In this issue of Blood, Brooks and colleagues report on a thromboresistant phenotype of venous valve sinus endothelium as compared to vein luminal endothelium.
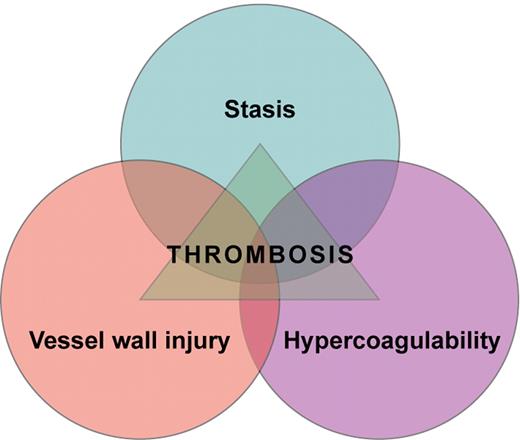
Venous thrombi are formed in the setting of low flow and low shear stress and mainly consist of fibrin strands, red blood cells, and a few platelets. In 1856, Rudolf Virchow postulated that abnormalities in blood flow, hypercoagulability of the blood, and injury to the vessel wall are causally related to thrombus formation (see figure). Reduced venous blood flow during, for instance, immobilization, prolonged bed rest, limb paresis, long-distance travel, or in obese or pregnant individuals, has been convincingly shown to increase the risk of deep vein thrombosis (DVT).1 In keeping with Virchow's concept, alterations of the coagulation system that induce a hypercoagulable state also confer an increased risk of DVT. For example, patients with high clotting factor levels have an increased risk of both a first and a recurrent DVT.2-5 Alterations of the vessel wall that lead to plaque formation and plaque rupture play a key role in the development and progression of arterial occlusive disease. However, whether the vessel wall itself plays a role in the development of venous thrombosis is less clear.
In an elegant study, Brooks et al address this important aspect. They explored the procoagulant and anticoagulant properties of the venous valve sinus endothelium in comparison to the vein lumenal endothelium.6 Compared with the vein lumenal endothelium, expression of endothelial protein C receptor (EPCR) and thrombomodulin (TM) was increased in valvular sinus endothelium while the expression of von Willebrand factor (VWF) was reduced. In other words, what they found was an up-regulation of anticoagulant (EPCR, TM) and a down-regulation of procoagulant (VWF) properties of the valvular sinus endothelium. From this observation, they conclude that valve pockets have a more thromboresistant phenotype. Along this line, they speculate that variations of this valvular sinus endothelial thromboresistance may be associated with a propensity toward venous thrombosis. This would further support the common notion that in patients with DVT of the lower limbs, thrombus formation preferentially starts in the valve pockets of the veins of the calf and then extends to the proximal veins.7
These findings are intriguing as they bring alterations of the vessel walls into focus when considering the multiple pathomechanisms leading to venous thrombosis. Nevertheless, whether a shift in the procoagulant and anticoagulant balance of particular sections of the endothelium will emerge as a novel risk factor of venous thrombosis is still an open question as there are some limitations to the study. The authors studied great saphenous veins, which were removed during coronary artery bypass crafting. Thrombosis can occur in various segments of the venous system including the cerebral sinus veins, splanchnic veins, and deep veins of the upper and lower extremities. These veins differ in flow conditions and anatomic features, mainly in the presence and number of valves. It needs to be investigated whether the findings of Brooks et al can be extrapolated to other parts of the venous system, in particular to the deep veins of the legs, where the majority of thrombotic events occur. A further limitation of the study is the rather small number of specimens investigated.
This study opens the door to further important research, as the mechanisms responsible for the procoagulant and anticoagulant properties of the endothelium are manifold. The authors studied some important components of the vessel wall, namely the anticoagulant protein C pathway and the procoagulant VWF. Many more vascular bed–specific pathways, including fibrinolysis, inflammation, and tissue factor to name just a few, should be considered in future studies.
Venous thrombosis is regarded as a multifactorial disease. However, in a substantial proportion of thrombosis patients, a risk factor of thrombosis is not detectable. It can easily be speculated that, at least in some of these patients, a shift in the balance between procoagulant and anticoagulant forces of the endothelium is responsible for their thrombotic tendency. This study should foster further research to unravel the role of the vessel wall in the pathogenesis of DVT, thereby bringing Virchow's triad finally to full realization.
Conflict-of-interest disclosure: The authors declare no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal