In this issue of Blood, Graham and colleagues demonstrate the importance of platelet dense granule secretion for in vivo platelet accumulation following laser injury, which is mediated by the SNARE protein Endobrevin/VAMP-8.
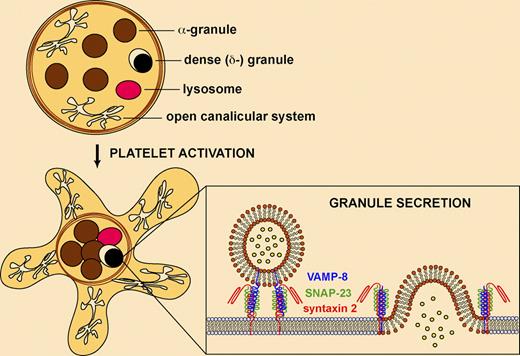
Each human platelet contains 50 to 80 α-granules, 3 to 8 dense (δ-) granules, and a few lysosomes. α-granules carry more than 300 releasable proteins, including adhesion molecules, chemokines, cytokines, fibrinolytic regulators, immunologic modulators, and an assortment of coagulation, complement, growth, and proangiogenic and antiangiogenic factors. Dense granules contain mostly small molecules, including calcium, magnesium, polyphosphate, ATP, ADP, GTP, GDP, and serotonin. Platelet granule release is important for hemostasis, because patients with inherited granule defects have bleeding problems. α-granules are absent in the gray platelet and ARC1 syndromes, while deficient δ-granules are observed in isolation, in combination with α-granule deficiency, or as part of a syndrome in the Hermansky-Pudlak, Chediak-Higashi, and Griscelli syndromes.2
Platelet granule secretion requires SNARE proteins. Platelets contain α-granules, dense (δ-) granules, and a few lysosomes. Platelet activation results in cytoskeletal rearrangements, centralization of granules, and fusion of granules with the target membrane (plasma membrane or the open canalicular system). Secretion involves the assembly of granule v-SNAREs (eg, VAMP-8) and target membrane t-SNAREs (eg, syntaxin 2, SNAP-23) into a complex allowing membrane fusion and exocytosis.
Platelet granule secretion requires SNARE proteins. Platelets contain α-granules, dense (δ-) granules, and a few lysosomes. Platelet activation results in cytoskeletal rearrangements, centralization of granules, and fusion of granules with the target membrane (plasma membrane or the open canalicular system). Secretion involves the assembly of granule v-SNAREs (eg, VAMP-8) and target membrane t-SNAREs (eg, syntaxin 2, SNAP-23) into a complex allowing membrane fusion and exocytosis.
The molecular mechanisms involved in platelet granule secretion are complex and incompletely understood.3,–5 Platelet activation involves several rapid events, including shape change, aggregation, and granule secretion, and it is difficult to discern the cellular mechanisms involved. Electron microscopy studies have revealed that platelet shape changes are accompanied by cytoskeletal rearrangements and centralization of granules. Platelet exocytosis involves fusion of granules with the plasma membrane or the open canalicular system (extensive channels of internal membranes that communicate with the outer surface) to release their contents into the extracellular space (see figure). Fusion of granule vesicles requires soluble N-ethylmaleimide–sensitive attachment protein receptors (SNAREs), lipid components, and SNARE regulatory proteins (eg, NSF, Sec1/Munc18 proteins).6 SNAREs from opposing membranes assemble into a complex consisting of parallel 4-helix bundles, which catalyzes the apposition and fusion of the vesicle with the target membrane. Platelets contain vesicle-membrane SNAREs (v-SNAREs), including VAMP-2, -3, -7, and -8, and target-membrane SNAREs (t-SNAREs), including syntaxin 2, 4, 7, and 11, and SNAP-23, -25 and -29.4 Studies have shown that syntaxin 2 and SNAP-23 are involved in all 3 platelet granule secretion events, whereas syntaxin 4 mediates α-granule and lysosome release. VAMP-8 is the primary v-SNARE required for secretion of all platelet granules.7
The studies by Graham et al in this issue of Blood reveal the importance of dense granule secretion for in vivo platelet accumulation following laser injury of cremaster arterioles.8 The most striking observation is that dense granule deficient mice (ruby-eye mice) show almost no platelet accumulation at injury sites, indicating that molecules released by dense granules are necessary for efficient thrombus formation. In VAMP-8−/− mice, thrombus formation is delayed and decreased (but not absent) following laser injury, suggesting that the v-SNARE Endobrevin/VAMP-8 is an important component of in vivo platelet granule secretion. The incomplete inhibition of platelet accumulation at sites of injury in VAMP-8−/− mice was somewhat surprising because VAMP-8 mediates the release of all platelet granules, hence an additive effect might have been expected. The results can be explained by functional redundancy of SNAREs, whereby VAMP-2 or -3 can mediate granule release in the absence of VAMP-8.7 The authors observe that VAMP-2 and VAMP-8 levels are comparable in mouse platelets, whereas VAMP-3 and VAMP-8 levels are comparable in human platelets. As VAMP-2 levels are greatly diminished in human platelets, this suggests that the major alternative granule v-SNARE in humans is VAMP-3.
The dense granule constituents responsible for mediating platelet accumulation in vivo were examined in vitro via aggregation studies of plasma-free washed platelets. Compared with wild-type, platelets from VAMP-8−/− and ruby-eye mice required 1.5- to 10-fold higher concentrations of thrombin for complete aggregation, while addition of exogenous ADP produced normal aggregation responses at low thrombin concentrations. The function of PKCα−/− mouse platelets (which have impaired dense granule biogenesis and defective secretion of both dense and α-granules) can also be restored by ADP in vitro.9 Thus, ADP is a key dense granule component, and its release during activation is likely required for efficient platelet accumulation at vascular injury sites. The thrombus formation defects observed in ruby-eye mice indicate that in vivo thrombin generation following laser injury cannot compensate for the absence of dense granule secretion.
Although these studies provide important new insights into the role of platelet granule secretion in vivo, some questions do remain. For example, which SNAREs and regulatory proteins control selective secretion from granules, including the recently discovered distinct populations of α-granules?10 What contribution does α-granule secretion make to thrombus formation in vivo? The availability of megakaryocyte-restricted gene knockout mice will undoubtedly shed light on some of these questions. Importantly, the current studies suggest that antiplatelet therapies targeting granule secretion could attenuate acute thrombus formation and also reduce the development of chronic atherosclerotic lesions by inhibiting the release of inflammatory and immune-modulating factors from platelets.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal