Abstract
Signaling through tumor necrosis factor receptor 1 (TNFR1) controls bacterial infections and the induction of inflammatory Th1 cell–mediated autoimmune diseases. By dissecting Th1 cell–mediated delayed-type hypersensitivity responses (DTHRs) into single steps, we localized a central defect to the missing TNFR1 expression by endothelial cells (ECs). Adoptive transfer and mast cell knockin experiments into KitW/KitW-v, TNF−/−, and TNFR1−/− mice showed that the signaling defect exclusively affects mast cell–EC interactions but not T cells or antigen-presenting cells. As a consequence, TNFR1−/− mice had strongly reduced mRNA and protein expression of P-selectin, E-selectin, ICAM-1, and VCAM-1 during DTHR elicitation. In consequence, intravital fluorescence microscopy revealed up to 80% reduction of leukocyte rolling and firm adhesion in TNFR1−/− mice. As substitution of TNF−/− mice with TNF-producing mast cells fully restored DTHR in these mice, signaling of mast cell-derived TNF through TNFR1-expressing ECs is essential for the recruitment of leukocytes into sites of inflammation.
Introduction
Tumor necrosis factor (TNF) signaling through the TNF receptors (TNFRs) TNFR1 and TNFR2 is critically involved in innate and adaptive T cell–mediated immune responses.1 The crucial role of TNF in inflammation is demonstrated by the improvement of severe inflammatory autoimmune diseases, such as rheumatoid arthritis, inflammatory bowel disease, or psoriasis, during treatment with either anti-TNF antibodies or TNF-binding fusion proteins.2 Concomitantly, inhibition of TNF increases susceptibility to infections with bacteria or mycobacteria, further emphasizing the essential role of TNF in critical steps of innate and adaptive immunity.2-5 Comparison of TNFR1−/− and TNFR2−/− mice revealed that TNF signaling through TNFR1 is essential in host defense against intracellular pathogens, such as Listeria monocytogenes6 and Mycobacterium tuberculosis,7 whereas TNF signaling through TNFR2 increases susceptibility to intracellular infections.8 Moreover, TNFR1−/− mice show that signaling through TNFR1 is involved in early phases of acute graft-versus-host disease,9 experimental autoimmune encephalitis (EAE),10 autoimmune diabetes,11 or arthritis.12,13
Even though the cascade of TNF signaling through TNFR1 has been studied extensively,14 and even though it is established that TNF signaling through TNFR1 plays an essential role in T cell–mediated inflammation,6,7,9-13,15 the underlying mechanism remains unknown. This is especially true for the TNFR1-signaling pathway that translates local T-cell responses into inflammation, which remains enigmatic. Various models have been proposed to explain the profound defect in early phases of T cell–mediated inflammation in the absence of TNFR1. The data focus on impaired activation of nuclear factor-κB–dependent genes, leading to inappropriate apoptosis induction13,16 or defective T-cell priming.17 Importantly, whereas CD4 T cell–mediated control of bacterial infections and CD4 T cell–mediated inflammation depend strictly on signaling through TNFR1, CD8 T cell–mediated control of viral infections with choriomeningitis virus is TNFR1-independent.18,19
Together, these data suggest that signaling through TNFR1 is necessary for the translation of CD4 T cell–mediated immune responses into local inflammation and pathogen control, whereas this signaling pathway is obviously not required for killing of virally infected target cells by CD8 T cells.18 To uncover the role of TNFR1 during T cell–mediated inflammation, we strictly studied T cell- and TNF-dependent delayed-type hypersensitivity reactions (DTHRs) in response to the hapten trinitrochlorobenzene (TNCB).20,21
Three major haptens are used to induce contact hypersensitivity reactions (CHSRs) to evaluate DTHRs. TNCB-induced CHSRs are among the best studied model diseases to investigate hapten-induced DTHRs as these reactions strictly depend on Th1 or Tc1 cells and can be attenuated either by interleukin-4 (IL-4) or by IL-4–producing Th2 cells.22 In these types of reactions, IL-4 initially promotes the activation of IL-12–producing antigen-presenting cells (APCs),23 yet, during periods of either T-cell priming or T-cell effector functions, IL-4 attenuates TNCB-induced CHSR.22,24,25 A second, frequently used hapten is Oxazolon. In contrast to DTHR induced by TNCB, IL-4 seems to play a role in the generation of DTHR induced by Oxazolon.24,26,27 A third frequently used hapten is dinitroflourobenzene (DNFB). DNFB exerts the strongest toxic reactions. DNFB-specific CHSRs also differ from TNCB-induced CHSRs as they also induce IL-10–producing mast cells that are required to attenuate local toxicity.28,29 Moreover, DNFB-induced CHSRs induce NK cell–mediated memory responses.28,29 As TNCB-induced CHSRs thus share most of the mechanisms characterized for conventional DTHRs, we studied the role of TNFR1 in response to TNCB, using the TNCB-induced CHSR as a model for the DTHR that provides the unique possibility to individually investigate antigen presentation, priming, and differentiation of CD4 and CD8 T cells, cell migration, T cell–mast cell interactions, and mast cell–endothelia interactions with the help of mast cell knock-in experiments.20,27,30-32 Importantly, it allows the study of these effects largely independently of phenomena, such as antigen processing by APCs. Here, we show that TNFR1 signaling is neither needed for in vivo priming nor for the induction of Th1 cells capable of inducing severe DTHRs.
Dissecting the single steps of CHSRs from the APC, T cell, and mast cell function to the local vascular endothelia, we discerned that TNFR1 expression exclusively by endothelial cells (ECs) is necessary for the translation of T cell–mediated immune responses into local inflammation. TNFR1 expression by EC was critically needed for the expression of adhesion molecules required for leukocyte rolling, adhesion, and migration in response to locally produced mast cell TNF.
Methods
Animals
TNFR1−/− C57BL/6 mice were from Technische Universität Munich, and TNF+/+ C57BL/6 mice were from Charles River Laboratories,6 whereas TNF−/− 129/Svx C57BL/6 mice,33 and TNF+/+ 129/Svx C57BL/6 mice, mast cell–deficient KitW/KitW-v mice, and congenic WBB6F1+/+ (Kit+/Kit+) mice34,35 were bred under specific pathogen-free conditions at the German Research Center for Environmental Health in Munich. KitW/KitW-v mice were originally from The Jackson Laboratory. All mice were between 8 and 12 weeks of age. Animal experiments were approved by the Bavarian Ministry and the Regierungspräsidium Tübingen (HT 1/03).
In vivo experiments
We sensitized TNFR1−/− C57BL/6 mice, TNF−/− 129/Svx C57BL/6 mice with 5% TNCB (80 μL of a 4:1 mixture of acetone/olive oil), or 0.5% DNFB (80 μL of a 4:1 mixture of acetone/olive oil), with the exception of experiments involving KitW/KitW-v mice, where we used 2% TNCB (20 μL of a 4:1 mixture of acetone/olive oil) on both sides of one ear. This modified protocol is required as KitW/KitW-v mice do not tolerate abdominal sensitization using higher concentrations of TNCB. Thus, they were sensitized at the nonreconstituted ear. To establish equal conditions for all experiments, we always analyzed only the right ear. The negative control is always a nonsensitized mouse, challenged with the hapten at the right ear. Sensitization at the ear induced equivalent DTHR in KitW/KitW-v and wild-type mice.20 One week after sensitization, we challenged mice with 1% TNCB (20 μL; 1:9 in acetone/olive oil) or 0.2% DNFB (20 μL of a 4:1 mixture of acetone/olive oil) on both sides of the previously untreated ear. Specific ear swelling was determined by measuring ear thickness with a micrometer (Oditest; Kroepelin) at the indicated time points before and after TNCB challenge. An irritant reaction, caused by 1% TNCB in naive animals, was used as the control. KitW/KitW-v mice were locally reconstituted with mast cells (derived from wild-type and TNF−/− mice) by injecting intracutaneously 5 × 105 bone marrow-derived mast cells (BMMCs), 5 weeks before sensitization, exclusively into the ear selected for the elicitation of a DTHR. Similarly, TNFR1−/− and TNF−/− mice were locally reconstituted with BMMCs derived from syngeneic TNF+/+ mice by injecting intracutaneously 5 × 105 cultured BMMCs, 5 weeks before sensitization, exclusively into the ear selected for elicitation of a DTHR. For adoptive T-cell transfer experiments, 2 × 104 cultured Th1, Tc1, CD4+, and CD8+ T cells were locally injected exclusively into one ear of either naive TNFR1+/+ or TNFR1−/− C57BL/6 mice, 0.5 hours before TNCB challenge.
Histology
Hematoxylin and eosin staining was performed according to standard procedures.20
Immunofluorescence staining and confocal microscopy
Frozen sections were fixed with periodate-lysine-paraformaldehyde. Sections were blocked using donkey serum and then incubated with primary rat monoclonal antibodies (mAbs) kindly provided by Prof Vestweber (Departement of Vascular Cell Biology, Max Planck Institute for Molecular Biomedicine, Münster, Germany): anti–P-selectin RB40 mAb (dilution 1:40), anti–E-selectin 10E9 mAb (1:50), anti–ICAM-1 KAT-1 mAb (1:200), and anti–VCAM-1 6C7.1 mAb (1:50). For each primary antibody, double staining was performed using goat anti–Typ-IV-Collagen Ab (1:20; Biozol). Bound mAb was visualized using Cy3-donkey anti–rat Ab and Cy5-donkey anti–goat Ab (Dianova). For nuclear staining, we used Yopro (1:2000; Invitrogen). Sections were analyzed using a Leica TCS-SP/Leica DM RB confocal laser scanning microscope, a HCX PL APO lens at 63×/1.132-0.6 oil CS, and Mowiol medium (Höchst). Images were processed with Leica Confocal Software LCS (Version 2.61). Original magnification, ×630.
Real-time polymerase chain reaction
Mouse ears were directly frozen in liquid nitrogen without or 4 hours after TNCB challenge and homogenized in lysis buffer (RNeasy kit; QIAGEN) as well as mouse heart ECs and brain ECs. During RNA purification, genomic DNA was digested with RNase-free DNase (QIAGEN). A total of 2 μg total RNA was reverse transcribed (Omniscript RT-kit; QIAGEN). For relative quantification by reverse-transcribed polymerase chain reaction, 20 ng of each cDNA was analyzed in a LightCycler Real Time PCR System (Roche Diagnostics). For each primer pair, a standard curve was developed.36 Relative mRNA expression levels of E-selectin, P-selectin, ICAM-1, and VCAM-1 were normalized with the expression level of aldolase. The following primers were used: aldolase (amplicon length 571 bp): 5′-AGCTGTCTGACATCGCTCACCG; reverse primer, 5′-CACATACTGGCAGCGCTTCAAG; E-selectin (632 bp): 5′-GCTGTCCAGTGTGAAGCCTTATC; reverse primer, 5′-GCAATGAGGACGATGTCAGGA; P-selectin (300 bp): 5′-GCTTCAGGACAATGGACAGC; reverse primer, 5′-CTTTCTTAGCAGAGCCAGGAGTG; ICAM-1 (463 bp):5′-GGAGACGCAGAGGACCTTAACAG; reverse primer, 5′-CATCTCCTGTTTGACAGACTTCACC; VCAM-1 (320 bp): 5′-AGAGAAACCATTTATTGTTGACATCTCCC; reverse primer, 5′-CAAGTGGCCCA-CTCATTTTAATTACTGG; TNFR1 (197 bp): 5′-CAGTCTGCAGGGAGTGTGAA; reverse primer, 5′-CACGCACTGGAAGTGTGTCT.
Protein analysis
Myeloperoxidase (MPO) activity was determined in protein extracts from ear tissues and directly frozen in liquid nitrogen 24 hours after TNCB challenge. Tissue was homogenized in extraction buffer. MPO activity was expressed as units per gram.20
Cell cultures
For adoptive transfer experiments, T cells were isolated from lymph nodes derived from either TNCB-sensitized or naive TNFR1+/+ or TNFR1−/− mice. CD4+ and CD8+ T cells were purified by negative selection. Lymph node cells were preincubated with anti-CD437 or anti-CD8 mAbs38 and purified using a mouse T-cell purification column (Mouse T; Cellect). CD4+ and CD8+ T cells were cultured for 12 to 14 days with anti-IL4 mAb39 (10 μg/mL), 5 U/mL (after day 3 with 50 U/mL) IL-2, and hapten-modified T cell–depleted splenocytes (5 × 105; APCs) in a total volume of 200 μL medium in 96-well plates in an incubator (37°C, 7.5% CO2). After 3 days, T cells were cultured in 24-well plates and after another 5 days in 100-mL cell-culture bottles; IL-2 (50 U/mL) was added every 3 days. Femoral TNF+/+ and TNF−/− bone marrow cells were cultured in the presence of 10 to 20 U/mL IL-340 and 200 ng/mL c-kit ligand.41 After 4 weeks of culture, BMMCs were determined to be more than 97% pure for mast cells and were used for adoptive transfer.42
Proliferation and cytokine assays
For cytokine assays, T cells (105) were cultured with either irradiated unmodified or hapten-modified APCs (5 × 105) in 96-well plates in a total volume of 200 μL medium. Supernatants were harvested for cytokine analysis after 48 hours. Interferon-γ (IFN-γ) was assayed by enzyme-linked immunosorbent assay (BD Biosciences PharMingen). To analyze T-cell proliferation, T cells isolated from draining lymph nodes derived from either TNFR1+/+ or TNFR1−/− mice (1.25 × 105 or 2.5 × 105 T cells) were stimulated with either irradiated unmodified or hapten-modified APC (5 × 105) in a total volume of 200 μL medium. After 72 hours of culture in Dulbecco modified Eagle medium containing 10% fetal calf serum (PAA), 2-mercaptoethanol (Sigma-Aldrich), and 2 mM glutamine (Invitrogen) at 37°C in a humidified atmosphere with 5% CO2 cells were pulsed with [3H] thymidine for the final 6 to 8 hours. For ELISpot analysis, we coated 96-well plates (Multiscreen-Millipore) with rat anti–mouse-IFN-γ mAb (4 μg/mL). T cells (5.0 and 2.5 × 105 well−1) and unmodified or hapten-modified irradiated APCs (1 × 105 well−1) were cultured with 5 U/mL IL-2 for 48 hours. Cytokine-producing T cells were stained with secondary mAb, and the number of dots analyzed with an ELISpot reader (Biosys). As positive controls, cells stimulated with 10 mg/mL concanavalin A were used.
In vivo neutralization of adhesion molecules
Rat RB40.34 mAb43 (anti–P-selectin; BD Biosciences PharMingen; 50 μg), 40 μg rat YN1/1.7.4 mAb44 (anti–ICAM-1; Biozol), and 100 μg rat M/K-2 mAb44 (anti–VCAM-1; Chemicon) or 100 μg rat IgG1 isotype control (BD Biosciences PharMingen), and 40 μg rat IgG2b isotype control (Biozol) were injected intravenously 1.5 to 2.0 hours after ear challenge. Rat UZ445 mAb (anti–E-Selectin; kindly provided by R.H.; 200 μg) or 100 μg rat IgM isotype control (BD Biosciences PharMingen) were injected intraperitoneally 1.0 to 1.5 hours after ear challenge.
Noninvasive intravital fluorescence microscopy
Leukocyte rolling and adhesion dynamics during DTHR were monitored in vivo by video fluorescence microscopy. First, mice were anesthetized with ether (Sigma-Aldrich) to cannulate the tail vein to administer rhodamine. Mice were anesthetized by inhalation of isoflurane-O2 (1.8%, Forene; Abbott) and fixed on a heating pad to maintain body temperature between 36°C and 37°C. The hapten-treated mouse ear was used to study the behavior of leukocytes in the microcirculation. Ears were fixed with 2 peripheral sutures (7-0, Prolene; Ethicon) on a black box. Leukocytes were stained in vivo by intravenous injection of 100 μL 0.02% rhodamine-6G (Invitrogen). Subsequently, skin venules were visualized 2.5 to 4.5 hours after TNCB challenge using a Zeiss Axiotech microscope (water immersion objective: 20×, W 20×/0.5; Carl Zeiss) with a 100-W HBO mercury lamp for epi-illumination. All images were videotaped and evaluated off-line, and single unbranched skin venules (15-60 μm diameter) were selected. The number of rolling and adherent leukocytes was determined offline during video playback analysis. Rolling leukocytes were defined as cells crossing an imaginary perpendicular line through the vessel at a velocity significantly lower than the centerline velocity. The number of adherent leukocytes was assessed by counting cells that did not move or detach from the endothelial surface within 20 seconds. Cells were quantified as the number of cells mm−2, calculated from the diameter and the length of the vessel segment observed, assuming cylindrical geometry.46
Statistical analysis
Differences in ear swelling responses, MPO concentration, and relative mRNA expression were compared using a 2-sample Student t test. Differences in leukocyte rolling and adhesion were analyzed by the Wilcoxon test. Data are represented by mean plus or minus SEM, and P values less than .05 were regarded as significant.
Results
A critical role for the TNFR1 in hapten-induced tissue damage
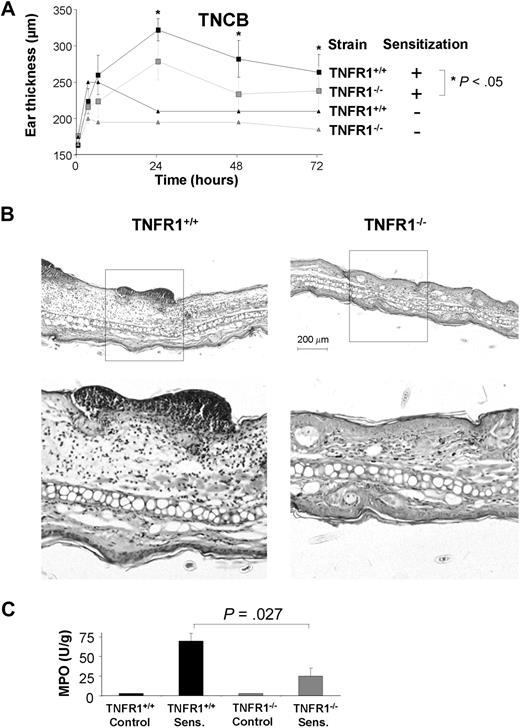
To address the role of TNFR1 signaling in DTHRs that are independent of infectious agents or antigen processing, we sensitized TNFR1−/− and TNFR1+/+ mice with DNFB and challenged mice 7 days later with the hapten to elicit a DNFB-specific DTHR. Hapten-specific DTHRs are dependent on IFN-γ–producing T cells.20,22,47 In agreement with previous studies,47 we found that TNFR1−/− mice had rather increased ear swelling in response to DNFB compared with TNFR1+/+ mice (Figure 1A). This finding is surprising in view of the overwhelming amount of data showing that TNFR1 is critical for the promotion of proinflammatory effects in DTHR in diseases leading to EAE,10 autoimmune diabetes,11 or arthritis.12,13 Therefore, we compared this DTHR in response to DNFB to the more widely used DTHR in response to TNCB, a hapten that also causes CHSRs that are dependent either on polarized Th1 cells or polarized Tc1 cells.20,48,49 In sharp contrast to DNFB-induced DTHR, TNCB-specific DTHRs were significantly diminished in TNFR1−/− mice compared with TNFR1+/+ mice (Figure 1B). Both haptens, TNCB or DNFB, induced T cell–dependent CHSRs that are dependent on IFN-γ–producing CD4+ Th1 and CD8+ Tc1 cells.29 To analyze why DNFB induced increased ear swelling whereas TNCB induced diminished ear swelling in TNFR1−/− mice, we determined tissue damage in TNFR1−/− and TNFR1+/+ mice by histopathology. In TNFR1+/+ mice, topical application of 0.5% DNFB to the skin induced severe necrosis, expanding into the mid-dermis (Figure 1C top right). In sharp contrast, little or no tissue necrosis occurred in TNFR1−/− mice exposed to 0.5% DNFB (Figure 1C bottom right). As a consequence, increased ear swelling in TNFR1−/− mice after DNFB application resulted from the reduced tissue damage that allowed the development of edema. This is in line with data showing that TNFR1 promotes cell death in mouse keratinocytes.50 In contrast to 0.5% DNFB, 5.0% TNCB induced very little cell death, but it did induce significant edema, a concomitant increase in dermal and epidermal thickness, and a strong inflammatory infiltrate (Figure 1C top left). In TNFR1−/− mice, all 3 TNCB-induced parameters of tissue damage (edema, infiltrate, and keratinocyte apoptosis) were significantly reduced (Figure 1C bottom left).
DNFB induces strong tissue necrosis in TNFR1+/+ mice. (A) DNFB-sensitized and -challenged TNFR1+/+ mice developed reduced ear swelling responses 24 hours after ear challenge compared with TNFR1−/− mice (n = 5-7). (B) TNCB-sensitized and -challenged TNFR1−/− mice developed reduced ear swelling responses 24 hours after ear challenge compared with TNFR1+/+ mice (n = 16-27). (C) Enhanced tissue necrosis in TNFR1+/+ mice after DNFB application. Hematoxylin and eosin–stained abdominal skin sections from TNCB-treated (top left) or DNFB-treated (top right) TNFR1+/+ and from TNCB-treated (bottom left) or DNFB-treated (bottom right) TNFR1−/− mice at day 5 after hapten application on the abdomen (n = 3-5).
DNFB induces strong tissue necrosis in TNFR1+/+ mice. (A) DNFB-sensitized and -challenged TNFR1+/+ mice developed reduced ear swelling responses 24 hours after ear challenge compared with TNFR1−/− mice (n = 5-7). (B) TNCB-sensitized and -challenged TNFR1−/− mice developed reduced ear swelling responses 24 hours after ear challenge compared with TNFR1+/+ mice (n = 16-27). (C) Enhanced tissue necrosis in TNFR1+/+ mice after DNFB application. Hematoxylin and eosin–stained abdominal skin sections from TNCB-treated (top left) or DNFB-treated (top right) TNFR1+/+ and from TNCB-treated (bottom left) or DNFB-treated (bottom right) TNFR1−/− mice at day 5 after hapten application on the abdomen (n = 3-5).
Reduced TNCB-induced DTHR in TNFR1−/− mice
We next analyzed the TNCB-specific DTHR to investigate the mechanisms underlying the phenomenon of strongly reduced DTHR in TNFR1−/− mice. We first examined the dynamics of ear swelling in TNFR1−/− and TNFR1+/+ mice. TNFR1−/− mice developed significantly reduced TNCB-specific DTHR at 24, 48, and 72 hours compared with TNFR1+/+ littermates (Figure 2A). To uncover the central mode of action, we compared hematoxylin and eosin–stained sections of ear tissue from TNFR1−/− with those from TNFR1+/+ mice. In TNFR1+/+ mice, TNCB-induced DTHRs resulted in strong edema, keratinocyte apoptosis, and abundant polymorphonuclear leukocyte (PMN) infiltrates with dermal and epidermal microabscesses. In sharp contrast, PMN infiltrates, keratinocyte apoptosis, and edema were virtually absent from TNFR1−/− mice (Figure 2B). Quantification of MPO activity in ear tissues confirmed that, in TNFR1−/− mice, PMN recruitment was reduced to less than 35% of TNFR1+/+ litter mice (Figure 2C). In contrast to the significant reduction in PMN, numbers of CD3+ cells were similar in TNFR1−/− and TNFR1+/+ mice (supplemental Figure 1A, available on the Blood website; see the Supplemental Materials link at the top of the online article) and the number of CD11c+ dendritic cells increased in TNFR1−/− mice. TNFR1−/− dendritic cells are largely apoptosis resistant51 (supplemental Figure 1B).
Impaired TNCB-specific DTHRs in TNFR1−/− mice. (A) TNCB-sensitized and naive TNFR1−/− ( ) and TNFR1+/+ (■) mice were challenged with TNCB. Ear thickness was measured before and at the indicated time points after TNCB challenge. Differences in ear thickness between TNFR1−/− (
) and TNFR1+/+ (■) mice were challenged with TNCB. Ear thickness was measured before and at the indicated time points after TNCB challenge. Differences in ear thickness between TNFR1−/− ( ) and TNFR1+/+ (■) were significant (P < .05) 24, 48, and 72 hours after ear challenge (24 hours: n = 16-27; 48 hours and 72 hours: n = 6 or 7). (B) Reduced PMN infiltrates, tissue necrosis, and edema in ear tissue from TNFR1−/− mice 24 hours after TNCB challenge. Hematoxylin and eosin–stained ear sections from TNFR1+/+ (left: top represents overview; bottom represents detail) and TNFR1−/− mice (right: top represents overview; bottom represents detail; n = 13-15). (C) PMN recruitment is TNFR1-dependent. MPO activity in protein extracts from ear tissue from TNFR1−/− and TNFR1+/+ mice (n = 3).
) and TNFR1+/+ (■) were significant (P < .05) 24, 48, and 72 hours after ear challenge (24 hours: n = 16-27; 48 hours and 72 hours: n = 6 or 7). (B) Reduced PMN infiltrates, tissue necrosis, and edema in ear tissue from TNFR1−/− mice 24 hours after TNCB challenge. Hematoxylin and eosin–stained ear sections from TNFR1+/+ (left: top represents overview; bottom represents detail) and TNFR1−/− mice (right: top represents overview; bottom represents detail; n = 13-15). (C) PMN recruitment is TNFR1-dependent. MPO activity in protein extracts from ear tissue from TNFR1−/− and TNFR1+/+ mice (n = 3).
Impaired TNCB-specific DTHRs in TNFR1−/− mice. (A) TNCB-sensitized and naive TNFR1−/− ( ) and TNFR1+/+ (■) mice were challenged with TNCB. Ear thickness was measured before and at the indicated time points after TNCB challenge. Differences in ear thickness between TNFR1−/− (
) and TNFR1+/+ (■) mice were challenged with TNCB. Ear thickness was measured before and at the indicated time points after TNCB challenge. Differences in ear thickness between TNFR1−/− ( ) and TNFR1+/+ (■) were significant (P < .05) 24, 48, and 72 hours after ear challenge (24 hours: n = 16-27; 48 hours and 72 hours: n = 6 or 7). (B) Reduced PMN infiltrates, tissue necrosis, and edema in ear tissue from TNFR1−/− mice 24 hours after TNCB challenge. Hematoxylin and eosin–stained ear sections from TNFR1+/+ (left: top represents overview; bottom represents detail) and TNFR1−/− mice (right: top represents overview; bottom represents detail; n = 13-15). (C) PMN recruitment is TNFR1-dependent. MPO activity in protein extracts from ear tissue from TNFR1−/− and TNFR1+/+ mice (n = 3).
) and TNFR1+/+ (■) were significant (P < .05) 24, 48, and 72 hours after ear challenge (24 hours: n = 16-27; 48 hours and 72 hours: n = 6 or 7). (B) Reduced PMN infiltrates, tissue necrosis, and edema in ear tissue from TNFR1−/− mice 24 hours after TNCB challenge. Hematoxylin and eosin–stained ear sections from TNFR1+/+ (left: top represents overview; bottom represents detail) and TNFR1−/− mice (right: top represents overview; bottom represents detail; n = 13-15). (C) PMN recruitment is TNFR1-dependent. MPO activity in protein extracts from ear tissue from TNFR1−/− and TNFR1+/+ mice (n = 3).
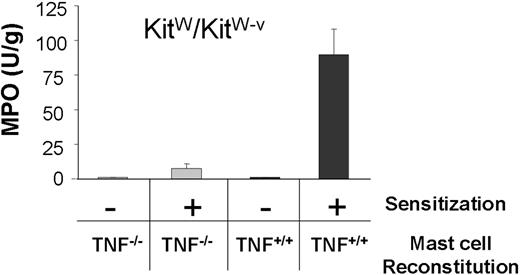
TNF+/+ mast cells reconstitute DTHR in TNF−/− mice, but not in TNFR1−/− mice
Mast cells secrete both TNF and the chemoattractant macrophage inflammatory protein 2. Both are critically needed for PMN recruitment and DTHR development.20,52 Neither T cells, nor macrophages, nor keratinocytes are capable of substituting for mast cells during this T cell–mediated DTHR in mast cell–deficient KitW/KitW-v mice.20,27 To formally prove the need for mast cell TNF for PMN recruitment, we first substituted mast cell–deficient KitW/KitW-v mice with either TNF+/+ or TNF−/− mast cells and quantified PMN recruitment during DTHR by determining MPO activity in ear tissues. As such, TNF+/+ and TNF−/− mast cells repopulate the ear tissue of mice with similar density.20 In line with previous data, KitW/KitW-v mice reconstituted with TNF+/+ mast cells developed strong DTHR and recruited large numbers of PMN, whereas mice substituted with TNF−/− mast cells failed to develop DTHR.20 As a consequence, MPO activity remained at background levels in mice reconstituted with TNF−/− mast cells (Figure 3).
Only TNF+/+ mast cells reconstitute PMN recruitment in KitW/KitW-v ears. MPO activity in protein extracts of ear tissue from KitW/KitW-v mice reconstituted with either TNF−/− ( ) or TNF+/+ (■) mast cells. Ear tissue was harvested 24 hours after TNCB challenge from sensitized and naive KitW/KitW-v mice (n = 2).
) or TNF+/+ (■) mast cells. Ear tissue was harvested 24 hours after TNCB challenge from sensitized and naive KitW/KitW-v mice (n = 2).
Only TNF+/+ mast cells reconstitute PMN recruitment in KitW/KitW-v ears. MPO activity in protein extracts of ear tissue from KitW/KitW-v mice reconstituted with either TNF−/− ( ) or TNF+/+ (■) mast cells. Ear tissue was harvested 24 hours after TNCB challenge from sensitized and naive KitW/KitW-v mice (n = 2).
) or TNF+/+ (■) mast cells. Ear tissue was harvested 24 hours after TNCB challenge from sensitized and naive KitW/KitW-v mice (n = 2).
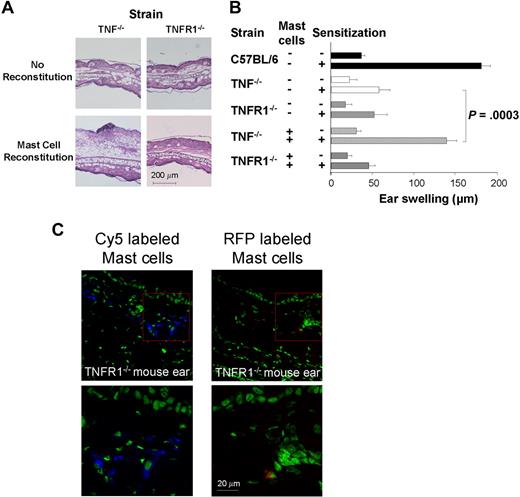
In TNFR1−/− mice, DTHRs were reduced to the same extent as in mast cell–deficient KitW/KitW-v or TNF−/− mice (Figure 4A-B). This finding is most easily understood in a model where TNFR1 expression is needed downstream of the T cell–mast cell cascade and is responsible for the induction of adhesion molecules that are required for PMN recruitment.
TNF+/+ mast cells reconstitute DTHRs in TNF−/− ears but not in TNFR1−/− ears. (A) Representative visualization of PMN infiltrates, tissue necrosis, and edema. Ear sections from TNF−/− (top left), TNFR1−/− (top right), mast cell-reconstituted TNF−/− mice (bottom left), and TNFR1−/− mice (bottom right) were fixed 24 hours after ear challenge and then stained with hematoxylin and eosin. (B) Hapten-specific DTHRs in C57BL/6 wild-type, TNF−/−, TNFR1−/−, mast cell–reconstituted TNF−/−, or mast cell–reconstituted TNFR1−/− mice. Ear swelling was measured 24 hours after TNCB challenge (n = 4-6). (C) Confocal microscopy of intracutaneously engrafted Cy5 (left) and red fluorescent protein (right) stained mast cells in ears of TNFR1−/− mice 5 days after mast cell engraftment (top represents overview; bottom represents detail).
TNF+/+ mast cells reconstitute DTHRs in TNF−/− ears but not in TNFR1−/− ears. (A) Representative visualization of PMN infiltrates, tissue necrosis, and edema. Ear sections from TNF−/− (top left), TNFR1−/− (top right), mast cell-reconstituted TNF−/− mice (bottom left), and TNFR1−/− mice (bottom right) were fixed 24 hours after ear challenge and then stained with hematoxylin and eosin. (B) Hapten-specific DTHRs in C57BL/6 wild-type, TNF−/−, TNFR1−/−, mast cell–reconstituted TNF−/−, or mast cell–reconstituted TNFR1−/− mice. Ear swelling was measured 24 hours after TNCB challenge (n = 4-6). (C) Confocal microscopy of intracutaneously engrafted Cy5 (left) and red fluorescent protein (right) stained mast cells in ears of TNFR1−/− mice 5 days after mast cell engraftment (top represents overview; bottom represents detail).
To experimentally address this point, we reconstituted TNF−/− and TNFR1−/− mice with mast cells from normal C57Bl/6 mice. In the absence of mast cell reconstitution, neither TNF−/− nor TNFR1−/− mice developed significant DTHRs. After mast cell reconstitution, TNF−/− mice developed strong TNCB-specific DTHR, equivalent to DTHRs in wild-type mice. In sharp contrast, mast cell reconstitution did not restore DTHRs in TNFR1−/− mice (Figure 4A-B), even though 0.5 × 106 directly (intracutaneously) into the right ear of naive TNFR1−/− mice transplanted mast cells engrafted selectively to the site of injection (Figure 4C, supplemental Figure 2). Together, these data demonstrate that TNFR1 expression by resident cells is needed for the development of strong, T cell–mediated inflammation in response to mast cell TNF.
TNFR1 expression by endothelia is needed for appropriate expression of adhesion molecules
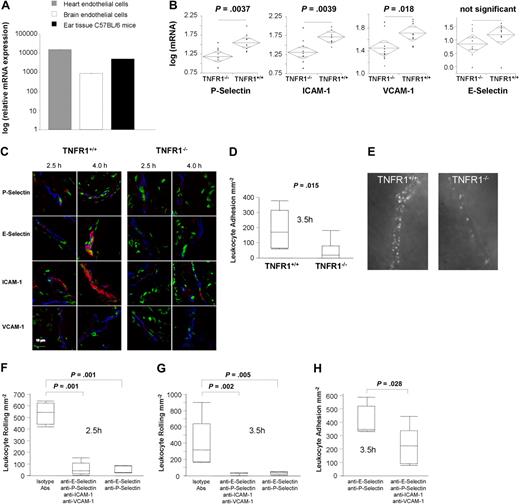
Endothelia of different origin express variable amounts of TNFR1 mRNA.53 TNFR1 mRNA is also expressed in normal ear tissue (Figure 5A). P-selectin and E-selectin are the first molecules expressed during DTHRs, followed by VCAM-1 and ICAM-1. The 2 selectins responsible for leukocyte rolling show peak expression after 2 to 4 hours,54-58 whereas the 2 major adhesion molecules responsible for leukocyte recruitment54,55,59 are strongly expressed starting at 3 to 4 hours of DTHR, with expression increasing over time.57,60 As TNF is capable of inducing all 4 adhesion molecules on ECs and as TNF is essential for PMN recruitment during TNCB-induced CHSR,20,61-63 TNFR1 expression by endothelia might provide the critical link that establishes crosstalk between somatic cells and the immune system in DTHRs.
Altered adhesion molecule expression and reduced leukocyte adhesion in TNFR1−/− mice. (A) mRNA expression of TNFR1 in mouse heart ECs, brain ECs, and mouse ear (data were normalized to aldolase expression; relative mRNA expression was calculated by dividing TNFR1 mRNA expression of TNFR1+/+ mice, mouse heart ECs, and brain ECs though the background TNFR1 mRNA in TNFR1−/− mice, corresponding to the detection limit, set as 1). (B) mRNA expression of P-selectin, E-selectin, ICAM-1, and VCAM-1 in TNFR1−/− mice and TNFR1+/+ mice, 4 hours after elicitation of DTHR (data were normalized to aldolase expression). Data are given in a decade log scale (n = 9 or 10). (C) Immunofluorescence staining of P-selectin, E-selectin, ICAM-1, and VCAM-1 (red) 2.5 hours and 4.0 hours after TNCB challenge in TNFR1+/+ and TNFR1−/− mice (green represents nuclei; blue represents type IV collagen; n = 3). (D) Leukocyte adhesion to vascular endothelia in TNFR1+/+ and TNFR1−/− mice 3.5 hours after elicitation of DTHR as determined by intravital fluorescent microscopy (n = 6 or 7). (E) Noninvasive intravital microscopy images of leukocyte adhesion to vascular endothelia in TNFR1+/+ and TNFR1−/− mice, 4.5 hours after elicitation of DTHR (supplemental Videos 1,2). (F) Leukocyte rolling and (G) firm adhesion of rhodamine-stained leukocytes to vascular endothelia in TNFR1+/+ mice after application of P-selectin, E-selectin, ICAM-1, and VCAM-1 blocking Abs or istotype control 2.5 and 3.5 hours after elicitation of DTHR. Analysis was performed by intravital fluorescent microscopy (n = 5-7).
Altered adhesion molecule expression and reduced leukocyte adhesion in TNFR1−/− mice. (A) mRNA expression of TNFR1 in mouse heart ECs, brain ECs, and mouse ear (data were normalized to aldolase expression; relative mRNA expression was calculated by dividing TNFR1 mRNA expression of TNFR1+/+ mice, mouse heart ECs, and brain ECs though the background TNFR1 mRNA in TNFR1−/− mice, corresponding to the detection limit, set as 1). (B) mRNA expression of P-selectin, E-selectin, ICAM-1, and VCAM-1 in TNFR1−/− mice and TNFR1+/+ mice, 4 hours after elicitation of DTHR (data were normalized to aldolase expression). Data are given in a decade log scale (n = 9 or 10). (C) Immunofluorescence staining of P-selectin, E-selectin, ICAM-1, and VCAM-1 (red) 2.5 hours and 4.0 hours after TNCB challenge in TNFR1+/+ and TNFR1−/− mice (green represents nuclei; blue represents type IV collagen; n = 3). (D) Leukocyte adhesion to vascular endothelia in TNFR1+/+ and TNFR1−/− mice 3.5 hours after elicitation of DTHR as determined by intravital fluorescent microscopy (n = 6 or 7). (E) Noninvasive intravital microscopy images of leukocyte adhesion to vascular endothelia in TNFR1+/+ and TNFR1−/− mice, 4.5 hours after elicitation of DTHR (supplemental Videos 1,2). (F) Leukocyte rolling and (G) firm adhesion of rhodamine-stained leukocytes to vascular endothelia in TNFR1+/+ mice after application of P-selectin, E-selectin, ICAM-1, and VCAM-1 blocking Abs or istotype control 2.5 and 3.5 hours after elicitation of DTHR. Analysis was performed by intravital fluorescent microscopy (n = 5-7).
To directly address this point, we first analyzed the mRNA expression of P-selectin, E-selectin, VCAM-1, and ICAM-1 in TNFR1−/−, TNFR1+/+, and TNF−/− (data not shown) mice. TNCB-induced DTHRs in TNFR1−/− mice resulted in significantly lower mRNA expression of P-selectin (P = .004), ICAM-1 (P = .004), or VCAM-1 (P = .018; Figure 5B). E-selectin showed a clear tendency toward reduced mRNA expression at 4 hours (Figure 5B) after elicitation of DTHR. Importantly, mRNA expression of P-selectin, E-selectin, VCAM-1, and ICAM-1 was reduced to the same degree in TNF−/− mice as it was in TNFR1−/− mice, directly supporting the central role of TNF and TNFR1 in the expression of these adhesion molecules (4 hours after challenge, data not shown). Equivalent findings for TNF−/− mice were reported by Nakae et al.64 Immunofluorescence staining ofP-selectin, E-selectin, ICAM-1, and VCAM-1 expression 2.5 hours and 4.0 hours after TNCB-ear challenge in TNFR1+/+ and TNFR1−/− mice (Figure 5C) and Western blot analysis (supplemental Figure 3) confirmed strongly reduced expression of all 4 adhesion molecules in TNFR1−/− mice (Figure 5C). Differences in protein expression were highest for P-selectin, E-selectin, and ICAM-1 (Figure 5C). Immunohistology underlined that only ECs express E-selectin; moreover, ECs and platelets are the only cells capable of producing P-selectin.58,62 Thus, our data can only be explained by a direct response of TNFR1-expressing ECs to mast cell–derived TNF.
Impaired leukocyte adhesion to TNFR1−/− endothelia in vivo
To formally prove that TNFR1-expressing ECs are needed to induce functionally active P-selectin, E-selectin, ICAM-1, or VCAM-1 protein during DTHR, we analyzed leukocyte rolling and leukocyte adhesion in TNFR1−/− mice and TNFR1+/+ mice using intravital fluorescence microscopy.46 At 2.5 hours, median P- and E-selectin–dependent leukocyte rolling was determined to be at 475 plus or minus 112 (± SEM) cells mm−1min−1 in TNFR1+/+ mice, but only 210 plus or minus 91 cells/mm−1min−1 in TNFR1−/− mice, which was close to background (114 ± 65 cells mm−1min−1). At 3.5 hours, this difference in rolling was lost (TNFR1+/+ mice: 384 ± 73 cells mm−1min−1; TNFR1−/− mice: 417 ± 92 cells mm−1min−1). Although rolling differed only initially, TNFR1−/− mice had a persistent defect in firm leukocyte adhesion. Firm leukocyte adhesion is a prerequisite for extravasation of PMN and T cells into the skin. Firm adhesion requires ICAM-1 and VCAM-1 interactions with lymphocyte function-associated antigen-1 (α1β2 integrin) and very late antigen-4 (α4β1 integrin).60,65,66 At 3.5 hours and 4.5 hours, when leukocytes had already infiltrated the connective tissue in TNFR1+/+ mice, adherent leukocytes were still strongly reduced to levels less than or equal to 12% in TNFR1−/− mice (P = .015; Figure 5D-E). Differential blood counts in TNFR1−/− mice and TNFR1+/+ mice confirmed a similar number of lymphocytes and PMNs in both strains (data not shown).
To definitely address the role of TNFR1-dependent expression of the adhesion molecules in TNCB-induced DTHR, we injected E-selectin, P-selectin, ICAM-1, and VCAM-1 blocking mAbs or corresponding isotype controls into TNFR1+/+ mice, 0.5 to 1.5 hours after challenge. Intravital fluorescence microscopy at 2.5 and 3.5 hours after the challenge demonstrated a nearly complete inhibition of leukocyte rolling when all 4 adhesion molecules were blocked; similar results were obtained by blocking P- and E-selectin only (Figure 5F). In contrast, P- and E-selectin mAbs had no effect on late adhesion. Only the combination of all 4 mAbs severely inhibited firm adhesion at 3.5 hours (Figure 5G), the difference was even significant compared with mice injected with P- and E-selectin mAbs only (Figure 5G).
Neither adequate T-cell priming nor induction of T-cell effector functions need TNFR1 expression
Others have suggested that TNFR1−/− mice develop deficient DTHR because of impaired T-cell priming resulting from defects in the functioning of T cells and dendritic cells.17,67 Therefore, we first determined the impact of TNFR1 expression on T-cell priming and Th1 cell differentiation. We analyzed freshly isolated CD4+ T cells from draining lymph nodes of TNCB-sensitized mice from both TNFR1+/+ and TNFR1−/− mice and stimulated the cells for T-cell proliferation and cytokine production using the Enzyme Linked Immuno Spot (ELISpot) technique.68 TNCB-specific CD4+ T cells from either TNFR1−/− mice or TNFR1+/+ mice proliferated equivalently in response to TNCB-modified APCs (Figure 6A), and both groups of mice had equal numbers of IFN-γ–producing TNCB-specific CD4+ T cells in draining lymph nodes (Figure 6B). This shows that TNFR1 expression was not needed for effective in vivo priming of CD4+ T cells as determined by their capacity to proliferate or to produce IFN-γ in response to specific stimulation by APCs. Yet, the signaling required for the induction of DTHR in vivo might be impaired.
TNFR1 expression is not needed for T-cell priming in vivo. (A) Proliferation of 2.5 × 105 (gray and black bars) or 1.25 × 105 (□ and  ) CD4+ T cells from either naive TNFR1+/+ or TNFR1−/− mice, or primed TNFR1+/+ or TNFR1−/− mice were stimulated for 72 hours with 5 × 105 of either unmodified (
) CD4+ T cells from either naive TNFR1+/+ or TNFR1−/− mice, or primed TNFR1+/+ or TNFR1−/− mice were stimulated for 72 hours with 5 × 105 of either unmodified ( and □) or TNCB-modified (■ and
and □) or TNCB-modified (■ and  ) APCs; [3H] thymidine was added for the final 6 hours. (B) Frequency of IFN-γ–producing TNCB-specific CD4+ T cells, 5 × 105 (■), or 2.5 × 105 (
) APCs; [3H] thymidine was added for the final 6 hours. (B) Frequency of IFN-γ–producing TNCB-specific CD4+ T cells, 5 × 105 (■), or 2.5 × 105 ( ) T cells from either naive or sensitized TNFR1+/+ or TNFR1−/− mice were stimulated on anti–IFN-γ mAb-coated 96-well plates with 5 × 105 either unmodified APCs (
) T cells from either naive or sensitized TNFR1+/+ or TNFR1−/− mice were stimulated on anti–IFN-γ mAb-coated 96-well plates with 5 × 105 either unmodified APCs ( , third column) or TNBS-modified APCs (■ and
, third column) or TNBS-modified APCs (■ and  ). After 48 hours of incubation, ELISPOT assay was developed. Control T cells were stimulated with 10 μg/mL concanavalin A and APCs (□); 3 independent experiments.
). After 48 hours of incubation, ELISPOT assay was developed. Control T cells were stimulated with 10 μg/mL concanavalin A and APCs (□); 3 independent experiments.
TNFR1 expression is not needed for T-cell priming in vivo. (A) Proliferation of 2.5 × 105 (gray and black bars) or 1.25 × 105 (□ and  ) CD4+ T cells from either naive TNFR1+/+ or TNFR1−/− mice, or primed TNFR1+/+ or TNFR1−/− mice were stimulated for 72 hours with 5 × 105 of either unmodified (
) CD4+ T cells from either naive TNFR1+/+ or TNFR1−/− mice, or primed TNFR1+/+ or TNFR1−/− mice were stimulated for 72 hours with 5 × 105 of either unmodified ( and □) or TNCB-modified (■ and
and □) or TNCB-modified (■ and  ) APCs; [3H] thymidine was added for the final 6 hours. (B) Frequency of IFN-γ–producing TNCB-specific CD4+ T cells, 5 × 105 (■), or 2.5 × 105 (
) APCs; [3H] thymidine was added for the final 6 hours. (B) Frequency of IFN-γ–producing TNCB-specific CD4+ T cells, 5 × 105 (■), or 2.5 × 105 ( ) T cells from either naive or sensitized TNFR1+/+ or TNFR1−/− mice were stimulated on anti–IFN-γ mAb-coated 96-well plates with 5 × 105 either unmodified APCs (
) T cells from either naive or sensitized TNFR1+/+ or TNFR1−/− mice were stimulated on anti–IFN-γ mAb-coated 96-well plates with 5 × 105 either unmodified APCs ( , third column) or TNBS-modified APCs (■ and
, third column) or TNBS-modified APCs (■ and  ). After 48 hours of incubation, ELISPOT assay was developed. Control T cells were stimulated with 10 μg/mL concanavalin A and APCs (□); 3 independent experiments.
). After 48 hours of incubation, ELISPOT assay was developed. Control T cells were stimulated with 10 μg/mL concanavalin A and APCs (□); 3 independent experiments.
Adequate activation of resident cells, namely, mast cells by Th1 and Tc1 cells, is essential for the recruitment of inflammation amplifying cells from the circulation and for the manifestation of DTHRs. Thus, priming T cells for specific proliferation and IFN-γ production is a prerequisite for inducing DTHRs, but it is not sufficient. To test the role of TNFR1 during the in vivo imprinting of T cells for the induction of effector cells capable of inducing tissue inflammation, we isolated Th1 or Tc1 effector cells from sensitized TNFR1−/− or TNFR1+/+ mice, expanded these cells in vitro, and tested their capacity to elicit DTHR. To overcome the defective expression of adhesion molecules in TNFR1−/− mice, we adoptively transferred the T-cell populations directly into the ears of either TNFR1−/− or TNFR1+/+ mice. Before injection, TNCB-specific Th1 or Tc1 cells proliferated equivalently and produced similar amounts of IFN-γ in response to hapten-modified APCs, irrespective of whether they were derived from TNFR1+/+ or TNFR1−/− mice (Figure 7A-D). Thirty minutes after transfer of these Th1 or Tc1 cells into the ears of either naive TNFR1−/− or naive TNFR1+/+ mice, we challenged their ear skin with TNCB. After transfer, T cells from either TNFR1−/− or TNFR1+/+ mice caused significant DTHR in TNFR1+/+ mice. Th1 cells induced stronger DTHRs than Tc1 cells, independently of whether or not the T cells expressed TNFR1 (Figure 7E-F). Thus, TNFR1 expression was not required either for in vivo priming of T cells for Th1 development or for the differentiation of naive T cells into a functionally, fully efficient Th1 or Tc1 effector phenotype. In sharp contrast, when transferred into the ears of TNFR1−/− mice, none of the Th1 or Tc1 cell lines, whether derived from TNFR1−/− or TNFR1+/+ mice, elicited TNCB specific effector functions, further underlining that establishment of local inflammation needed TNFR1 expression exclusively by resident cells (Figure 7E-F).
TNFR1 expression is needed neither for T-cell imprinting nor for T-cell effector functions. (A-D) Proliferation and IFN-γ production by TNFR1+/+ or TNFR1−/− Th1 and Tc1 cells. (A-B) TNFR1+/+ (■) or TNFR1−/− ( ) TNCB-specific T cells (105) of the indicated origin were stimulated for 24 hours with 5 × 105 hapten-modified APCs. [3H] thymidine was added for the final 6 hours. (C-D) TNFR1+/+ (■) or TNFR1−/− (
) TNCB-specific T cells (105) of the indicated origin were stimulated for 24 hours with 5 × 105 hapten-modified APCs. [3H] thymidine was added for the final 6 hours. (C-D) TNFR1+/+ (■) or TNFR1−/− ( ) T cells (105) from the indicated origin were stimulated for 24 hours with 5.0 × 105 hapten-modified APCs. Supernatants were harvested after 24 hours, and the IFN-γ content was determined by enzyme-linked immunosorbent assay. (E-F) Efficient DTHRs require TNFR1-expressing resident cells. Th1 or Tc1 cell lines that were either TNFR1−/− or TNFR1+/+ were transferred intracutaneously into ears of naive TNFR1−/− or TNFR1+/+ mice, 0.5 hours before challenge with TNCB (n = 3-7). Ear swelling was determined 24 hours later.
) T cells (105) from the indicated origin were stimulated for 24 hours with 5.0 × 105 hapten-modified APCs. Supernatants were harvested after 24 hours, and the IFN-γ content was determined by enzyme-linked immunosorbent assay. (E-F) Efficient DTHRs require TNFR1-expressing resident cells. Th1 or Tc1 cell lines that were either TNFR1−/− or TNFR1+/+ were transferred intracutaneously into ears of naive TNFR1−/− or TNFR1+/+ mice, 0.5 hours before challenge with TNCB (n = 3-7). Ear swelling was determined 24 hours later.
TNFR1 expression is needed neither for T-cell imprinting nor for T-cell effector functions. (A-D) Proliferation and IFN-γ production by TNFR1+/+ or TNFR1−/− Th1 and Tc1 cells. (A-B) TNFR1+/+ (■) or TNFR1−/− ( ) TNCB-specific T cells (105) of the indicated origin were stimulated for 24 hours with 5 × 105 hapten-modified APCs. [3H] thymidine was added for the final 6 hours. (C-D) TNFR1+/+ (■) or TNFR1−/− (
) TNCB-specific T cells (105) of the indicated origin were stimulated for 24 hours with 5 × 105 hapten-modified APCs. [3H] thymidine was added for the final 6 hours. (C-D) TNFR1+/+ (■) or TNFR1−/− ( ) T cells (105) from the indicated origin were stimulated for 24 hours with 5.0 × 105 hapten-modified APCs. Supernatants were harvested after 24 hours, and the IFN-γ content was determined by enzyme-linked immunosorbent assay. (E-F) Efficient DTHRs require TNFR1-expressing resident cells. Th1 or Tc1 cell lines that were either TNFR1−/− or TNFR1+/+ were transferred intracutaneously into ears of naive TNFR1−/− or TNFR1+/+ mice, 0.5 hours before challenge with TNCB (n = 3-7). Ear swelling was determined 24 hours later.
) T cells (105) from the indicated origin were stimulated for 24 hours with 5.0 × 105 hapten-modified APCs. Supernatants were harvested after 24 hours, and the IFN-γ content was determined by enzyme-linked immunosorbent assay. (E-F) Efficient DTHRs require TNFR1-expressing resident cells. Th1 or Tc1 cell lines that were either TNFR1−/− or TNFR1+/+ were transferred intracutaneously into ears of naive TNFR1−/− or TNFR1+/+ mice, 0.5 hours before challenge with TNCB (n = 3-7). Ear swelling was determined 24 hours later.
Discussion
Using knockin experiments with selective reconstitution of either distinct T-cell populations (Th1, Tc1) or distinct mast cell populations into control, TNFR1−/−, TNF−/−, or KitW/KitW-v mice, we here found that differentiation of IFN-γ–producing Th1 or Tc1 cells was normal in TNFR1−/− mice. Th1 or Tc1 cells from TNFR1−/− mice were also fully capable of inducing DTHRs. Yet, TNFR1−/− mice had a profound defect in developing Th1- or Tc1-mediated DTHRs, as reported for other disease models of T cell–dependent DTHRs.6,10-13 Dissecting the single steps of DTHRs starting with in vivo T-cell priming through T-cell differentiation and T-cell effector functions, we localized the defect downstream of the T cell–mast cell interaction. Importantly, TNF-producing mast cells were unable to translate T-cell responses into DTHRs in TNFR1−/− mice exclusively. This defect was associated with defective PMN recruitment and reduced expression of the 4 critical adhesion molecules at the site of inflammation. In contrast to the other adhesion molecules analyzed, P-selectin and E-selectin are highly specific for endothelia.58 We propose that P-selectin and E-selectin expression resulted from ECs, as the only other cells capable of expressing P-selectin mRNA or protein are platelets.58 This was confirmed by immunohistology. To directly determine whether TNFR1−/− endothelia were capable of inducing functional adhesion molecule expression, we performed intravital fluorescence microscopy. Knockout experiments have shown that only the combined loss of E-selectin and P-selectin impairs hapten-induced T cell–mediated skin inflammation.69 In line with this, we found strongly impaired leukocyte rolling and adhesion in TNFR1−/− mice, directly underlining that E-selectin and P-selectin were not only reduced at the mRNA level but were also functionally ineffective. Thus, direct crosstalk between TNF produced by mast cells in response to locally activated Th1 cells and TNFR1, expressed by endothelia, ultimately controlled PMN recruitment into the site of inflammation.
These findings help explain various biologically important questions that arose during the characterization of TNFR1−/− mice and the introduction of anti-TNF mAb or soluble TNF receptors into therapy for autoimmune disease in humans.
Elimination of lymphocytic choriomeningitis virus-infected cells, which strictly depends on the functioning of CD8 T cells, is completely unaffected in TNFR1−/− mice.18 Although others have suggested that T-cell priming would be impaired in TNFR1−/− mice, the analysis of CD8 T cells in lymphocytic choriomeningitis virus infection and the analysis of either CD4 or CD8 T cells in adoptive T-cell transfer clearly show that T-cell priming and T-cell effector function develop normally in TNFR1−/− mice. Although TNFR1 signaling does not seem to be needed for these direct T cell–mediated immune functions, TNFR1 signaling is obviously crucial for the development of DTHR effector functions where, in addition to T cells, macrophages or PMNs are involved. Thus, TNFR1−/− mice infected with intracellular bacteria, such as Listeria monocytogenes6 or Mycobacterium tuberculosis,7 are highly susceptible and cannot cope with these infections. Similarly, the acute phases of collagen-induced arthritis12,13 or EAE10,70 are attenuated in TNFR1−/− mice. Importantly, comparing disease development in TNFR1−/− and TNFR2−/− mice demonstrates that efficient DTHRs in infectious and autoimmune diseases depend on TNFR1, as TNFR2-deficient mice show a normal phenotype in these models of human disease.6,7,9-13
Here, we have shown that T cell–mediated inflammation strictly requires a direct crosstalk between mast cell-TNF and TNFR1-expressing endothelia, as TNFR1 signaling by ECs is essential for PMN recruitment into sites of inflammation. This seems to be of great relevance for understanding the mode of action of anti-TNF therapy in humans. Blocking TNF action in humans with either anti-TNF mAbs or TNFR fusion proteins is highly effective in diseases that critically involve PMN recruitment. In consequence, blocking TNF aggravates the course of sepsis71 but improves PMN-associated autoinflammatory diseases, such as rheumatoid arthritis,2,72-74 psoriasis, psoriasis arthritis,2,74-77 or inflammatory bowel disease.2,78 In sharp contrast, no consistent effects have been reported for PMN-independent inflammation, such as chronic phases of multiple sclerosis.2,74 This central role of TNF in PMN recruitment is underlined by reports showing that concomitant infections with bacteria or mycobacteria may be severely aggravated and difficult to treat in patients treated with TNF-blocking agents,2,72-78 whereas defense against viral diseases seems to be largely unaffected.2,72-78
The critical role for TNF-mediated PMN recruitment in host protection against bacteria was originally unexpected and first observed in humans that received TNF blockade as a treatment for sepsis.71 In this study, TNFR fusion protein was given to reduce fever and TNF-induced symptoms of septic shock in the hope of reducing morbidity and lethality. TNFR fusion protein, however, significantly increased the lethality of septic shock.71 The critical role of TNF-mediated PMN recruitment for the control of sepsis was also shown in mice with either bacterial pneumonitis or septic peritonitis. In the absence of TNF, the mice failed to recruit PMN, failed to control expansion of the bacteria, and succumbed to these bacterial infections.4,79 In sharp contrast, viral diseases seem to be well controlled in anti-TNF–treated humans.2,72-78 Together the data show that TNF induces many symptoms of sepsis, such as fever or hypotonia, but is essential for efficient control of bacterial expansion in patients.
Even though previous data have independently shown that TNF and TNFR1 signaling is essential for establishing DTHRs that involve PMNs and macrophages, the underlying biologic mechanism remained enigmatic. Most authors on the subject have speculated that TNFR1 signaling may be involved in either T-cell priming or macrophage functioning. Here, we show that the priming of effector T cells capable of inducing DTHR is TNFR1-independent and that the central TNFR1-expressing cell required for PMN extravasation and development of DTHR is the EC. Thus, TNFR1-expressing ECs establish the molecular crosstalk between the intravascular leukocytes and TNF-producing mast cells. As TNFR1-expressing ECs respond to mast cell-TNF by expressing adhesion molecules required for leukocyte rolling and firm adhesion, TNFR1 signaling is responsible for the critical step that initiates local inflammation in protective and harmful T cell–mediated immune responses. As such, the data provided here not only unravel the long-sought molecular basis for understanding the well-described defects of TNFR1−/− mice in developing efficient DTHRs, but they also propose a concept to explain the risks and opportunities for anti-TNF therapy in humans.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank U. Bamberg, N. Bauer, H. Bischof, C. Bodenstein, F. Cay, D. Dick, B. Fehrenbacher, O. Hallmaier, S. Harrasser, H. Hinze-Heyn, D. Jakob, H. Möller, R. Nordin, and U. Schmidt for technical support and Prof D. Vestweber (Departement of Vascular Cell Biology, Max Planck Institute for Molecular Biomedicine) for advice and help with immunohistology.
This work was supported by Deutsche Forschungsgemeinschaft (SFB 685 A6, B6, and C1 Bi 696/3-1, BI 696/5-1, Sch 897/3, and SFB 773 Z), Wilhelm Sander-Stiftung (97.041.3), Deutsche Krebshilfe (10-1917-M02) and the National Institutes of Health (grant RO3AIO59791).
This work is part of the doctoral thesis of M.K. and K.F.
National Institutes of Health
Authorship
Contribution: M.K., R.M., L.H., T.S., M.S., S.M., K.L.M., R. Hallmann, B.J.P., R. Haubner, M.G., K.P., T.B., and M.R. designed the study; M.K., R.M., L.H., T.S., K.F., D.B., C.A.S., and B.J.P performed the experiments; M.K., R.M., L.H., T.S., K.F., M.S., D.B., S.M., C.A.S., M.E., K.L.M., R. Hallmann, B.J.P., R. Haubner, M.G., K.P., T.B., H.B., and M.R. analyzed data; M.K., T.B., and M.R. wrote the manuscript; all authors approved the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Manfred Kneilling, Department of Dermatology, Eberhard Karls University Tübingen, Liebermeisterstr 25, 72076 Tübingen, Germany, e-mail: manfred.kneilling@med.uni-tuebingen.de; or Martin Röcken, Department of Dermatology, Eberhard Karls University Tübingen, Liebermeisterstr 25, 72076 Tübingen, Germany, e-mail: mrocken@med.uni-tuebingen.de.


![Figure 6. TNFR1 expression is not needed for T-cell priming in vivo. (A) Proliferation of 2.5 × 105 (gray and black bars) or 1.25 × 105 (□ and ) CD4+ T cells from either naive TNFR1+/+ or TNFR1−/− mice, or primed TNFR1+/+ or TNFR1−/− mice were stimulated for 72 hours with 5 × 105 of either unmodified ( and □) or TNCB-modified (■ and ) APCs; [3H] thymidine was added for the final 6 hours. (B) Frequency of IFN-γ–producing TNCB-specific CD4+ T cells, 5 × 105 (■), or 2.5 × 105 () T cells from either naive or sensitized TNFR1+/+ or TNFR1−/− mice were stimulated on anti–IFN-γ mAb-coated 96-well plates with 5 × 105 either unmodified APCs (, third column) or TNBS-modified APCs (■ and ). After 48 hours of incubation, ELISPOT assay was developed. Control T cells were stimulated with 10 μg/mL concanavalin A and APCs (□); 3 independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/114/8/10.1182_blood-2008-11-187682/4/m_zh89990940510006.jpeg?Expires=1767712607&Signature=mz7Ieos23i6yaGnwnp8YYEggr5sELsGvqv2ySQL48GKxbTqNSYxFQ5T7hgYdNh4dMsS5BkuKpnCnWeHcqS1qDNybsEmRzoWCJ21oJP7dPvARS5-wPv3I3n-Vg~xJPZnRVNj8kJxZyzQjqgmEA87kwd2ZwrU6asQVPlcDsQgTvI4-351On0Z8V4KSidoe1k17Sbu2Q85kbwBiYKLjp1Jgq0ZUVC8kDMCp2aeI47LS3v0UcR4kzeOVgHHTFo-jrxg4d~G3422hj46JYPS~2mn21p37lI0radmvdK5EF-MIvELXo2J19ctchATe5GH1~voltIjtvlbP3sE6S3hp6A6B3w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 7. TNFR1 expression is needed neither for T-cell imprinting nor for T-cell effector functions. (A-D) Proliferation and IFN-γ production by TNFR1+/+ or TNFR1−/− Th1 and Tc1 cells. (A-B) TNFR1+/+ (■) or TNFR1−/− () TNCB-specific T cells (105) of the indicated origin were stimulated for 24 hours with 5 × 105 hapten-modified APCs. [3H] thymidine was added for the final 6 hours. (C-D) TNFR1+/+ (■) or TNFR1−/− () T cells (105) from the indicated origin were stimulated for 24 hours with 5.0 × 105 hapten-modified APCs. Supernatants were harvested after 24 hours, and the IFN-γ content was determined by enzyme-linked immunosorbent assay. (E-F) Efficient DTHRs require TNFR1-expressing resident cells. Th1 or Tc1 cell lines that were either TNFR1−/− or TNFR1+/+ were transferred intracutaneously into ears of naive TNFR1−/− or TNFR1+/+ mice, 0.5 hours before challenge with TNCB (n = 3-7). Ear swelling was determined 24 hours later.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/114/8/10.1182_blood-2008-11-187682/4/m_zh89990940510007.jpeg?Expires=1767712607&Signature=EIY7H5tgzVYKV~~aYq8nLFKcLbwd8n-CsKgCvv1L6Tf7hMW7ZPJ9AbQ08sUzpCHyodcct0DK1xo0kGiluLysCPM5Uf7kCXkDK6M1KOP5pXVSzM1XoYf-WEx9gdhh7RWXSGLNP9j80Vmr5K40pKMhaSdzlavs6CrJ9Y7hf479SOG2~I~edghCnG6z7ihx2TsekMHPZnV6hWBnfoolciW0QymuYZBZ~GSGW4DOWNsHCUf1~QXrEQTsOgXYmV6G-AbZ5YIO3IDRv1TEEul2piCoSeyCjlJ7~BxqZ~F8IzyV41r-sFaFzv67oX8-AXZU~ChS09aEuHOCG10aqVo7PPajwA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal