||
| 0 | 83 | 88 | 20 | 11-55 | |
| 1 + 2 | 69 | 90 | 20 | 11-44 | |
| HLA-A, -B (antigen), -DR (allele) | .5 | ||||
| 0 + 1 | 13 | 92 | 18 | 14-30 | |
| 2 | 63 | 84 | 20 | 11-47 | |
| 3 + 4 | 44 | 86 | 20 | 11-49 | |
| HLA-A, -B (antigen, HVG), -DR (allele, HVG) | .2 | ||||
| 0 + 1 | 25 | 96 | 18 | 11-32 | |
| 2 | 54 | 80 | 20 | 11-44 | |
| 3 + 4 | 41 | 90 | 20 | 11-49 | |
| HLA-A, -B (antigen, GVH), -DR (allele, GVH) | .05 | ||||
| 0 + 1 | 26 | 96 | 18 | 11-36 | |
| 2 | 57 | 84 | 19.5 | 11-49 | |
| 3 + 4 | 37 | 84 | 20 | 11-34 | |
| HLA-A, -B, -DR (allele) | .4 | ||||
| 0 + 1 | 10 | 90 | 18 | 14-30 | |
| 2 | 36 | 86 | 20 | 11-44 | |
| 3 + 4 + 5 | 56 | 84 | 19 | 11-49 | |
| HLA-A, -B, -DR (allele, HVG) | .3 | ||||
| 0 + 1 | 19 | 94 | 19 | 11-32 | |
| 2 | 34 | 79 | 20 | 13-44 | |
| 3 + 4 + 5 | 49 | 86 | 21 | 11-49 | |
| HLA-A, -B, -DR (allele, GVH) | .05 | ||||
| 0 + 1 | 16 | 94 | 17 | 11-30 | |
| 2 | 40 | 88 | 20 | 11-44 | |
| 3 + 4 + 5 | 46 | 80 | 20 | 11-49 | |
Cumulative incidence of neutrophil engraftment. MM indicates mismatch. (A) Effect of HLA antigen mismatch. (B) Effect of HLA antigen mismatch in the GVH direction. (C) Effect of HLA antigen mismatch according to mismatch both in the GVH and the HVG directions. 2GVH indicates 2 antigens mismatch in the GVH direction; 2HVG, 2 antigens mismatch in the HVG direction; 01GVH, 0 or 1 antigen mismatch in the GVH direction; 01HVG, 0 or 1 antigen mismatch in the HVG direction. (D) Effect of HLA class I antigen mismatch in the GVH direction.
Cumulative incidence of neutrophil engraftment. MM indicates mismatch. (A) Effect of HLA antigen mismatch. (B) Effect of HLA antigen mismatch in the GVH direction. (C) Effect of HLA antigen mismatch according to mismatch both in the GVH and the HVG directions. 2GVH indicates 2 antigens mismatch in the GVH direction; 2HVG, 2 antigens mismatch in the HVG direction; 01GVH, 0 or 1 antigen mismatch in the GVH direction; 01HVG, 0 or 1 antigen mismatch in the HVG direction. (D) Effect of HLA class I antigen mismatch in the GVH direction.
In addition to the degree of mismatch, we analyzed the significance of class I (HLA-A, -B) or class II (HLA-DR) mismatch (Table 2). The number of class I antigens mismatched in the GVH direction showed a negative correlation with the probability and the speed of engraftment (P = .006, Figure 2D), but not in the HVG or both directions. More specifically, the presence of HLA-B antigens mismatched in the GVH direction was significantly associated with inferior engraftment kinetics (P = .04). To the contrary, HLA-DR antigen mismatch did not influence engraftment kinetics in either the HVG or the GVH direction.
The cumulative incidence of engraftment was also assessed using 120 pairs who had HLA-A, -B antigens and -DRB1 allele information available (Table 2). Patients with 0 or 1 mismatch showed better engraftment kinetics compared with those with 2, 3, or 4 mismatches in the GVH direction, which was about to be significant statistically (P = .05), whereas HLA mismatch in the HVG direction did not show significant impact on engraftment.
HLA allele mismatch at the HLA-A, -B, and -DR was examined in 102 pairs. In the GVH direction, the cumulative incidence of engraftment was 94% in 0 or 1 allele mismatch, 88% in 2 alleles mismatched, and 80% in 3 to 5 alleles mismatched (P = .05), showing that alleles mismatched in the GVH direction could be inversely associated with engraftment kinetics (Table 2). In contrast, allele disparity in the HVG direction did not affect engraftment (Table 2). When HLA-A, -B, and -DR alleles were analyzed independently, no statistically significant differences were observed in any allele tested in either the GVH or HVG direction (data not shown).
Multivariate analyses revealed that low TNC dose (< 3 × 107/kg) and HLA antigens mismatched in the GVH direction (0 or 1 vs 2 antigens mismatched) were significantly associated with inferior engraftment kinetics, when age, recipient sex, risk of underlying disease, GVHD prophylaxis, and blood type mismatch were included as covariates (P = .002 and P = .004, respectively).
Clinical features of graft failure
There were 17 patients who failed to achieve engraftment: 8 males and 9 females, median age of 55 years (range, 17-68 years), high-risk diseases in 12 patients. Median TNC dose of CB grafts was 2.36 × 107/kg (range, 2.01-3.40 × 107/kg), and median CD34+ cell dose was 0.59 × 105/kg (range, 0.30-1.38 × 105/kg). Nine of them died before engraftment because of disease progression (n = 2), infection (n = 5), multiple organ failure (n = 1), and idiopathic pneumonia syndrome (n = 1). The remaining 8 patients received a second RI-CBT at a median of 34 days (range, 28-49 days) after first RI-CBT, and 3 of them were alive in remission.
Among those who did not achieve engraftment, chimerism analyses in the BM early after transplantation were performed on 8 patients (median, 12 days; range, 10-17 days). Of those,4 achieved complete donor chimerism, one had mixed chimerism (60% donor type), and 3 patients showed recipient chimerism. Four of 5 patients with donor dominant chimerism showed hemophagocytosis in the BM. On the other hand, all 3 patients with recipient chimerism did not show hemophagocytosis.
GVHD and survival
Among 134 evaluable patients, the cumulative incidence of acute GVHD of grade II to IV was 43%. The incidence of acute GVHD according to HLA disparity in the GVH direction was summarized in Table 3. Patients with 2 antigens mismatched showed a trend toward higher incidence of acute GVHD II-IV (P = .08). The number of class I or class II antigens mismatched had no correlation with the incidence of acute GVHD. Similarly, HLA disparity in the allele level was not significantly associated with the incidence of acute GVHD. Among 66 evaluable patients, the cumulative incidence of chronic GVHD was 51%. The degree of HLA mismatch was not significantly associated with the incidence of chronic GVHD (data not shown). Other pretransplantation factors, including age, infused cells, and GVHD prophylaxis, did not affect the incidence of GVHD. Overall survival and event-free survival at 2 years were 35% and 30%, respectively. HLA disparity in the GVH direction, as well as in the HVG direction, did not influence overall survival and event-free survival (Table 3; and data not shown).
Univariate analyses of acute GVHD and survival according to HLA disparity in the GVH direction
| No. of HLA mismatches in the GVH direction . | Acute GVHD II-IV . | 2-year overall survival . | ||||
|---|---|---|---|---|---|---|
| n . | Cumulative incidence, % . | P . | n . | Survival rate, % . | P . | |
| HLA-A, -B, -DR (antigen) | .08 | .5 | ||||
| 0 + 1 | 50 | 33 | 59 | 36 | ||
| 2 | 84 | 48 | 104 | 35 | ||
| HLA-A, -B (class I antigen) | .5 | .2 | ||||
| 0 | 22 | 36 | 24 | 54 | ||
| 1 | 80 | 42 | 96 | 32 | ||
| 2 | 32 | 46 | 43 | 32 | ||
| HLA-DR (class II antigen) | .5 | .9 | ||||
| 0 | 71 | 38 | 91 | 32 | ||
| 1 + 2 | 63 | 47 | 72 | 38 | ||
| HLA-A, -B (antigen), -DR (allele) | .4 | 1.0 | ||||
| 0 + 1 | 25 | 32 | 29 | 38 | ||
| 2 | 48 | 51 | 60 | 38 | ||
| 3 + 4 | 30 | 44 | 38 | 39 | ||
| HLA-A, -B, -DR (allele) | .3 | .4 | ||||
| 0 + 1 | 15 | 27 | 16 | 56 | ||
| 2 | 35 | 49 | 41 | 37 | ||
| 3 + 4 + 5 | 36 | 51 | 50 | 35 | ||
| No. of HLA mismatches in the GVH direction . | Acute GVHD II-IV . | 2-year overall survival . | ||||
|---|---|---|---|---|---|---|
| n . | Cumulative incidence, % . | P . | n . | Survival rate, % . | P . | |
| HLA-A, -B, -DR (antigen) | .08 | .5 | ||||
| 0 + 1 | 50 | 33 | 59 | 36 | ||
| 2 | 84 | 48 | 104 | 35 | ||
| HLA-A, -B (class I antigen) | .5 | .2 | ||||
| 0 | 22 | 36 | 24 | 54 | ||
| 1 | 80 | 42 | 96 | 32 | ||
| 2 | 32 | 46 | 43 | 32 | ||
| HLA-DR (class II antigen) | .5 | .9 | ||||
| 0 | 71 | 38 | 91 | 32 | ||
| 1 + 2 | 63 | 47 | 72 | 38 | ||
| HLA-A, -B (antigen), -DR (allele) | .4 | 1.0 | ||||
| 0 + 1 | 25 | 32 | 29 | 38 | ||
| 2 | 48 | 51 | 60 | 38 | ||
| 3 + 4 | 30 | 44 | 38 | 39 | ||
| HLA-A, -B, -DR (allele) | .3 | .4 | ||||
| 0 + 1 | 15 | 27 | 16 | 56 | ||
| 2 | 35 | 49 | 41 | 37 | ||
| 3 + 4 + 5 | 36 | 51 | 50 | 35 | ||
Discussion
Delayed hematopoietic recovery and graft failure are significant concerns in adult CBT. In the present study, median time to engraftment was 20 days, which was comparable with that reported in previous studies.1,4,7,19 These data indicate that our pretransplantation conditioning regimens, consisting mainly of fludarabine, melphalan, and 4 Gy TBI, along with single calcineurin inhibitors for GVHD prophylaxis, can exert reasonable immunosuppressive effects that allow rapid hematopoietic recovery after CBT. The engraftment was durable except for disease progression.
Almost all reports on CBT have demonstrated the profound impact of infused cell dose on engraftment.13,14,20 We showed that both high numbers of TNCs and CD34+ cells were favorably associated with time to engraftment and the probability of engraftment, confirming previous findings on the association of cell dose with neutrophil recovery. Considering that CD34+ cell dose reflects stem cell contents in the CB unit, stem cell dose is one of the major determinants of successful engraftment, as has been observed in the xenogeneic transplantation model.21-23
Although our results, demonstrating that HLA disparity in the GVH direction affected engraftment kinetics more than HLA disparity in the HVG direction, may seem paradoxical to the former notion of graft failure that results from graft rejection in most cases, they suggest a novel mechanism of graft failure in CBT. Previously, we have reported that a high incidence of noninfectious high-grade fever often coexisted with eruption, diarrhea, and weight gain, starting on a median of day 9 in more than 50% of the patients receiving CBT.8,24 We regarded this reaction as early onset of acute GVHD in which activated donor T cells secreted various cytokines.25 HLA disparity in the GVH direction may augment alloimmune reactions, which evoke hypercytokinemia and macrophage activation and occasionally result in establishment of hemophagocytic syndrome, one of the major complications directly related to graft failure in recipients.26-28 Indeed, a considerable number of patients showed hemophagocytosis in the BM with donor dominancy, leading to graft failure, even though we cannot exclude the possibility of graft rejection caused by recipient lymphocytes in some cases. In addition, among those who achieved donor cell engraftment, delayed neutrophil recovery was prominent for those with more HLA mismatch in the GVH direction rather than in the HVG direction. Myelosuppression is commonly observed during acute or chronic GVHD, indicating that GVHD can negatively affect hematopoietic function of the graft, possibly because of an attack on the hematopoiesis-supporting recipient stromal cells29 or production of cytokines from immune cells, such as transforming growth factor-β, known to regulate hematopoiesis negatively.30 The delayed engraftment observed in our study may have been caused by similar mechanisms during the recovery of donor cells. Furthermore, our results demonstrated that HLA class I antigen mismatch in the GVH direction was associated with inferior engraftment. Higher impact of HLA class II disparity on the development of acute GVHD has been reported in National Marrow Donor Program data.31 On the contrary, the Japan Marrow Donor Program registry data showed that mismatch in class I had higher impact than that in class II.32 The discrepancy may be explained by unique ethnic background of the Japanese population. The observation shown here may further strengthen our hypothesis that GVH reactions play a crucial role in engraftment process. In the analysis using allele data, the statistical power of HLA disparity in the GVH direction on engraftment had decreased. This discrepancy probably results from the small sample size in each mismatched category but may be suggestive of more powerful immunogenicity of mismatch in antigen rather than allele level.
In the Eurocord registry data, which includes 550 CBTs, HLA disparity was shown to have a negative impact on engraftment, although the effect of direction of mismatch was not described.14,33 More specifically, it was reported from the Düsseldorf Cord Blood Bank and Eurocord-Netcord Registry that HLA-A locus high-resolution typing in the HVG direction was associated with reduced cumulative incidence of engraftment in 122 patients receiving CBT.34 Several reasons may explain this discrepancy from our observations. First, patients included in our study received relatively uniform pretransplantation conditioning regimens consisting mainly of fludarabine, melphalan, and TBI, whereas those in the Eurocord database had more variable pretransplantation conditioning regimens. Second, all of our patients had GVHD prophylaxis using single calcineurin inhibitors, whereas most of those in the Eurocord Registry received additional chemicals or anti-thymocyte globulin. Many institutes use methotrexate,35,36 mycophenolate mofetil,19,37 corticosteroids,13 or anti-thymocyte globulin38,39 in combination with a calcineurin inhibitor as GVHD prophylaxis in CBT. Narimatsu et al demonstrated that use of short-term methotrexate was associated with a lower rate of posttransplantation immune reactions without compromising engraftment.36 Thus, more intensive immunosuppression may be beneficial for controlling early immune reactions and overcoming the issue of HLA mismatch. In addition, the unavoidable high incidence of gastrointestinal tract damage caused by TBI or melphalan in preparative regimens may have increased the chance of triggering GVH reactions.40
In the present study, HLA disparity had little association with the development of GVHD and survival, despite its obvious impact on engraftment. According to the Eurocord Registry data, better HLA match was not associated with better outcome in hematologic malignancies receiving CBT.20 Further analyses are required to determine whether this is the result of the unique immunologic immaturity of CB or to the heterogeneous patient population with the majority being in the high-risk disease status.
In conclusion, HLA disparity in the GVH direction, especially class I disparity, was found to have a significant impact on engraftment. These results shed light on a novel mechanism responsible for graft failure in CBT and add a valuable clue for choosing a better CB unit to avoid graft failure.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank data coordinators Kaori Kobayashi and Naomi Yamada for their invaluable help in making this study possible; Dr Akinori Kimura, Department of Molecular Pathogenesis, Division of Pathophysiology, Medical Research Institute, Tokyo Medical and Dental University, for critical review of the manuscript; and the physicians, nurses, pharmacists, and support personnel for their care of patients in this study.
This work was supported in part by a Research Grant for Tissue Engineering (H17-014) from the Japanese Ministry of Health, Labor, and Welfare.
Authorship
Contribution: N.M. and A.W. performed research and extracted data; A.Y. reviewed histopathologic methods; N.M. and Y.K. performed statistical analysis; N.U. and S. Taniguchi reviewed study design and methods; and K.I., H.A., S. Takagi, M.T., H.Y., D.K., Y.M., S.S., K.M., S. Miyakoshi, and S. Makino contributed to the writing of the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Naoyuki Uchida, 2-2-2 Toranomon, Minato-Ku, Tokyo 105-8470; e-mail: nuchida@toranomon.gr.jp.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal