Abstract
Tumor antigen–specific T cells are found within melanomas, yet tumors continue to grow. Although the tumor microenvironment is thought to influence the suppression of tumor-reactive T cells, the underlying mechanisms for this T-cell dysfunction are not clear. Here, we report that the majority of tumor infiltrating T lymphocytes (TIL), including MART-1/Melan-A melanoma antigen–specific CD8 T cells, predominantly expressed PD-1, in contrast to T cells in normal tissues and peripheral blood T lymphocytes (PBL). PD-1+ TIL expressed CTLA-4 and Ki-67, markers that were not expressed by PD-1− TIL and T cells in the normal tissues and PBL. Moreover, PD-1+ TIL were primarily HLA-DR+ and CD127−, in contrast to PD-1− TIL. Effector cytokine production by PD-1+ TIL was impaired compared with PD-1− TIL and PBL. Collectively, the phenotypic and functional characterizations of TIL revealed a significantly higher frequency and level of PD-1 expression on TIL compared with normal tissue T-cell infiltrates and PBL, and PD-1 expression correlated with an exhausted phenotype and impaired effector function. These findings suggest that the tumor microenvironment can lead to up-regulation of PD-1 on tumor-reactive T cells and contribute to impaired antitumor immune responses.
Introduction
The programmed death 1 receptor (PD-1, also known as CD279) is an immunoinhibitory receptor that is expressed by chronically stimulated CD4 and CD8 T cells after T-cell activation.1-3 PD-1 knockout mice display a variety of autoimmune diseases depending on the strains of mice,4,5 suggesting that this inhibitory receptor plays a critical role in lymphocyte homeostasis.6 Moreover, the high expression of PD-1 on virus-specific CD8 T cells has been reported to correlate with increased viremia in patients with chronic HIV7,8 and hepatitis C virus infections9 and in chronic infection in a murine model.10 These PD-1+ CD8 T cells had reduced capacity to proliferate and produce effector cytokines and thus the term “functional exhaustion” was attributed to T cells that expressed PD-1. Murine studies have demonstrated that blocking the interaction of PD-1 with its ligand reversed the course of infection by restoring effector function in the “exhausted” T cells.10 These preclinical studies indicated that PD-1 expression on virus-specific CD8 T cells could inhibit the effectiveness of an immune response that was required to control a viral infection, and thus they provided an incentive to conduct clinical trials of anti–PD-1 mAb in patients with chronic viral infections.6
Induction and expression of PD-1 on antigen-specific T cells is thought to regulate the immune responses to foreign as well as self-antigens.3,6 Many tumors continue to grow despite the presence of tumor antigen–specific T cells infiltrating into the tumor stroma. The underlying mechanism for this tumor-specific T-cell dysfunction is not well understood. It is postulated that the tumor microenvironment may contribute to a weakened antitumor immune response.11,12 One of the ligands of PD-1 is PDL-1 (also known as B7-H1) that is expressed by nonlymphoid tissues as well as by activated antigen-presenting cells (APC).13 The engagement of PD-1 with PDL-1 inhibits TCR-mediated proliferation and cytokine production.13 Interestingly, expression of PDL-1 is reported on a variety of human tumors, including melanomas.14 In addition to tumors, tumor-associated myeloid dendritic cells can also up-regulate PDL-1 and its blockade can enhance T-cell activation in vitro.15 Although PDL-1 expression on tumors correlates with unfavorable prognosis for many human tumors,6,16 little is known about the pattern expression of PD-1 and its phenotypic and functional characteristics on tumor infiltrating T lymphocytes (TIL).
To address this issue, we examined PD-1 expression on TIL in metastatic melanoma lesions and contrasted their phenotypic and functional profile with T cells in normal tissues and peripheral blood. We found that both the frequency and the level of expression of PD-1 were significantly higher on CD4 and CD8 TIL compared with T cells in normal tissues and peripheral blood in the same patients and healthy donors. The overwhelming majority of CD8 T cells specific for the tumor differentiation antigen MART-1/Melan-A (hereafter, MART-1) expressed high levels of PD-1 in tumors compared with MART-specific T cells in peripheral blood in the same patients. PD-1+ TIL also expressed CTLA-4 and HLA-DR, and lacked expression of CD127, a phenotypic characteristic of “exhausted” T cells. Furthermore, PD-1+ TIL displayed an impaired effector function compared with PD-1− TIL and peripheral blood T lymphocytes (PBL) in the same patients. Our findings suggest that the tumor microenvironment may play a role in the induction and maintenance of PD-1 expression on tumor-reactive T cells and that expression of PD-1 on TIL impairs the antitumor immune responses in humans.
Methods
Patients, PBMC, and normal and tumor samples
Tumor and normal tissue specimens and peripheral blood samples were collected from the same patients with metastatic melanoma. Peripheral blood mononuclear cells (PBMC) from patients with metastatic melanoma (n = 14) and healthy donors (n = 7) were obtained by either leukapheresis or venipuncture and were prepared over Ficoll-Hypaque (LSM; ICN Biomedicals Inc) gradient and cryopreserved until analyzed. Tumor specimens from melanoma patients (n = 28) were processed by sterile mechanical dissection followed by enzymatic digestion as previously described.17 Normal tissues (liver, lung, or spleens) were obtained from patients who had tumor metastasis in these tissues. The normal tissues were at least 5 centimeters away from the visible tumor mass. To process cells from tumor-free tissues (liver, lung, and spleen), specimens were mechanically dissected and digested overnight with 33% enzymatic cocktail. Because splenocytes from tumor-free spleens were easily released from the splenic capsule by the mechanical pressure, we used mincing followed with either mechanical pressure using a syringe plunger and/or digesting overnight with a 10% enzymatic cocktail. Single-cell suspensions were filtered and separated on a Ficoll-Hypaque gradient and cryopreserved until analyzed. In general, patients had a wide range of prior therapies that might have included 2 or more of the following treatments: surgery, chemotherapy, radiotherapy, and immunotherapy or none of the above. The patients' characteristics are provided in Table 1. Although a fraction of patients had received immunotherapy, patients who had received nonmyeloablated adoptive cell transfers before tumor resection were excluded, with the exception of 1 patient who had received this treatment 1 year earlier. All protocols were approved by the Institutional Review Board of the National Cancer Institute and patient informed consent was obtained in accordance with the Declaration of Helsinki.
Patient characteristics
| Variable/trait . | Total (%) . |
|---|---|
| Total no. of patients | 28 (100) |
| Sex | |
| Male | 16 (57) |
| Female | 12 (43) |
| Age | |
| 11-20 | 1 (4) |
| 31-40 | 5 (18) |
| 41-50 | 7 (25) |
| 51-60 | 12 (43) |
| 61-70 | 3 (11) |
| ECOG | |
| 0 | 17 (61) |
| 1 | 5 (18) |
| Not available | 6 (21) |
| Prior Rx | |
| Surgery | 22 (79) |
| Chemotherapy | 8 (29) |
| Radiotherapy | 6 (21) |
| Immunotherapy | 17 (61) |
| Any 2 or more | 18 (64) |
| Any 3 or more | 11 (39) |
| Not available | 6 (21) |
| Variable/trait . | Total (%) . |
|---|---|
| Total no. of patients | 28 (100) |
| Sex | |
| Male | 16 (57) |
| Female | 12 (43) |
| Age | |
| 11-20 | 1 (4) |
| 31-40 | 5 (18) |
| 41-50 | 7 (25) |
| 51-60 | 12 (43) |
| 61-70 | 3 (11) |
| ECOG | |
| 0 | 17 (61) |
| 1 | 5 (18) |
| Not available | 6 (21) |
| Prior Rx | |
| Surgery | 22 (79) |
| Chemotherapy | 8 (29) |
| Radiotherapy | 6 (21) |
| Immunotherapy | 17 (61) |
| Any 2 or more | 18 (64) |
| Any 3 or more | 11 (39) |
| Not available | 6 (21) |
Antibodies and reagents
The following monoclonal antibodies (mAbs) specific for human antigens were purchased from BD Biosciences: PerCP-conjugated anti-CD3 (SK7), FITC-conjugated anti-CD8 (SK1), and PE-conjugated anti-CD25 (2A3), anti-CD27 (M-T271), anti-CD127 (hIL-7R-M21), anti–IFN-γ (25 723.11), anti–IL-2 (MQ1-17H12), anti–Ki-67 (B56), and anti–CTLA-4 (BNI3). Anti–human PD-1 mAb (MIH4) and its isotype control Ab conjugated to FITC, PE, or APC were purchased from BD Biosciences. MART-1:26-35(27L)/HLA-A*0201 tetramers (Beckman Coulter) were used to measure surface levels of MART-1 TCR.
Intracellular cytokine induction
Cryopreserved tumor digests and PBMC were thawed into complete medium consisting of RPMI-1640 (Gibco) supplemented with 10% heat-inactivated human AB serum (Gemini Bio-products), 100 U/mL penicillin and 100 μg/mL streptomycin (Biofluids), 2 mM HEPES buffer (Biofluids), 2 mM l-glutamine (Biofluids), and 50 μM 2-mercaptoethanol (Gibco). The complete medium was supplemented with 1% (10 μg/mL) DNase (Dornase Alfa Pulmozyme; Genentech Inc) to prevent cell clumping. T cells were stimulated with or without PMA (2 ng/mL) and ionomycin (1 μM) in the presence of 1 μM monensin (GolgiStop; BD Biosciences) for 6 to 8 hours as previously described.18 Cells were subsequently centrifuged and resuspended in complete medium and stained for surface markers followed by fixation with Fix/Perm solution (eBioscience) for 40 to 50 minutes. Cells were washed once and stored at 4°C overnight before intracellular staining for cytokine.
Flow cytometric analysis
Cells were resuspended in staining buffer (PBS containing 3% FBS) and blocked with mouse IgG mAbs (Caltag Labs) for 15 to 30 minutes at room temperature. Cells were stained with mAbs against surface antigens for 30 minutes at 4°C in the dark. For intracellular staining, cells were subsequently washed in staining buffer twice before fixation and permeabilization using eBioscience's buffers (FOXP3 Kit) for intracellular staining to stain for CTLA-4 and Ki-67. Cells were analyzed on FACSCalibur or FACSCanto flow cytometry instruments (BD Biosciences) and data were analyzed using CellQuest software (BD Biosciences). The quadrants were set based on isotype control antibody staining, and the number in each quadrant represents the percentage of cells.
Statistical analysis
Statistical comparisons of different T-cell subsets in the same patients were calculated using the paired t test. P values of .05 or less were considered significant.
Results
Expression of PD-1 by CD4 and CD8 T cells infiltrating into tumors
Although melanoma lesions typically contain tumor-reactive T cells with the capacity to proliferate when cultured in high-dose IL-2 and result in objective clinical responses upon adoptive cell transfers,19,20 tumors can grow despite the presence of tumor-reactive T cells.21 To elucidate the mechanisms underlying T-cell dysfunction within tumors, we decided to assess expression of inhibitory receptor, PD-1, and phenotypically and functionally contrast TILs with T cells in normal tissues and peripheral blood. Single-cell suspensions of enzymatically digested tumors were analyzed directly ex vivo. As demonstrated in Figure 1A, PD-1 expression could be detected in both CD4 and CD8 T cells infiltrating into metastatic melanoma lesions. The frequency of PD-1 expression was variable among patients (n = 28) and T-cell subsets; the frequency of expression ranged from 9.8% to 71.8% with mean of 38.0% for CD4 T cells, and 12.9% to 91.2% with mean 53.2% for CD8 T cells (Figure 1B). Interestingly, the frequency of PD-1+ cells was significantly higher in CD8 than CD4 TILs (P < .001, n = 28, Figure 1B). In contrast, there was no significant difference in the PD-1 expression level (MFI) between CD4 and CD8 TIL (P < .07, n = 28; data not shown).
Expression of PD-1 by CD4 and CD8 T cells infiltrating into tumors. Cryopreserved tumor digests from patients with metastatic melanoma (Pt) were thawed and immediately stained with CD3, CD8, and PD-1 mAbs. (A) The dot plots were gated on CD3+ lymphocytes. Quadrants were set based on isotype control mAbs. The percentages represent the fraction of PD-1+ T cells in CD8 T cells (top right) or CD4 T cells (top left). CD3+ CD8− T cells were considered CD4 T cells throughout the study. (B) The percentage of PD-1+ CD4 T cells per total CD4 T-cell population and PD-1+ CD8 T cells per total CD8 T-cell population in tumor digests from 28 patients with metastatic melanoma is shown. P value was calculated using the paired t test.
Expression of PD-1 by CD4 and CD8 T cells infiltrating into tumors. Cryopreserved tumor digests from patients with metastatic melanoma (Pt) were thawed and immediately stained with CD3, CD8, and PD-1 mAbs. (A) The dot plots were gated on CD3+ lymphocytes. Quadrants were set based on isotype control mAbs. The percentages represent the fraction of PD-1+ T cells in CD8 T cells (top right) or CD4 T cells (top left). CD3+ CD8− T cells were considered CD4 T cells throughout the study. (B) The percentage of PD-1+ CD4 T cells per total CD4 T-cell population and PD-1+ CD8 T cells per total CD8 T-cell population in tumor digests from 28 patients with metastatic melanoma is shown. P value was calculated using the paired t test.
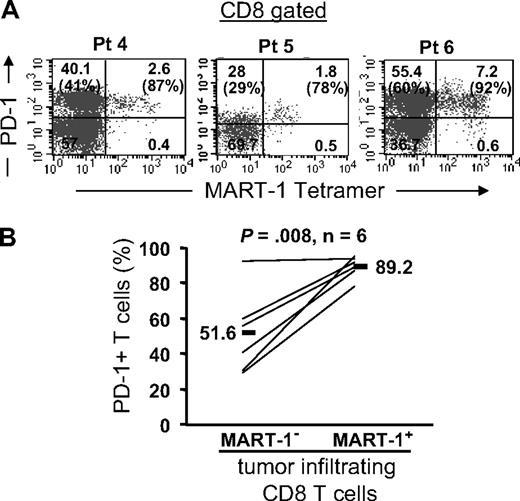
PD-1 expression on MART-1–specific CD8 T cells in tumors
Next, we examined PD-1 expression on tumor antigen–specific CD8 T cells reactive for the melanoma shared antigen, MART-1, using MART-1 tetramers. We found that PD-1 was expressed by the overwhelming majority of MART-1 tetramer+ CD8 T cells in tumors (Figure 2). Representative fluorescence-activated cell sorting (FACS) plots from 3 patients and a summary from 6 patients are shown in Figure 2A and B, respectively. PD-1 expression was significantly higher on MART-1 tetramer+ (range: 78%-95.5%, mean: 89.2%) than MART-1 tetramer–negative (range: 29%-92.7%, mean: 51.6%) CD8 T cells present in metastatic melanoma lesions in the same patients (P = .008; Figure 2B). The higher expression of PD-1 on MART-1 tetramer+ CD8 T cells raised the possibility that PD-1 expression was induced on tumor-reactive T cells after tumor-antigen recognition either on the tumor and/or antigen-presenting cells within the tumor microenvironment.
Tumor antigen–specific CD8 T cells express PD-1 in tumors. Tumor digests from patients were thawed and immediately stained with anti-CD3, anti-CD8, and anti–PD-1 mAb and MART-1 tetramer. (A) Three representative patients are shown. The dot plots were gated on CD3+ CD8+ lymphocytes. The numbers represent the percentage of CD3+ CD8+ T cells for each quadrant, and the percentage values represent the fraction of PD-1+ T cells in MART-1 tetramer+ CD8 T cells (top right) and in MART-1 tetramer− CD8 T cells (bottom left). (B) The overall frequency of PD-1+ T cells in MART-1 tetramer− (MART-1−) and MART-1 tetramer+ (MART-1+) tumor-infiltrating CD8 T cells is shown for 6 patients. P value was calculated using the paired t test.
Tumor antigen–specific CD8 T cells express PD-1 in tumors. Tumor digests from patients were thawed and immediately stained with anti-CD3, anti-CD8, and anti–PD-1 mAb and MART-1 tetramer. (A) Three representative patients are shown. The dot plots were gated on CD3+ CD8+ lymphocytes. The numbers represent the percentage of CD3+ CD8+ T cells for each quadrant, and the percentage values represent the fraction of PD-1+ T cells in MART-1 tetramer+ CD8 T cells (top right) and in MART-1 tetramer− CD8 T cells (bottom left). (B) The overall frequency of PD-1+ T cells in MART-1 tetramer− (MART-1−) and MART-1 tetramer+ (MART-1+) tumor-infiltrating CD8 T cells is shown for 6 patients. P value was calculated using the paired t test.
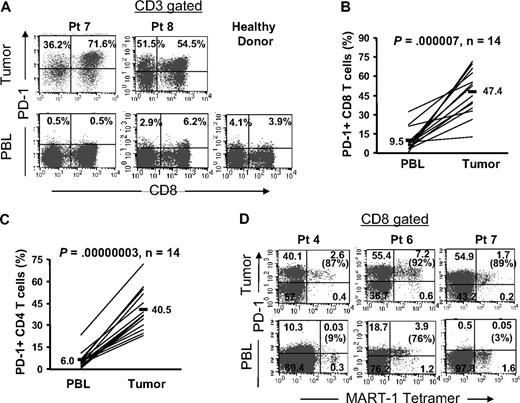
PD-1 is predominantly expressed by tumor-infiltrating T cells compared with peripheral blood T cells
Thus far, we have found that a substantial proportion of TIL expressed PD-1. Subsequently, we compared the proportion and level of PD-1 expression on circulating peripheral blood T cells compared with TIL from the same patients. In contrast to TIL, circulating peripheral blood T cells exhibited a minimal level of PD-1 expression that was comparable with the level expressed by peripheral blood T cells from healthy donors (Figure 3A). The frequency of PD-1+ CD8 T cells was significantly (P < .001) higher within tumors (range: 12.4%-71.6%, mean: 47.4%, n = 14) than in peripheral blood in the same patients (range: 0.5%-32.2%, mean: 9.5%, n = 14; Figure 3B) or healthy donors (range: 3.0%-19.5%, mean, 9.1%, n = 7; data not shown). Similarly, the frequency of PD-1+ CD4 T cells was significantly (P < .001) higher within tumors (range: 23.0%-56.2%, mean: 40.5%, n = 14) than in peripheral blood in the same patients (range: 0.4%-23.2%, mean: 6.0%, n = 14; Figure 3C) or healthy donors (range: 3.8%-13.1%, mean: 6.7%, n = 7; data not shown). Furthermore, both the frequency and the level of expression of PD-1 were higher on MART-1 tetramer+ CD8 T cells in metastatic melanoma tumors than in circulating MART-1 tetramer+ CD8 T cells in peripheral blood (Figure 3D). Although a relatively higher proportion of circulating MART-1 tetramer+ CD8 T cells were PD-1+ in 1 patient (pt 6, 76%) compared with the other 2 patients (pt 4, 9% and pt 7, 3%), the frequency (92%) and the level of expression of PD-1 were higher on MART-1 tetramer+ CD8 T in the tumor in this patient (pt 6) and were comparable with the other 2 patients (pt 4, 87% and pt 7, 89%; Figure 3D). The differential expression of PD-1 on MART-1–specific CD8 T cells in the tumor versus peripheral blood suggests that the tumor microenvironment mediates the induction of PD-1 on T cells that infiltrate into tumors.
PD-1 is predominantly expressed by tumor-infiltrating T cells compared with peripheral blood T cells. Cryopreserved tumor digests from patients with metastatic melanoma (Pt) and PBL from the same patients and healthy donors were thawed and immediately stained with CD3, CD8, and PD-1 mAbs. (A) Dot plots were gated on CD3+ lymphocytes. The percentage for each quadrant represents the fraction of PD-1+ T cells in CD8 T cells (top right quadrant) or in CD4 T cells (top left quadrant). These were representative of 14 patients and 7 healthy donors. (B) The percentage of PD-1+ CD8 T cells per total CD8 T-cell population and (C) the percentage of PD-1+ CD4 T cells per total CD4 T-cell population were quantified in PBL and tumor digests from the same patients (n = 14). P value was calculated using the paired t test. (D) In 3 patients who had detectable circulating MART-1 tetramer CD8 T cells in their peripheral blood, we compared PD-1 expression on MART-1 tetramer+ CD8 T cells in tumor digest samples versus those in peripheral blood in the same patients as described in Figure 2.
PD-1 is predominantly expressed by tumor-infiltrating T cells compared with peripheral blood T cells. Cryopreserved tumor digests from patients with metastatic melanoma (Pt) and PBL from the same patients and healthy donors were thawed and immediately stained with CD3, CD8, and PD-1 mAbs. (A) Dot plots were gated on CD3+ lymphocytes. The percentage for each quadrant represents the fraction of PD-1+ T cells in CD8 T cells (top right quadrant) or in CD4 T cells (top left quadrant). These were representative of 14 patients and 7 healthy donors. (B) The percentage of PD-1+ CD8 T cells per total CD8 T-cell population and (C) the percentage of PD-1+ CD4 T cells per total CD4 T-cell population were quantified in PBL and tumor digests from the same patients (n = 14). P value was calculated using the paired t test. (D) In 3 patients who had detectable circulating MART-1 tetramer CD8 T cells in their peripheral blood, we compared PD-1 expression on MART-1 tetramer+ CD8 T cells in tumor digest samples versus those in peripheral blood in the same patients as described in Figure 2.
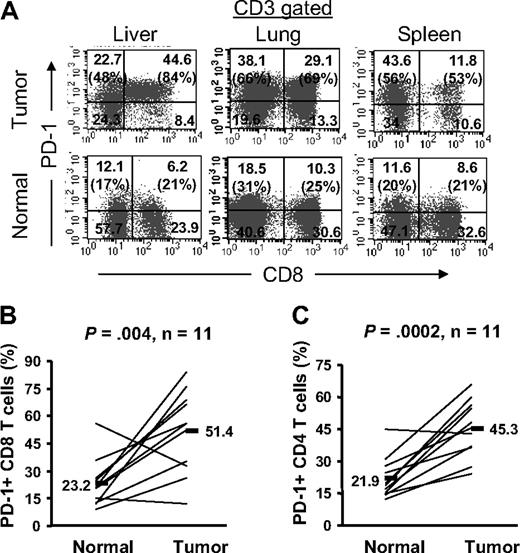
PD-1 expression is significantly higher on T cells infiltrating into tumors compared with normal tissue
We asked whether expression of PD-1 was unique to T cells infiltrating into tumors, or was an intrinsic feature of T cells that infiltrate into any tissues, including tumor-free normal tissues. To address this question, we compared PD-1 expression on T cells infiltrating into tumor metastases and into normal tissues (liver, lung, and spleen) where tumors were resected in the same patients. We found that not only the frequency of PD-1+ T cells was higher on TIL compared with T-cell infiltrates in normal tissue but also their level of expression was higher in the same patients (Figure 4). The differential expression of PD-1 on TIL versus normal tissues T-cell infiltrates was detected in all 3 organs (liver, n = 3; lung, n = 6; spleen, n = 2) that were examined. Furthermore, this differential PD-1 expression was observed in both CD4 and CD8 T-cell subsets; the frequency of PD-1+ CD8 T cells was significantly (P = .004) higher on CD8 TIL (range: 12.4%-84.0%, mean: 51.4%) than normal tissue infiltrates (range: 9.0%-55.7%, mean: 23.2%) in the same patients (n = 11; Figure 4B). Similarly, the frequency of PD-1+ CD4 T cells was significantly (P < .001) higher on CD4 TIL (range: 24.3%-66.1%, mean: 45.3%) than normal tissue infiltrates (range: 12.1%-44.8%, mean: 21.9%) in the same patients (n = 11; Figure 4C). These data collectively suggest that the tumor microenvironment enhances the expression of PD-1 on TIL, and that PD-1 expression on T cells displays a tissue hierarchy that was expressed more highly in tumors than normal tissues, and least expressed by circulating peripheral blood T cells.
The frequency of PD-1+ T cells is significantly higher in tumor than adjacent normal tissue. Cryopreserved tumor digests (Tumor) and normal tissue digests (Normal) isolated from the same patients were thawed and immediately stained with CD3, CD8, and PD-1 mAbs. (A) The dot plots were gated on CD3+ lymphocytes. Quadrants were set based on isotype control mAbs. Numbers represent the percentages of T cells in each quadrant and the percentage values represent the fraction of PD-1+ T cells in CD8 T cells (top right quadrant) and CD4 T cells (top left quadrant). The percentage of PD-1+ CD8 T cells per total CD8 T-cells population (B) and PD-1+ CD4 T cells per total CD4 T-cell population (C) in tumors and normal tissues for 11 patients with metastatic melanoma are shown. P value was calculated using the paired t test.
The frequency of PD-1+ T cells is significantly higher in tumor than adjacent normal tissue. Cryopreserved tumor digests (Tumor) and normal tissue digests (Normal) isolated from the same patients were thawed and immediately stained with CD3, CD8, and PD-1 mAbs. (A) The dot plots were gated on CD3+ lymphocytes. Quadrants were set based on isotype control mAbs. Numbers represent the percentages of T cells in each quadrant and the percentage values represent the fraction of PD-1+ T cells in CD8 T cells (top right quadrant) and CD4 T cells (top left quadrant). The percentage of PD-1+ CD8 T cells per total CD8 T-cells population (B) and PD-1+ CD4 T cells per total CD4 T-cell population (C) in tumors and normal tissues for 11 patients with metastatic melanoma are shown. P value was calculated using the paired t test.
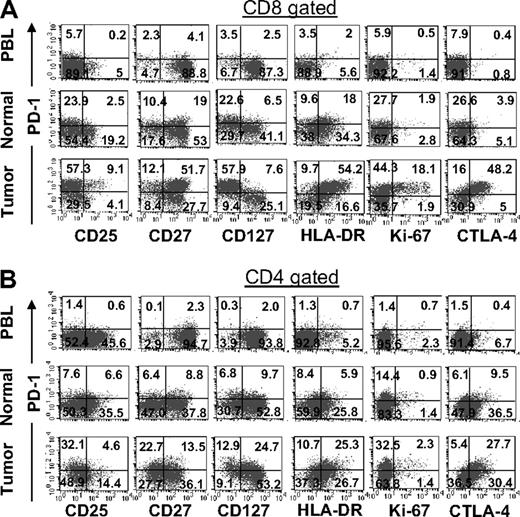
Phenotypic comparison of CD8 and CD4 T cells infiltrating into tumor, normal tissue, and peripheral blood
A comparison of the phenotype of CD8 T cells infiltrating tumors with those from normal tissues and circulating blood indicated an overall up-regulation of markers typically associated with T-cell exhaustion (Figure 5A). In contrast to CD8 T cells in normal tissues and peripheral blood that primarily lacked CTLA-4 expression, a large fraction of CD8 TIL were CTLA-4+, which was expressed mainly by PD-1+ CD8 TIL. Despite up-regulation of CTLA-4, the overwhelming majority of PD-1+ CD8 T cells lacked CD25 expression and were primarily CD27+. Interestingly, PD-1+ CD8 TIL lacked mainly expression of IL-7 receptor α chain (CD127), a phenotype that is associated with T cells' inability to proliferate and produce effector cytokines in chronic viral infections in vivo22-24 as well as human FOXP3+ CD4 T regulatory cells (Treg).25,26 In addition to the coexpression of CTLA-4, PD-1+ CD8 TIL also expressed high levels of HLA-DR, a marker of T-cell activation. Although HLA-DR expression was detected only in a very small number of peripheral blood CD8 T cells, a larger number of normal tissue CD8 T-cell infiltrates also expressed this marker. However, the expression of HLA-DR was not associated with PD-1 expression on CD8 T cells in normal tissues, as it was in tumors (Figure 5A). Similar to CTLA-4 expression, Ki-67, a protein expressed by dividing cells, was also exclusively expressed by a fraction of PD-1+CD8 T cells found in tumors and not by T cells in normal tissues or peripheral blood. Collectively, these results reveal significant phenotypic differences between PD-1+ CD8 TIL and T cells in normal tissues and peripheral blood that are characteristics of an exhausted phenotype and further suggest that their effector function might be impaired.
Phenotypic comparison of CD8 and CD4 T cells infiltrating into tumor, normal tissue, and peripheral blood in the same patient. Cryopreserved digests from a tumor metastasized into lung (Tumor), normal lung tissue (Normal), and peripheral blood lymphocytes (PBL) from the same patient were thawed and immediately stained with CD3, CD8, PD-1 mAbs, and appropriate marker as described in “Flow cytometric analysis.” For CD8 T cells, dot plots were gated on CD3+CD8+ lymphocytes (A) and for CD4 T cells dot plots were gated on CD3+CD8− lymphocytes (B). The numbers represent the percentages of CD8 or CD4 T cells in each quadrant. This is representative of 8 independent experiments from patients with metastatic melanoma lesions and normal tissue isolated from lung (n = 6), liver (n = 1), and spleen (n = 1).
Phenotypic comparison of CD8 and CD4 T cells infiltrating into tumor, normal tissue, and peripheral blood in the same patient. Cryopreserved digests from a tumor metastasized into lung (Tumor), normal lung tissue (Normal), and peripheral blood lymphocytes (PBL) from the same patient were thawed and immediately stained with CD3, CD8, PD-1 mAbs, and appropriate marker as described in “Flow cytometric analysis.” For CD8 T cells, dot plots were gated on CD3+CD8+ lymphocytes (A) and for CD4 T cells dot plots were gated on CD3+CD8− lymphocytes (B). The numbers represent the percentages of CD8 or CD4 T cells in each quadrant. This is representative of 8 independent experiments from patients with metastatic melanoma lesions and normal tissue isolated from lung (n = 6), liver (n = 1), and spleen (n = 1).
Phenotypic comparisons of CD4 T cells infiltrating different tissue compartments were characteristically similar to CD8 T cells (Figure 5B). PD-1+ CD4 T cells in tumors typically lacked CD25 expression, whereas CD4 T cells in peripheral blood and normal tissues expressed CD25 without PD-1 expression. Similar to PD-1+ CD8 TIL, the majority of PD-1+ CD4 TIL also expressed HLA-DR and CTLA-4. Consistent with CD8 TIL, Ki-67 expression was primarily detected in PD-1+ CD4 TIL. In contrast to PD-1+ CD8 TIL that were primarily CD127− and CD27+, PD-1+ CD4 TIL were heterogeneous for the expression of these 2 markers in some patients.
PD-1+ TILs display an impaired effector function
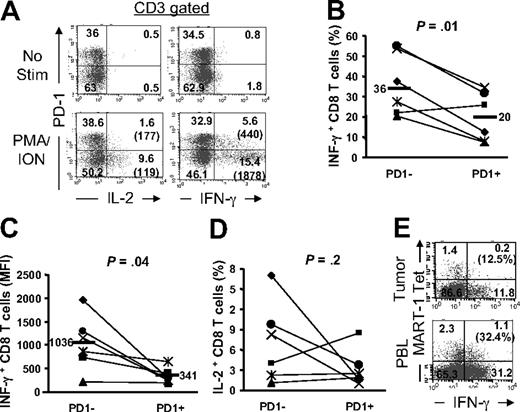
Expression of PD-1 on viral-specific human CD8 T cells has correlated with the reduced effector function in vitro.7-9 To determine whether the expression of PD-1 on TIL impaired their effector function, we stimulated uncultured freshly thawed TILs with a potent stimulus, phorbol myristate acetate (PMA) and ionomycin (I), and assessed the production of IL-2 and IFN-γ using intracellular staining at a single-cell level ex vivo. As demonstrated in Figure 6A, PMA/I activation resulted in the production of IL-2 and IFN-γ by a large fraction of PD-1− TIL (IFN-γ, 37.7%; IL-2, 17%). In contrast, not only did a smaller fraction of PD-1+ T cells produce these cytokines (IFN-γ, 12.6%; IL-2, 1.6%), but they also exhibited markedly reduced levels of IFN-γ production as quantified by mean fluorescence intensity (MFI; 440 for PD-1+ compared with 1878 for PD-1− T-cell subsets; Figure 6A). Similar results were measured in tumor lesions from multiple patients (n = 6); the percentage (Figure 6B, P = .01) and the amount of cytokine production (MFI; Figure 6C, P = .04) were reduced in PD-1+ CD8 T cells compared with PD-1− CD8 T cells. These results indicate that the ability to produce IFN-γ was inversely correlated with the expression of PD-1 by CD8 TIL. We have also found that the proportion of CD8 TIL producing IL-2 was reduced in PD-1+ compared with PD-1− subset in 3 patients (1.6% vs 17%; 1% vs 8.3%; and 3.7% vs 9.7%; PD-1+ vs PD-1− CD8 TIL, respectively, Figure 6D). However, we did not detect striking differences in 3 other patients. It is notable that the frequency of IL-2–producing CD8 TIL was too low in PD-1− subset (≤ 4%) in these latter patients (Figure 6D), providing a plausible explanation as to not finding a noticeable difference in IL-2 production between the PD-1 subsets in these patients.
PD-1 expression on tumor-infiltrating T cells correlates with impaired effector function. Tumor digests and peripheral blood sample from patients with metastatic melanoma were thawed and immediately stimulated with PMA/I for 6 to 8 hours in the presence of monensin. Cells were subsequently stained with anti-CD3, anti-CD8, and anti–PD-1 mAb along with anti–IL-2 and anti–IFN-γ mAbs. (A) Dot plots were gated on CD3+ T cells. The numbers represent the percentages of T cells in each quadrant and the value in parentheses represents the MFI for each quadrant. (B) The percentage of CD3+CD8+ T cells that were IFN-γ+ is depicted for PD-1+ and PD-1− CD8 TILs. (C) The MFI for IFN-γ+ CD3+CD8+ T cells are depicted for PD-1+ and PD-1− CD8 TILs. (D) The percentage of CD3+CD8+ T cells that were IL-2+ is depicted for PD-1+ and PD-1− CD8 TILs for 6 patients. P values are calculated based on the paired t test. (E) IFN-γ production by MART-1 tetramer+ CD8 T cells in tumor digests versus peripheral blood (PBL) from the same patient is shown. The percentage values represent the fraction of MART-1 tetramer+ CD8 T cells that produced IFN-γ.
PD-1 expression on tumor-infiltrating T cells correlates with impaired effector function. Tumor digests and peripheral blood sample from patients with metastatic melanoma were thawed and immediately stimulated with PMA/I for 6 to 8 hours in the presence of monensin. Cells were subsequently stained with anti-CD3, anti-CD8, and anti–PD-1 mAb along with anti–IL-2 and anti–IFN-γ mAbs. (A) Dot plots were gated on CD3+ T cells. The numbers represent the percentages of T cells in each quadrant and the value in parentheses represents the MFI for each quadrant. (B) The percentage of CD3+CD8+ T cells that were IFN-γ+ is depicted for PD-1+ and PD-1− CD8 TILs. (C) The MFI for IFN-γ+ CD3+CD8+ T cells are depicted for PD-1+ and PD-1− CD8 TILs. (D) The percentage of CD3+CD8+ T cells that were IL-2+ is depicted for PD-1+ and PD-1− CD8 TILs for 6 patients. P values are calculated based on the paired t test. (E) IFN-γ production by MART-1 tetramer+ CD8 T cells in tumor digests versus peripheral blood (PBL) from the same patient is shown. The percentage values represent the fraction of MART-1 tetramer+ CD8 T cells that produced IFN-γ.
In addition to differential IFN-γ production that inversely correlated with PD-1 expression on CD8 TIL, we were able to directly compare the effector function of tumor Ag-specific MART-1 tetramer+ CD8 T cells in tumors with those in the peripheral blood in the same patient. We found that a smaller fraction of MART-1 tetramer+ CD8 T cells in metastatic melanoma lesions (12.5%) produced IFN-γ upon PMA/I stimulation compared with those circulating in peripheral blood (32.4%) in the same patient (Figure 6E). This reduced ability to produce IFN-γ by MART-1 tetramer+ CD8 TIL correlated with their very high percentage of PD-1–expressing cells (86%) compared with low percentage of PD-1–expressing cells on MART-1 tetramer+ CD8 T cells in the peripheral blood (16%; data not shown). Evaluation of additional patients has been limited due to scarce number of patients with circulating MART-1 tetramer+ CD8 T in their peripheral blood. The higher functional capacity and low expression of PD-1 on circulating tumor Ag-specific MART-1 tetramer+ CD8 T cells in peripheral blood compared with those in tumors strongly suggested that the induction of PD-1 expression by tumor-infiltrating MART-1 tetramer+ CD8 T cells impaired the ability of these cells to produce IFN-γ even in response to a potent stimulus such as PMA/I, which bypasses T-cell receptor (TCR) stimulation.
Discussion
In the present study, we report the expression of high levels of PD-1 on a large proportion of T cells infiltrating into tumors compared with those in normal tissue and peripheral blood in the same patients and healthy donors. This novel finding reveals a tissue hierarchy for the expression of PD-1 on T cells (tumor > normal tissue > peripheral blood), indicating that the tumor microenvironment plays a pivotal role in the induction and maintenance of PD-1 expression on TIL. We also demonstrate that expression of PD-1 on TIL correlates with an exhausted phenotype and an impaired effector function. These results suggest that the induction of PD-1 on TIL undermines their ability to mount an effective antitumor immune response, and provides a plausible explanation for tumor growth despite the presence of tumor-reactive T cells infiltrating into the tumor stroma. However, we could not evaluate whether there was any correlation between the frequency of PD-1+ T cells in tumors and clinical outcome of patients because patients received various treatments after their tumor resections.
Although a large proportion of TIL expressed PD-1, the level of expression and the percentage of PD-1+ T cells were variable among patients. The mechanism and factors that lead to PD-1 expression on TIL are unclear. Because our findings also reflect a higher proportion of MART-1 tetramer+ CD8 T cells that expressed PD-1 compared with the rest of CD8 TIL, it is likely that Ag encounter within the tumor can result in the expression of PD-1 on TIL. Thus, the frequency and recognition of tumor-Ag specific T cells within the tumor may determine the frequency as well as the level of expression of this inhibitory receptor on T cells. Because the level of PD-1 expression on T cells may regulate the activation threshold in T cells and their cytokine production,3 the level of functional exhaustion on TIL may also be variable among patients and depend on the level of PD-1 expression.
Although immunohistochemical analyses in non–small cell lung cancer27 and renal cell carcinoma28 have detected PD-1 expression on mononuclear immune cells, our study is the first demonstration of PD-1 expression on TIL in metastatic melanoma lesions. In addition, we report on significant differences in phenotype and function of T cells in different tissue compartments that have not being previously reported.
A phenotypic comparison of CD8 TIL with those in normal tissues and circulating blood indicated an overall up-regulation of markers typically associated with T-cell exhaustion. The selective high expression of CTLA-4, another inhibitory receptor,6 on PD-1+ CD8 TIL and not on PD-1− CD8 TIL, normal tissue infiltrates, and PBL suggests that the tumor and its microenvironment may mediate the induction of these inhibitory receptors on T cells. Similar to CTLA-4, Ki-67 was also selectively expressed by PD-1+ CD8 TIL, suggesting that T-cell activation leading to cell division within tumors might be a prerequisite to up-regulation of PD-1 on TIL. Alternatively, the data may also suggest that a fraction of PD-1+ TIL was capable of undergoing cell division even though PD-1 expression was typically associated with the inability to proliferate.22,29 Although HLA-DR was expressed by CD8 T cells in normal tissues, these cells did not express high levels of PD-1 as TIL did, suggesting that T-cell activation and up-regulation of HLA-DR did not necessarily result in PD-1 expression. It is plausible that T-cell activation under a suppressive microenvironment such as in a tumor may induce expression of PD-1 on T cells.
We have previously reported that IL-2 leads to down-regulation of CD27 on CD8 T cells during in vitro activation.30 This cytokine is also known to up-regulate its own receptor, CD25. Because PD-1+ CD8 TIL typically expressed CD27 and lacked CD25 expression, it suggests that these cells might have been activated in a microenvironment that might not have provided the sufficient IL-2 to up-regulate CD25 and down-regulate CD27 expression. In fact, the low frequency of IL-2–producing TIL even in PD-1− subset after PMA/I stimulation (Figure 6D) indirectly supports this hypothesis.
Our study also demonstrated that in contrast to PD-1− CD8 TIL and PBL, the overwhelming majority of PD-1+ CD8 TIL lacked CD127 expression. Although T-cell activation can down-regulate CD127 on effector T cells in vitro,31,32 low expression of CD127 is associated with impaired memory T-cell differentiation in vivo.22-24,33 In addition to chronically stimulated virus-specific CD8 T cells in vivo,22,23,34,35 human FOXP3+ CD4 Treg25,26 also express low levels of CD127. Although its functional significance may not be clear in CD4 Treg, low expression of CD127 on antigen-specific CD8 T cells has been reported to correlate with T cells' inability to proliferate and produce effector cytokines in chronic viral infectious settings.22-24 The differential expression of CD127 on PD-1+ compared with PD-1− TIL was indirectly correlated with impaired IFN-γ production in PD-1+ CD8 TIL (Figure 6). It is not clear whether the low expression of CD127 results in T-cell effector impairment or whether it merely serves as a phenotypic marker to identify these cells in vivo.33
Although PD-1+ TIL share similar phenotypic markers as CD4 Treg in tumors such as the high expression of CTLA-4 and lack of CD127 expression, there are distinct phenotypic differences that clearly distinguish CD4 Treg from PD-1+ T cells. Unlike CD4 Treg, PD-1+ CD8 TIL primarily lacked CD25 (Figure 5A) and FOXP3 expression (data not shown), a phenotype shared with normal tissue CD8 T-cell infiltrates and peripheral blood CD8 T cells. This is in agreement with our recent study that found FOXP3 was expressed primarily by CD4 and not CD8 TIL in metastatic melanoma lesions.18
It has been reported that PD-1 ligation can inhibit antigen receptor signaling by dephosphorylation of TCR signaling intermediates such as CD3ζ, ZAP70, and PKCθ36 to suppress the activation of PI3K and Akt.6 These findings explain the inability of viral-specific CD8 T cells that express PD-1 to produce effector cytokine in response to Ag restimulation in vitro.7,8 Our study demonstrates that PD-1+ TIL exhibited a diminished cytokine production in response to PMA/ionomycin (PMA/I), a potent T-cell stimulus that bypasses TCR signaling to activate DAG and open calcium channels.37 Studies in primary human T cells isolated from peripheral blood have suggested that PD-1 engagement has to be in the proximity of either TCR complex or CD28 molecule to inhibit T-cell activation.38 Although there are inherent differences between TIL (current study) versus PBL,38 our results do not directly dispute these results. In fact, we propose that the diminished effector cytokine production by PD-1+ TIL, even in response to a potent stimulus, reflects on their suppressed effector function compared with PD-1− TIL. Accordingly, we speculate that Ag stimulation of PD-1+ TIL would have failed to produce any detectable IFN-γ because PD-1 expression would have inhibited not only the induction of cytokine production but also the activation of TCR signaling intermediates. We also demonstrate that the impaired cytokine production by MART-1 tetramer+ CD8 T cells in tumors compared with those in peripheral blood circulation was associated with high expression of PD-1 on the majority of MART-1 tetramer+ TIL. The reduced effector function by MART-1–reactive TIL is in accord with a previous study, though it had not examined the role of PD-1 on these cells.39 These data further indicate that PBL do not share similar phenotypic and functional characteristics as TIL, as we have recently reported with regard to selective accumulation of CD4 Treg in tumors.18
Given that PD-1+ TIL displayed an impaired capacity to produce IFN-γ, an essential cytokine required for an effective antitumor immune response,40 their abilities to destroy tumor cells are undermined and may lead to tumor immune escape. Whether the expression of PD-1 is directly responsible for this functional exhaustion or merely serves as a phenotypic marker for T cells exhibiting the suppressed effector function is yet unclear. Although the addition of anti–PD-1 blocking antibody to Ag-stimulated T cells in vitro has previously demonstrated the inhibitory role of PD-1 in T-cell function,7,8,34,41,42 those functional assays were dependent on TCR stimulation for several days. Using PMA/I, we have bypassed TCR signaling and avoided long-term stimulation, allowing us to determine the functional state of TIL ex vivo without long-term culturing. The addition of anti–PD-1 blocking antibody with PMA/I stimulation may not reveal any significant functional changes due to the short duration of the assay (5-8 hours). Furthermore, in vitro activation and expansion of TILs are dependent on the addition of high-dose IL-2.17 In fact, our preliminary results indicate that PD-1 expression was reduced on TILs that were expanded from tumor digest samples upon culturing in high-dose IL-2 for 2 weeks (M.A. and S.A.R., unpublished data, January 2008). Although we cannot exclusively determine whether IL-2 leads to down-regulation of PD-1 or expansion of PD-1− T cells in cultures, it suggests that the addition of exogenous cytokines such as IL-2 may alter the phenotype and function of cells at the population level.
In summary, we report that PD-1 is expressed by a larger number of T cells infiltrating metastatic melanoma lesions than T cells in normal tissues and peripheral blood in the same patients as well as healthy donors. The PD-1+ TIL displayed an exhausted phenotype and an impaired effector function compared with PD-1− TIL and PBL, supporting a critical role for PD-1 pathway to suppress T-cell effector function. These preclinical findings have important implications for modulating the antitumor immune responses in patients with cancer. These results have also provided us with the rationale to conduct a clinical study to evaluate the potential impact of anti–PDL-1 mAb in a clinical trial in patients with metastatic melanoma.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors are grateful to A. Mixon and S. Farid for performing flow cytometry.
Authorship
Contribution: M.A. designed and performed research and wrote the paper; L.A.J. assisted with phenotypic analysis; B.H. assisted with performing functional assays; J.R.W. processed tumor digests; M.E.D. assisted with identifying patients with tumor antigen reactivity; D.E.W. prepared the patient characteristics table and provided statistical consultation; and S.A.R. designed and supervised the project.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Steven A. Rosenberg or Mojgan Ahmadzadeh, Surgery Branch, NCI, NIH, CRC Bldg, Rm 3W-3940, 10 Center Dr, Bethesda, MD 20892; e-mail: sar@nih.gov or mojgan_ahmadzadeh@nih.gov.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal