Bortezomib's unprecedented antitumor activity in myeloma has long been attributed to NF-κB inhibition. A new study from the group that put the drug on the map directly challenges this assumption.
Bortezomib (Velcade, formerly PS-341) is a peptide boronate inhibitor of the proteasome that was developed for cancer therapy by Julian Adams, Peter Elliott, and their colleagues.1 At the time, this was considered a very bold move, since loss of proteasome function was thought to be incompatible with viability in normal cells. However, using a novel assay that directly measures 20S proteasome activity, they demonstrated that rodents and primates tolerated doses of bortezomib resulting in up to 80% proteasome inhibition without obvious toxicity. They also used this assay to guide dose escalation in cancer patients in phase 1 clinical trials in solid and hematologic malignancies.1 Although single-agent activity was modest in most tumors, Anderson's group led a phase 2 clinical trial that showed that bortezomib was active in relapsed refractory multiple myeloma (MM),2 leading to FDA approval of the drug for the treatment of MM in 2003.
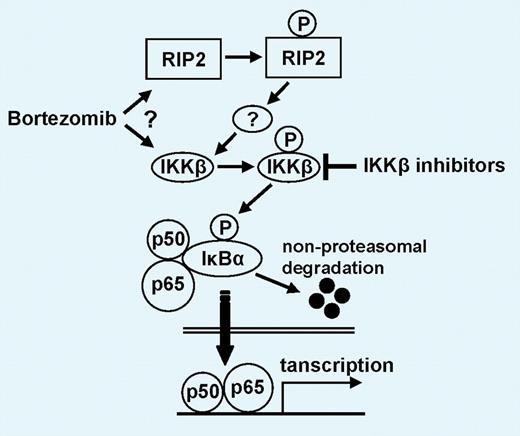
Possible mechanism whereby bortezomib triggers canonical NF-κB activation. Bortezomib either directly or indirectly (via RIP2) activates IKKβ, which subsequently phosphorylates IκBα, an inhibitor of p50/p65. After nonproteasomal degradation of IκBα, p50/p65 translocates to nucleus. IKKβ inhibitors block down-regulation of IκBα and NF-κB activity as well as enhance bortezomib-induced cytotoxicity. See the complete figure in the article beginning on page 1046.
Possible mechanism whereby bortezomib triggers canonical NF-κB activation. Bortezomib either directly or indirectly (via RIP2) activates IKKβ, which subsequently phosphorylates IκBα, an inhibitor of p50/p65. After nonproteasomal degradation of IκBα, p50/p65 translocates to nucleus. IKKβ inhibitors block down-regulation of IκBα and NF-κB activity as well as enhance bortezomib-induced cytotoxicity. See the complete figure in the article beginning on page 1046.
Although it was assumed from the start that bortezomib would have diverse effects on cancer cell biology, the most common mechanism attributed to its antitumor actions was inhibition of the inflammation-associated transcription factor, NF-κB. Work performed by several groups implicated NF-κB activation in the maintenance of cancer cell survival,3 and other studies demonstrated that conventional cancer therapies also commonly activated NF-κB, undermining their therapeutic potential.4 Preclinical studies and clinical trials confirmed that bortezomib down-regulated tumor cell expression of known NF-κB transcriptional targets (IL-6, etc), consistent with the hypothesis. Furthermore, a more recent high-profile study demonstrated that the subset of primary MMs that possesses activating mutations within the so-called noncanonical NF-κB pathway was especially sensitive to bortezomib,5 adding further support to the concept.
A cautionary note was introduced by work performed several years ago, showing that NF-κB inhibition did not fully account for bortezomib's cytotoxic effects in MM cells.6 Nonetheless, it is still generally accepted that bortezomib is a potent and general NF-κB inhibitor and that these effects contribute to cytotoxicity. In this issue of Blood, the new work by Hideshima et al turns this idea on its head.7 Using human MM cell lines and primary tumor specimens, they now show that bortezomib actually activates 2 upstream NF-kB–activating kinases (RIP2 and IKKβ), promotes down-regulation of NF-κB's inhibitor (IκBα), and increases NF-κB DNA binding in vitro (see figure). Another structurally unrelated proteasome inhibitor (lactacystin) induces the same effects, strongly suggesting that NF-κB activation is an “on-target” effect of the drug. Furthermore, they present evidence that bortezomib also fails to block NF-κB in vivo, determined by measuring nuclear localization of NF-κB's RelA/p65 subunit.7 Exposure of cells to bortezomib in the presence of a selective IKK antagonist (MLN120B) blocks NF-κB activation and promotes cell death. Together, these results provide compelling evidence that NF-κB inhibition is probably irrelevant to the effects of bortezomib in MM cells.
Why did it take so long for this long-held assumption to be overturned? One reason is that bortezomib's documented NF-κB inhibitory effects were almost always measured in cells exposed to cytokines that are known to promote proteasome-dependent degradation of IκBα (ie, TNFα), while investigators largely ignored bortezomib's lack of inhibitory effects on basal NF-κB activity. In addition, the possibility that nonproteasomal degradation of IκBα might contribute to NF-κB activation was not explored. So, if NF-κB inhibition is not involved in bortezomib's cytotoxic effects, what other mechanisms might explain its unique potency in MM cells? One attractive explanation (and the simplest) is that cell death results from protein build-up and aggregation,8,9 as is the case in neurodegenerative diseases.10 In this model, the high levels of immunoglobulin production and ER-Golgi protein transport would sensitize MM cells to proteotoxic stress, providing an attractive explanation for bortezomib's clinical activity and a potential means of identifying bortezomib-based combination approaches that will display even greater antitumor effects.
Conflict-of-interest disclosure: D.J.M. has received honoraria from Millennium Pharmaceuticals and Proteolix and research support from Nereus Pharmaceuticals and Astra-Zeneca. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal