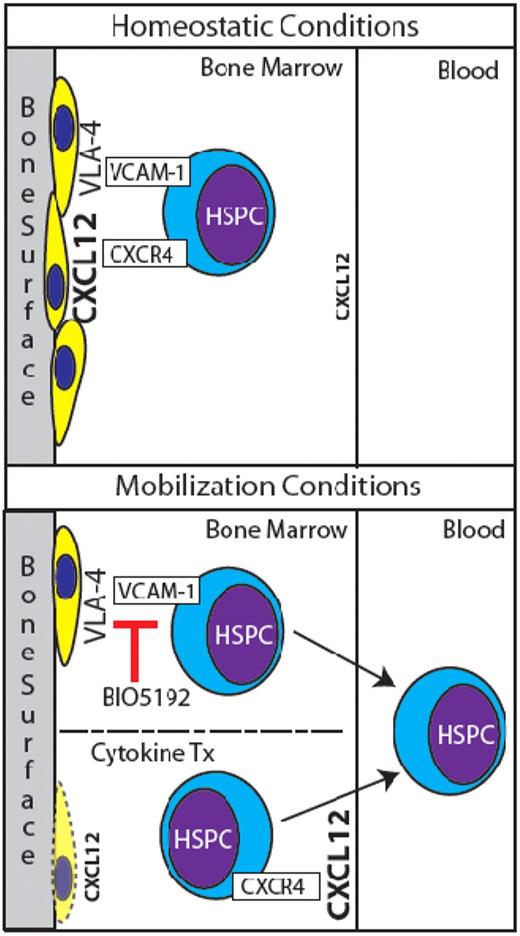
In this issue of Blood, Ramirez and colleagues unveil a new small molecule antagonist of VCAM-1/VLA-4 signaling, BIO5192, that rapidly mobilizes HSPCs. Also in this issue, Christopher and colleagues show that suppression of CXCL12 (SDF-1) production by osteoblasts is essential for cytokine-mediated mobilization of HSPCs.
Throughout adult life, the majority of hematopoietic stem cells (HSCs) are located within specialized niches in the bone marrow (BM) where their activity is tightly controlled.1,2 However, under certain circumstances, HSCs can be induced to egress from the BM and enter into the peripheral circulation. From here, in a still relatively ill-understood process termed “mobilization,” HSCs are free to move throughout the body and transiently lodge in peripheral organs such as the spleen. This interesting natural phenomenon, that often accompanies various hematopologic disorders, has been adapted as a useful component of therapy, given the discovery of agents that artificially incite mobilization of HSCs into the bloodstream where they can be collected and used for purposes such as transplantation. Mobilizing agents include chemotherapeutic drugs, such as cyclophosphamide and 5-fluorouracil, various cytokines and chemokines, such as stem cell factor (SCF), stromal derived factor-1 (SDF-1 or CXCL12), interleukin-8 (IL-8), granulocyte-colony stimulating factor (G-CSF), and synthesized small molecules, such as AMD3100 (plerixafor).3
Normally, HSPCs are retained in BM niches in part by CXCR4/CXCL12 and VCAM-1/VLA-4 signaling (top panel). HSPCs are mobilized by BIO5192's antagonism of VCAM-1, and perhaps in part by suppression of osteoblasts and redistribution of CXCL12 levels via cytokine treatment (bottom panel).
Normally, HSPCs are retained in BM niches in part by CXCR4/CXCL12 and VCAM-1/VLA-4 signaling (top panel). HSPCs are mobilized by BIO5192's antagonism of VCAM-1, and perhaps in part by suppression of osteoblasts and redistribution of CXCL12 levels via cytokine treatment (bottom panel).
Although clinically G-CSF is the most extensively used mobilization agent, its drawbacks include potentially toxic side effects, a relatively long course of treatment (5-7 days of consecutive injections), and variable responsiveness of patients. There is a need for additional and/or alternative methods of HSPC mobilization. Ramirez et al have characterized a novel small molecule antagonist to VCAM-1/VLA-4 niche interaction termed “BIO5192.”4 The authors observe that a single dose of BIO5192 given to wild-type mice resulted in a 30-fold increase of circulating hematopoietic stem and progenitor cells (HSPCs), as measured by colony forming unit (CFU) assays. Serial transplantation of mobilized mononuclear cells demonstrated that BIO5192 truly mobilizes HSPCs capable of long-term multilineage reconstitution. When used in combination with plerixafor and G-CSF, additive effects of mobilization were observed (> 100-fold increases of circulating HSPCs).
Additionally, in the Ramirez study, these different mobilizing agents were administered to splenectomized mice. While the efficacy of BIO5192 was not affected, G-CSF treatment showed increased levels of circulating HSPCs, supporting the spleen's role as a transient reservoir for mobilized cells. On the other hand, plerixafor had a diminished ability to mobilize HSPCs in mice lacking a spleen. The authors hypothesize this may indicate that plerixafor acts to mobilize HSPCs associated with vascular niches, as it is well-known that vascular niches exist within the BM as well as the spleen.5 However, this theory requires further investigation. Nevertheless, BIO5192 represents an exciting new addition to the arsenal of tools available to clinicians for therapeutic mobilization of HSPCs.
Given the extensive use of G-CSF for mobilization, numerous insights have been made into its mechanism of action. Three main processes have been implicated: activation of proteases, inhibition of adhesion molecules, and attenuation of CXCR4/CXCL12 signaling. Christopher et al investigate these processes by attempting to mobilize CXCR4-/- BM chimeras with G-CSF and other agents.6 These mice failed to mobilize in response to G-CSF. Interestingly, the levels of activated metalloproteinases in their BM were up-regulated at levels similar to wild-type controls, indicating that proteolytic activity is not an independent contributor to HSPC mobilization. In contrast, these chimeric animals successfully mobilized in response to BIO5192, indicating that disruption of HSPC adhesion via antagonizing VCAM-1/VLA-4 signaling is both independent of CXCR4/CXCL12 signaling and essential for HSPC mobilization. Building upon previous work, Christopher et al validate the importance of osteoblast loss during G-CSF–(and other cytokine-) mediated mobilization by observing concurrent decreases in CXCL12 levels (specifically at the bone surface) and diminished expression of CXCL12 mRNA by the remaining osteoblasts.
Is inhibition of CXCR4 essential for mobilization of HSPCs? While some data support this,7 current findings may support the opposing argument that CXCR4 expression on HSPCs is critical for proper mobilization by facilitating cellular responses to changing CXCL12 gradients within BM during mobilization.8 The debate continues. In these 2 papers, Ramirez and Christopher highlight the complexity of HSPC mobilization, make substantial contributions to both its application and understanding, and hopefully will stimulate additional research into this dynamic phenomenon.
Conflict-of-interest disclosure: The authors declare no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal