Abstract
Klimchenko and colleagues use human embryonic stem cells to define a novel bipotential hematopoietic progenitor that gives rise to primitive (yolk sac–type) erythrocytes and megakaryocytes.1
Studies of hematopoiesis have led the way in advancing our knowledge of human stem cell biology.2 This is partly because adult hematopoietic stem cells are easily sampled from peripheral blood or bone marrow. Unraveling the origins of early hematopoiesis through analysis of human embryos is more difficult, both practically and ethically. Although such studies have yielded valuable information,3 most data on the ontogeny of vertebrate blood formation have been gained through analysis of model organisms including mice, fish, chickens, and frogs.
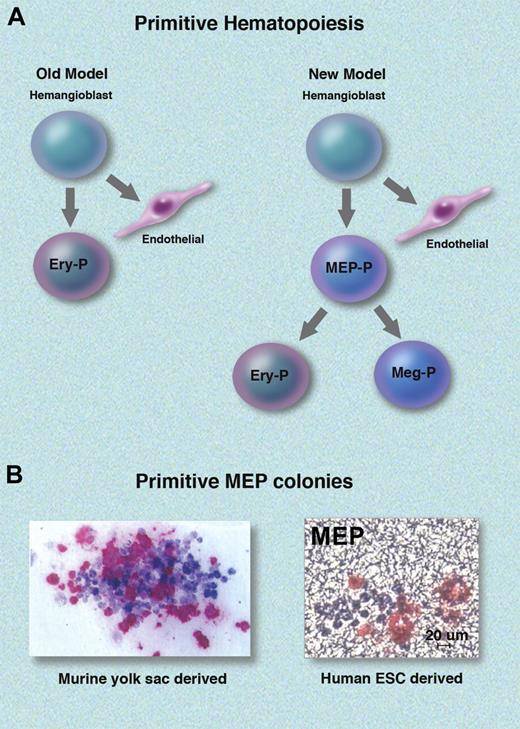
Old and new models for yolk sac primitive hematopoiesis. (A) Previous models indicated that primitive (P) erythrocytes develop directly from a yolk sac vascular-endothelial precursor termed the hemangioblast.2 New data extend the hematopoietic repertoire of yolk sac hematopoiesis by defining a bipotent primitive MEP that gives rise to erythrocytes (Ery) or megakaryocytes (Meg), which then mature into platelets (not shown). Data by Tober et al indicate that the primitive MEP is hemangioblast derived.6 (B) Bi-lineage erythro-megakaryocytic colonies arising from single primitive MEPs. The right panel shows a colony derived from human ESCs (adapted from Figure 4 of the article beginning on page 1506). Megakaryocytes are stained with anti-CD41 (pink). The left panel shows a colony derived from mouse yolk sac. Erythrocytes are stained with anti-βH1 globin (blue); megakaryocytes are stained with anti-GP1bβ (red). Adapted with permission from Tober et al.6 Professional illustration by Marie Dauenheimer.
Old and new models for yolk sac primitive hematopoiesis. (A) Previous models indicated that primitive (P) erythrocytes develop directly from a yolk sac vascular-endothelial precursor termed the hemangioblast.2 New data extend the hematopoietic repertoire of yolk sac hematopoiesis by defining a bipotent primitive MEP that gives rise to erythrocytes (Ery) or megakaryocytes (Meg), which then mature into platelets (not shown). Data by Tober et al indicate that the primitive MEP is hemangioblast derived.6 (B) Bi-lineage erythro-megakaryocytic colonies arising from single primitive MEPs. The right panel shows a colony derived from human ESCs (adapted from Figure 4 of the article beginning on page 1506). Megakaryocytes are stained with anti-CD41 (pink). The left panel shows a colony derived from mouse yolk sac. Erythrocytes are stained with anti-βH1 globin (blue); megakaryocytes are stained with anti-GP1bβ (red). Adapted with permission from Tober et al.6 Professional illustration by Marie Dauenheimer.
In mammalian embryos, yolk sac–derived erythrocytes are among the first blood cells to form. These cells, termed “primitive,” differ from adult-type fetal liver or bone marrow–derived “definitive” erythrocytes in many respects, including morphology, gene expression, and cytokine requirements.4 In 2001, Xu et al showed that early murine embryonic yolk sac also produces primitive megakaryocytes that differ distinctly from their definitive counterparts in adult bone marrow.5 More recently, Tober et al found that yolk sac megakaryocytes arise from a bipotential primitive megakaryocyte-erythroid precursor (MEP), analogous to the well-known definitive MEP present in later-stage fetal liver and adult bone marrow. They also showed that primitive megakaryocytes give rise to circulating platelets with different structural features from those found in later-stage embryos and adults.6 Whether primitive MEPs exist in human hematopoiesis has been an open question that Klimchenko et al addressed using embryonic stem cells (ESCs).
In vitro differentiation of ESCs closely mimics aspects of early embryonic development, including hematopoiesis.7 Thus, primitive and definitive erythrocytes arise sequentially in human and mouse ESC differentiation cultures, similar to the order of events in embryos. Several years ago, Vodyanik et al showed that the very first blood lineages to arise in human ESC hematopoietic induction cultures are erythroid and megakaryocytic progenitors, which reside within a population of cells co-expressing the surface markers CD43 (leukosialin), CD41a (GPIIb, a progenitor and megakaryocytic antigen), and CD235a (glycophorin A, an erythrocyte antigen).8 Now, work published in this issue of Blood extends previous studies by showing that single human ESC-derived CD41+/CD235a+ cells can give rise to primitive erythroblasts and megakaryocytes in culture, thus defining a human primitive MEP.1 Gene expression analysis showed that the differentiation of this bipotential progenitor into single lineages is accompanied by signature changes in the expression of key transcription factors that have been previously implicated in definitive erythrocyte and megakaryocyte production. This is the first report of primitive human MEPs and fits well with the original identification of the same progenitor in mouse embryos by Tober et al.6 Together, these studies provide new concepts on the origins of mammalian blood (see figure). Specifically, yolk sac primitive hematopoiesis is not restricted to erythrocytes, as posed in older models.1 Rather, the process produces at least 2 cell types (erythroid and megakaryocytic) that are intimately linked by a common bipotential progenitor. More generally, the current study illustrates the utility of ESCs as a model system for human hematopoiesis, particularly for investigating early embryonic events.
As for all interesting studies, new questions arise. Why are platelets produced in early embryos? NF-E2–null embryos, which exhibit low (but not absent) platelet levels, do not bleed until they experience birth trauma.9 However, platelets express many potent signaling molecules that may exert currently unrecognized functions in development of the vasculature or other embryonic tissues.10 There are also important issues of therapeutic relevance. For example, it may be possible to use human ESCs or related induced pluripotent stem cells (iPSCs) to generate platelets and erythrocytes for transfusion therapies. For this strategy to succeed, it is necessary to develop new culture methods that optimize the purity and yield of megakaryocytes, platelets, and erythrocytes from human ESCs or iPSCs. It may also be important to control the production of primitive versus definitive erythro-megakaryocytic cells and to better understand the functional differences between these related but distinct lineages.
Conflict-of-interest disclosure: The authors declare no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal