Bortezomib is a proteasome inhibitor with remarkable preclinical and clinical antitumor activity in multiple myeloma (MM) patients. The initial rationale for its use in MM was inhibition of nuclear factor (NF)-κB activity by blocking proteasomal degradation of inhibitor of κBα (IκBα). Bortezomib inhibits inducible NF-κB activity; however, its impact on constitutive NF-κB activity in MM cells has not yet been defined. In this study, we demonstrate that bortezomib significantly down-regulated IκBα expression and triggered NF-κB activation in MM cell lines and primary tumor cells from MM patients. Importantly, no inhibition of p65 (RelA) nuclear translocation was recognized after bortezomib treatment in a murine xenograft model bearing human MM cells. Bortezomib-induced NF-κB activation was mediated via the canonical pathway. Moreover, other classes of proteasome inhibitors also induced IκBα down-regulation associated with NF-κB activation. Molecular mechanisms whereby bortezomib induced IκBα down-regulation were further examined. Bortezomib triggered phosphorylation of IκB kinase (IKKβ) and its upstream receptor-interacting protein 2, whereas IKKβ inhibitor MLN120B blocked bortezomib-induced IκBα down-regulation and NF-κB activation, indicating receptor-interacting protein 2/IKKβ signaling plays crucial role in bortezomib-induced NF-κB activation. Moreover, IKKβ inhibitors enhanced bortezomib-induced cytotoxicity. Our studies therefore suggest that bortezomib-induced cytotoxicity cannot be fully attributed to inhibition of canonical NF-κB activity in MM cells.
Introduction
Multiple myeloma (MM) is a malignant plasma cell proliferation in the bone marrow (BM) associated with monoclonal protein in the serum and/or urine. It has a prevalence of 50 000 patients in the United States, occurring in approximately 16 000 new individuals each year.1 The BM microenvironment plays a crucial role in MM cell pathogenesis. Specifically, adhesion of tumor cells to both BM cellular components and cytokines trigger signaling cascades mediating MM cell proliferation, survival, drug resistance, and migration, including the following: phosphatidylinositide-3 kinase/Akt (also known as protein kinase B); Ras/Raf/mitogen-activated protein kinase kinase/extracellular signal-related kinase; Janus kinase 2/signal transducers and activators of transcription 3; and nuclear factor (NF)-κB cascades.2 Although MM remains incurable, novel agents targeting MM cells in the BM milieu, such as thalidomide, lenalidomide, and bortezomib, when used alone or in combination, can overcome conventional drug resistance and improve patient outcome
NF-κB is a member of the Rel family proteins, including RelA (p65), RelB, c-Rel, p50 (NFκB1), and p52 (NFκB2), which regulates protein expression mediating cell cycle/proliferation, antiapoptosis, and cytokine secretion.3 It is typically a heterodimer composed of p50 and p65 subunits and constitutively present in the cytosol and nucleus. In the cytosol, NF-κB is inactivated by its association with family inhibitor of κBα (IκB),4 which therefore has a crucial role in regulating NF-κB activation. After stimulation (ie, tumor necrosis factor [TNF]–α), IκBα is phosphorylated by IκB kinases (IKKs) followed by its proteasomal degradation, thereby allowing nuclear translocation of NF-κB via either canonical or noncanonical cascades. Although the precise role of NF-κB activation in the pathogenesis of MM has not been fully characterized, we have previously shown that MM cell adhesion to BM stromal cells (BMSCs) induces NF-κB–dependent up-regulation of interleukin-6 transcription.5 In addition, we have shown that intracellular adhesion molecule-1 (CD54) and vascular cell adhesion molecule-1 (CD106) expression on both MM cells and BMSCs are regulated by NF-κB.
Bortezomib is a 26S proteasome inhibitor that was approved by the Food and Drug Administration in 2003, 2005, and 2008 for the treatment of relapsed/refractory, relapsed, and newly diagnosed MM, respectively.6,–8 Because IκBα is a substrate of the proteasome, the initial rationale for use of bortezomib in MM was inhibition of NF-κB activity. Although 20S proteasome activity in peripheral blood mononuclear cells (PBMCs) is inhibited in phase 1 studies,9 to date bortezomib-induced NF-κB inhibition in patient MM cells has not yet been shown. In this study, we therefore examined whether the growth-inhibitory effect of bortezomib was associated with canonical NF-κB inhibition in MM cells.
Methods
Cells
MM cell lines were obtained from ATCC or the German Collection of Microorganisms and Cell Cultures and maintained, as previously described.10 After Dana-Farber Cancer Institute Institutional Review Board approval and informed consent in accordance with the Declaration of Helsinki protocol, BM specimens were obtained from patients with MM. Primary CD138+ plasma cells were obtained using negative selection as previously described.11
Reagents
Bortezomib was commercially obtained from Millennium Pharmaceuticals Inc. IKKβ-specific inhibitors PS-114512 and MLN120B13,14 were provided by Millennium Pharmaceuticals Inc. Lactacystin, Z-VAD-FMK, N-(4-aminobutyl)-5-chloro-2-naphthalenesulfonamide (W-13), Na-tosyl-phe chloromethyl ketone, 1,2-bis(o-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid tetra (acetoxymethyl) ester, N-acetyl-L-cysteine (LNAC), SP600125, calpeptine, okadaic acid, granzyme inhibitors (I and IV), and BMS345541 were purchased from Calbiochem. Z-DEVD-FMK was purchased from MBL. TNFα was purchased from R&D Systems.
Electrophoretic mobility shift analysis
Electrophoretic mobility shift analysis (EMSA) was carried out for detection of NF-κB activity, as previously described.15 Briefly, nuclear extracts from MM cells were obtained using a nuclear extraction kit (Panomics). Double-stranded NF-κB and Oct-1 consensus oligonucleotide probes (Promega) were end labeled with [γ32P] adenosine triphosphate (10 mCi/mL; PerkinElmer). Binding reactions containing 0.035 pmol/μL oligonucleotide and 8 μg of nuclear protein were conducted at room temperature for 20 minutes in binding buffer (10 mM Tris-HCl, pH 7.5, 50 mM NaCl, 1 mM MgCl2, 0.5 mM ethylenediaminetetraacetic acid, 0.5 mM dithiothreitol, 4% glycerol [vol/vol], and 0.5 μg poly[dI-dC]; Pharmacia). The samples were loaded onto a 4% polyacrylamide gel, transferred to Whatman paper (Whatman), and visualized by autoradiography. For supershift analysis, 1 mg of anti-p65, RelB, c-Rel (Santa Cruz Biotechnology), p50 (ABcam), or p52 (Rockland) antibodies (Abs) was incubated for 5 minutes before adding the reaction mixtures.
Preparation of biotinylated probes and hybridization on microarrays
RPMI 8226 cells were incubated with 20 nM bortezomib for 6 hours. mRNA was extracted using TRIzol reagent (Invitrogen), according to manufacturer's protocol. Gene expression profiling was performed and analyzed, as previously described.16
Cell proliferation assay
MM cell growth was assessed by measuring 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) dye absorbance, as described previously.11 DNA synthesis was measured by bromodeoxyuridine (BrdU) cell proliferation assay kit (Calbiochem), according to the manufacturer's protocol. All experiments were performed 3 times in quadruplicate.
Immunoblotting and immunoprecipitation
Immunoblotting was performed, as previously described.11,15 Antibodies included: anti-phospho (p)-receptor-interacting protein 2 (RIP2; Ser176), TNF receptor-associated factor (TRAF) 6, p-TGT-β–activated kinase 1 (Ser412), p-IKKα/β (Ser176/180), p-p65 (Ser536), p-IκBα (Ser32/36), IκBα, and β-catenin (Cell Signaling Technology); as well as anti-RIP2, p65, p50, p52, RelB, c-Rel, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH; Santa Cruz Biotechnology).
Immunohistochemistry
Immunohistochemistry was performed, as described previously.17 The anti-human p65 Ab was obtained from Cell Signaling Technology. The primary Abs were visualized with the corresponding biotinylated Ab coupled to streptavidin-peroxidase complex (Vector Laboratories). All Abs, conditions, and reactivities were tested in positive control slides. Histologic micrographs were taken using a Leica DM200 microscope (aperture HC PLANs 10×/22, objective lenses: N PLAN 100×/1.25 oil) and a SPOT Insight QE Model camera with SPOT Advanced acquisition software (Diagnostic Instruments).
Real-time quantitative polymerase chain reaction
IκBα gene expression was examined by real-time polymerase chain reaction (PCR) and normalized to expression of GAPDH, as previously described.18 Briefly, total RNA was extracted from untreated or bortezomib-treated RPMI 8226 cells using the RNeasy kit and RNase-free DNase set (QIAGEN). cDNA was synthesized using the first-strand cDNA synthesis kit (SuperArray Bioscience). Quantitative real-time PCR was carried out in a 7500 real-time PCR system (Applied Biosystems). Gene-specific oligonucleotide primers (Invitrogen) included the following: GAPDH, forward 5′-AATCCCATCACCATCTTCCA-3′ and reverse 5′-TGGACTCCACGACGTACTCA-3′; as well as IκBα, forward 5′-ATGATGTTGACCTTTCCAGGG-3′ and reverse 5′-GCAACTGATGAAAAGTTACAGAA-3′. Data represent mean plus or minus SD of triplicate wells.
Xenograft murine model of human MM
To assess in vivo activity, severe combined immunodeficiency mice bearing human MM cells were treated with bortezomib (1 mg/kg), as in previous studies.19,20 Twenty-four hours after bortezomib injection, tumor samples were excised for immunohistochemical analysis. All animal studies were conducted according to protocols approved by the Animal Ethics Committee of Dana-Farber Cancer Institute.
Statistical analysis
Statistical significance of differences observed in drug-treated versus control cultures was determined using the Wilcoxon signed-rank test. The minimal level of significance was P value less than .05.
Results
Bortezomib down-regulates IκBα expression
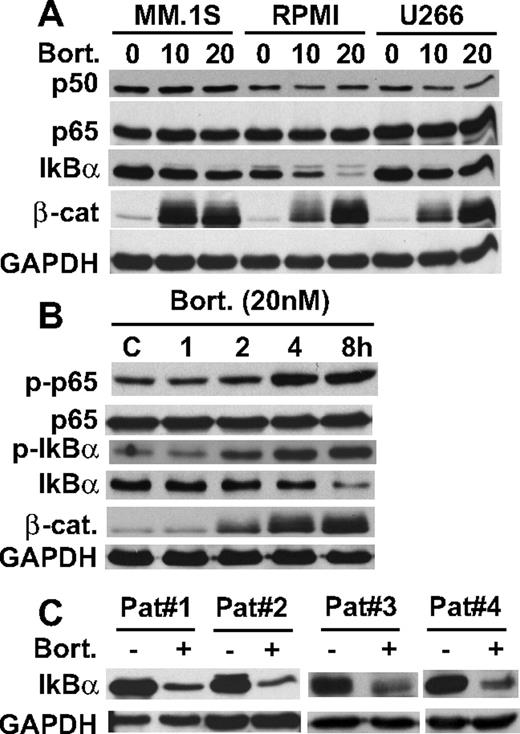
Proteasomes play a major role in protein catabolism; conversely, their inhibitors induce accumulation of ubiquitinated proteins. Although IκBα is a substrate of the proteasome, we unexpectedly showed down-regulation of IκBα protein expression in MM.1S, RPMI 8226, and U266 MM cell lines induced by bortezomib treatment in a dose-dependent fashion, without alteration of p50 or p65 expression (Figure 1A). Among these MM cell lines, RPMI 8226 showed most significant down-regulation of IκBα triggered by bortezomib. Inhibition of proteasome activity was confirmed by accumulation of a known proteasome substrate, β-catenin (Figure 1A). Because both phosphorylation and ubiquitination are required for proteasomal degradation of IκBα, we next examined whether bortezomib triggers phosphorylation of IκBα in RPMI 8226 cells. Bortezomib treatment in a time- and dose-dependent fashion markedly increased phosphorylation of IκBα. Bortezomib also triggers p65 (RelA) phosphorylation (Figure 1B), suggesting that bortezomib activated their upstream molecule IKKβ.21 Importantly, bortezomib also triggered IκBα down-regulation in primary tumor cells from patients with MM (Figure 1C). We next determined whether bortezomib-induced IκBα down-regulation occurred at a transcriptional or posttranscriptional level. In contrast to decreased protein levels, real-time reverse transcription-PCR showed that bortezomib in a dose-dependent fashion significantly increased mRNA level of IκBα in RPMI 8226 cells (supplemental Figure 1A, available on the Blood website; see the Supplemental Materials link at the top of the online article). These data indicate that bortezomib-induced down-regulation of IκBα is posttranscriptional. These results suggest that bortezomib blocks TNFα-induced,22,23 but down-regulates constitutive, IκBα expression in MM cells.
Bortezomib down-regulates IκBα expression. (A) MM.1S, RPMI 8226, and U266 cells were cultured with bortezomib (10 nM and 20 nM) for 8 hours. (B) RPMI 8226 cells were cultured with bortezomib (20 nM) for the indicated time periods. (C) Primary tumor cells from MM patients were cultured with bortezomib (20 nM) for 8 hours. Whole-cell lysates (A-C) were immunoblotted with indicated Abs.
Bortezomib down-regulates IκBα expression. (A) MM.1S, RPMI 8226, and U266 cells were cultured with bortezomib (10 nM and 20 nM) for 8 hours. (B) RPMI 8226 cells were cultured with bortezomib (20 nM) for the indicated time periods. (C) Primary tumor cells from MM patients were cultured with bortezomib (20 nM) for 8 hours. Whole-cell lysates (A-C) were immunoblotted with indicated Abs.
Bortezomib activates canonical NF-κB pathway in MM cell lines
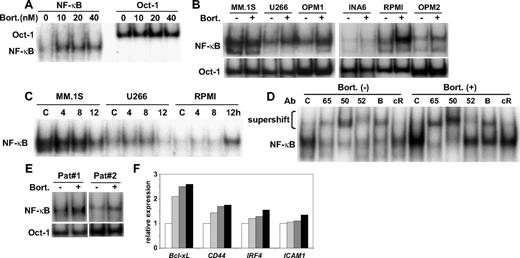
We next examined whether down-regulation of IκBα altered NF-κB activity in MM cell lines. Consistent with down-regulation of IκBα, bortezomib markedly enhanced NF-κB activity in a dose-dependent fashion in RPMI 8226 cells (Figure 2A). Importantly, bortezomib (20 nM for 8-hour treatment) triggered NF-κB activation in wide range of MM cell lines (Figure 2B). Time course studies showed that the pattern of NF-κB activation by bortezomib also varied between each cell line (Figure 2C). For example, NF-κB activation triggered by bortezomib peaked at 8 hours in U266 cells, whereas no NF-κB activation was triggered by bortezomib in MM.1S cells. NF-κB activation is mediated via both canonical and noncanonical pathways, and we hypothesized that bortezomib triggered canonical NF-κB activation, because IκBα is a major inhibitor of p50/65 nuclear translocation. Supershift assays confirmed that bortezomib triggered canonical NF-κB activation, evidenced by markedly enhanced supershifted bands in the presence of anti-p65 and p50 Abs in RPMI 8226 cells (Figure 2D). Bortezomib also activated NF-κB in patient MM cells (Figure 2E). Moreover, expression of known NF-κB–targeted genes (Bcl-xL, CD44 IRF4, and ICAM1) was up-regulated by bortezomib treatment (Figure 2F). In contrast, bortezomib markedly inhibited conversion of p100 to p52 (supplemental Figure 2A) and its nuclear translocation in MM.1S cells (supplemental Figure 2B), indicating that bortezomib could inhibit the noncanonical NF-κB activation pathway in cells expressing p100/p52 (supplemental Figure 2C).
Bortezomib activates NF-κB in MM cell lines. (A) RPMI 8226 cells were cultured with increasing doses (10-40 nM) of bortezomib for 8 hours. (B) MM.1S, U266, OPM1, INA6, RPMI 8226, and OPM2 cells were treated with bortezomib (20 nM) for 8 hours. (C) MM.1S, U266, and RPMI 8226 cells were treated with bortezomib (10 nM) for indicated time periods. (D) RPMI 8226 cells were treated with bortezomib (20 nM) for 8 hours. Nuclear extracts were subjected to EMSA (A-C) or supershift assay (D) using anti-p65, (65) p50, (50) p52, (52) RelB (B), and cRel (cR) Abs. Oct-1 served as a loading control for EMSA. Exposure time of autoradiography varied for each cell line. (E) Alteration of gene expression after bortezomib treatment (20 nM, 6 hours) in RPMI 8226 cells. (F) RPMI8226 cells were cultured with DMSO control medium (□) or bortezomib (20 nM) for 1 hour ( ), 3 hours (
), 3 hours ( ) and 6 hours (■). Gene expression of Bcl-xL, CD44, IRF4, and ICAM1 was analyzed by DNA microarray.
) and 6 hours (■). Gene expression of Bcl-xL, CD44, IRF4, and ICAM1 was analyzed by DNA microarray.
Bortezomib activates NF-κB in MM cell lines. (A) RPMI 8226 cells were cultured with increasing doses (10-40 nM) of bortezomib for 8 hours. (B) MM.1S, U266, OPM1, INA6, RPMI 8226, and OPM2 cells were treated with bortezomib (20 nM) for 8 hours. (C) MM.1S, U266, and RPMI 8226 cells were treated with bortezomib (10 nM) for indicated time periods. (D) RPMI 8226 cells were treated with bortezomib (20 nM) for 8 hours. Nuclear extracts were subjected to EMSA (A-C) or supershift assay (D) using anti-p65, (65) p50, (50) p52, (52) RelB (B), and cRel (cR) Abs. Oct-1 served as a loading control for EMSA. Exposure time of autoradiography varied for each cell line. (E) Alteration of gene expression after bortezomib treatment (20 nM, 6 hours) in RPMI 8226 cells. (F) RPMI8226 cells were cultured with DMSO control medium (□) or bortezomib (20 nM) for 1 hour ( ), 3 hours (
), 3 hours ( ) and 6 hours (■). Gene expression of Bcl-xL, CD44, IRF4, and ICAM1 was analyzed by DNA microarray.
) and 6 hours (■). Gene expression of Bcl-xL, CD44, IRF4, and ICAM1 was analyzed by DNA microarray.
We next examined whether other classes of proteasome inhibitors could down-regulate IκBα expression. Similar to bortezomib, lactacystin significantly down-regulated IκBα protein expression in RPMI 8226 cells, associated with increased IκBα phosphorylation and accumulation of ubiquitinated proteins (supplemental Figure 3A). Similar results were observed after MG132 treatment (data not shown). Moreover, lactacystin markedly enhanced NF-κB activation in a dose-dependent fashion (supplemental Figure 3B). These results indicate that other classes of proteasome inhibitors also down-regulate IκBα, associated with enhanced NF-κB activation.
Bortezomib-induced cytotoxicity in MM cells is not associated with NF-κB inhibition
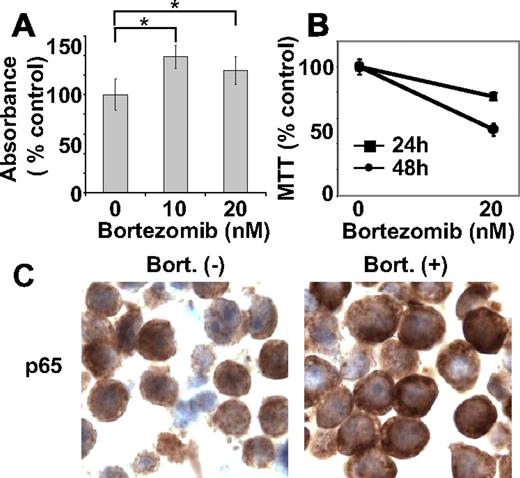
Because NF-κB can modulate cell survival and/or proliferation, we next examined whether bortezomib-induced NF-κB activation was associated with cell proliferation, evaluated by BrdU incorporation assay. RPMI 8226 cells treated with bortezomib (10 nM and 20 nM for 8 hours) showed significantly higher (P < .01) absorbance compared with control cells. For example, 10 nM and 20 nM bortezomib induced 39% and 25% increases in BrdU incorporation, respectively (Figure 3A), indicating that bortezomib did not inhibit cell proliferation during this time period. We have shown that bortezomib shows significant antitumor effects against MM cells in vitro,16,22,24 in vivo using xenograft mouse models,19 and in patients.6,7 Indeed, treatment of RPMI 8226 cells with bortezomib (20 nM for 8 hours), followed by washout and culture for another 24 hours and 48 hours, triggered cytotoxicity (Figure 3B). These results indicate that cells are already committed to death within 8-hour treatment with bortezomib, even though NF-κB is activated. We also examined NF-κB activity in RPMI 8226 cells in vivo using a mouse xenograft model. Importantly, treatment with bortezomib (1.0 mg/kg) did not inhibit p65 nuclear translocation in MM cells (Figure 3C). Taken together, our results suggest that bortezomib-induced antitumor activities in RPMI 8226 cells may not be mediated by NF-κB inhibition.
Bortezomib induces DNA synthesis. (A) RPMI 8226 cells were treated with bortezomib (10 and 20 nM) for 8 hours. Cells were labeled with BrdU for an additional 2 hours and assessed for growth by DNA synthesis. (B) RPMI 8226 cells were incubated with bortezomib (20 nM) for 8 hours. Cells were washed with media to remove bortezomib, and then cultured for another 24 hours or 48 hours. Cell survival was determined by MTT assay. (C) Immunohistochemical analysis for p65 expression was performed on tumor tissue from RPMI 8226 xenograft mice, untreated and treated with bortezomib. *P < .01.
Bortezomib induces DNA synthesis. (A) RPMI 8226 cells were treated with bortezomib (10 and 20 nM) for 8 hours. Cells were labeled with BrdU for an additional 2 hours and assessed for growth by DNA synthesis. (B) RPMI 8226 cells were incubated with bortezomib (20 nM) for 8 hours. Cells were washed with media to remove bortezomib, and then cultured for another 24 hours or 48 hours. Cell survival was determined by MTT assay. (C) Immunohistochemical analysis for p65 expression was performed on tumor tissue from RPMI 8226 xenograft mice, untreated and treated with bortezomib. *P < .01.
Inhibitors are not able to block bortezomib-induced IκBα down-regulation
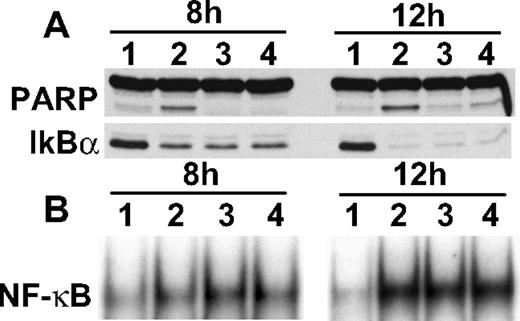
To determine whether proteases are involved in bortezomib-induced IκBα down-regulation, we next cultured RPMI 8226 cells with bortezomib in the presence and absence of inhibitors against proteases and kinases, including chymotrypsin, calmodulin, calpain, protein kinase C, granzyme, and c-Jun N-terminal kinase. Importantly, none of these inhibitors blocked bortezomib-induced IκBα down-regulation (supplemental Figure 4). Because previous studies have shown that cysteine protease caspases cleave/down-regulate many proteins in MM cells,16,18,25 we examined whether caspase inhibitors block bortezomib-induced IκBα down-regulation. Although pan-caspase inhibitors (Z-VAD-FMK and Z-DEVD-FMK) inhibited bortezomib-induced poly(adenosine 5′-diphosphate-ribose) polymerase cleavage, they did not block either IκBα down-regulation or NF-κB activation triggered by bortezomib (Figure 4A). We did show that IκBα down-regulation and NF-κB activation induced by bortezomib were inhibited by LNAC (supplemental Figure 5), consistent with a prior report that LNAC inhibits bortezomib-induced cytotoxicity in leukemia cells.26 Interestingly, bortezomib-induced phosphorylation of IκBα was further enhanced in a dose-dependent manner by protein phosphatase inhibitor okadaic acid (OA), associated with significant down-regulation of IκBα (data not shown). Taken together, these results suggest that IκBα down-regulation is not mediated by proteases.
Neither caspase nor c-Jun N-terminal kinase inhibitors block bortezomib-induced IκBα down-regulation. (A) RPMI 8226 cells were cultured with bortezomib (20 nM for 8 hours and 12 hours) in the presence of control media (lane 2), Z-VAD-FMK (lane 3), or Z-DEVD-FMK (lane 4). Whole-cell lysates were subjected to immunoblotting with indicated Abs (A), and nuclear extracts were subjected to EMSA (B).
Neither caspase nor c-Jun N-terminal kinase inhibitors block bortezomib-induced IκBα down-regulation. (A) RPMI 8226 cells were cultured with bortezomib (20 nM for 8 hours and 12 hours) in the presence of control media (lane 2), Z-VAD-FMK (lane 3), or Z-DEVD-FMK (lane 4). Whole-cell lysates were subjected to immunoblotting with indicated Abs (A), and nuclear extracts were subjected to EMSA (B).
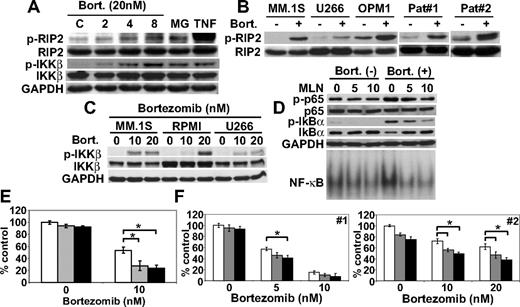
Bortezomib triggers phosphorylation of RIP2 and IKKβ; conversely, inhibition of IKKβ blocks bortezomib-induced IκBα down-regulation and NF-κB activation
We have previously shown that canonical NF-κB pathway in MM cells is activated by TNFα,12,23 CD40,27 and B cell–activating factor.10 Because bortezomib triggered NF-κB activation via canonical pathway mediated via phosphorylation and down-regulation of IκBα, we next examined upstream molecules involved in this process. Although neither TRAF6 nor phosphorylation of TGF-β–associated kinase 1 (TAK1) was altered (data not shown), phosphorylation of IKKβ and its upstream RIP2 was induced by bortezomib treatment in a time-dependent fashion. MG132 similarly induced phosphorylation of RIP2 and IKKβ (Figure 5A). Bortezomib also induced phosphorylation of RIP2 in MM.1S, U266, OPM1 cell lines, as well as primary tumor cells (Figure 5B). Consistent with RIP2, bortezomib also triggered IKKβ phosphorylation in MM.1S and U266 cells in a dose-dependent fashion (Figure 5C). To confirm the role of IKKβ in IκBα down-regulation, RPMI 8226 cells were cultured with bortezomib in the presence and absence of specific IKKβ inhibitor MLN120B. MLN120B inhibited both phosphorylation and protein expression of IκBα in a dose-dependent fashion. Moreover, enhanced phosphorylation (Ser536) of p65 triggered by bortezomib was also inhibited by MLN120B. NF-κB activation induced by bortezomib was blocked by MLN120B (Figure 5D), further confirming that activation of IKKβ plays a key role in bortezomib-induced NF-κB activation. Moreover, inhibition of canonical NF-κB activity by MLN120B enhanced bortezomib-induced cytotoxicity in RPMI 8226 cells (Figure 5E). Importantly, MLN120B also significantly enhanced bortezomib-induced cytotoxicity against primary tumor cells from MM patients (Figure 5F). To confirm that this effect was due to specific inhibition of IKKβ, we also used other IKKβ inhibitors (PS-114512,28 and BMS-24554129 ). Consistent with MLN120B, these agents also enhanced bortezomib-induced cytotoxicity in a dose-dependent fashion (supplemental Figure 6).
Bortezomib triggers IKKβ phosphorylation; conversely, inhibition of IKKβ blocks bortezomib-induced IκBα down-regulation and NF-κB activation. (A) RPMI 8226 cells were cultured with bortezomib (20 nM) or MG132 (MG, 0.5 μM). TNFα (TNF, 5 ng/mL) served as a positive control for p-RIP2 and p-IKKβ. (B) MM.1S, U266, and OPM1 MM cell lines, as well as primary tumor cells from MM patients, were cultured with bortezomib (20 nM for 8 hours). (C) MM.1S, RPMI 8226, and U266 cells were cultured with bortezomib (10 nM and 20 nM) for 8 hours. (D) RPMI 8226 cells were cultured with bortezomib (20 nM) for 8 hours in the presence (5 and 10 μM) or absence of MLN120B (MLN). Whole-cell lysates and nuclear extracts were immunoblotted with indicated Abs and EMSA, respectively. RPMI 8226 cells (E) and primary tumor cells (F) from MM patients (no. 1 and 2) were cultured with bortezomib (10 nM) for 24 hours, in the presence (5 μM,  ; 10 μM, ■) or absence (□) of MLN120B, and cell growth was assessed by MTT assay. * P < .01.
; 10 μM, ■) or absence (□) of MLN120B, and cell growth was assessed by MTT assay. * P < .01.
Bortezomib triggers IKKβ phosphorylation; conversely, inhibition of IKKβ blocks bortezomib-induced IκBα down-regulation and NF-κB activation. (A) RPMI 8226 cells were cultured with bortezomib (20 nM) or MG132 (MG, 0.5 μM). TNFα (TNF, 5 ng/mL) served as a positive control for p-RIP2 and p-IKKβ. (B) MM.1S, U266, and OPM1 MM cell lines, as well as primary tumor cells from MM patients, were cultured with bortezomib (20 nM for 8 hours). (C) MM.1S, RPMI 8226, and U266 cells were cultured with bortezomib (10 nM and 20 nM) for 8 hours. (D) RPMI 8226 cells were cultured with bortezomib (20 nM) for 8 hours in the presence (5 and 10 μM) or absence of MLN120B (MLN). Whole-cell lysates and nuclear extracts were immunoblotted with indicated Abs and EMSA, respectively. RPMI 8226 cells (E) and primary tumor cells (F) from MM patients (no. 1 and 2) were cultured with bortezomib (10 nM) for 24 hours, in the presence (5 μM,  ; 10 μM, ■) or absence (□) of MLN120B, and cell growth was assessed by MTT assay. * P < .01.
; 10 μM, ■) or absence (□) of MLN120B, and cell growth was assessed by MTT assay. * P < .01.
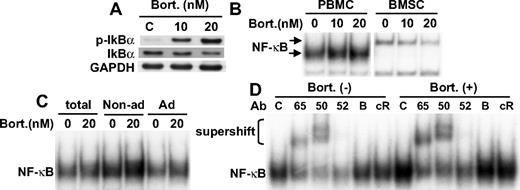
Bortezomib activates NF-κB in PBMCs
We next examined whether bortezomib similarly induced IκBα down-regulation and NF-κB activation in PBMCs and BMSCs. As in MM cell lines, bortezomib induced phosphorylation and down-regulation of IκBα in PBMCs in a dose-dependent fashion (Figure 6A). Consistent with previous studies,23 bortezomib markedly inhibited NF-κB activity in BMSCs. In contrast, bortezomib in a dose-dependent fashion significantly enhanced NF-κB activity in PBMCs (Figure 6B). No significant difference in NF-κB activation triggered by bortezomib was observed in nonadherent versus adherent cell populations (Figure 6C). Supershift assays indicated that bortezomib induced canonical NF-κB pathway, evidenced by increased intensity of p50 and p65 supershifted bands (Figure 6D). These results demonstrate that bortezomib also triggers NF-κB activation in normal cell populations.
Bortezomib activates NF-κB in normal healthy donor-derived PBMCs. (A) PBMCs were cultured with bortezomib (10 nM and 20 nM) for 8 hours. Whole-cell lysates were immunoblotted with indicated Abs. (B) PBMCs and BMSCs from MM patient were cultured with bortezomib (10 nM and 20 nM) for 8 hours. (C) PBMCs were separated into adherent and nonadherent subsets, followed by bortezomib (20 nM) treatment for 8 hours. Nuclear extracts were subjected to EMSA. (D) PBMCs were cultured with or without bortezomib (20 nM for 8 hours). Nuclear extracts were subjected to supershift assays using anti-p65 (65), p50 (50), p52 (52), RelB (B), and cRel (cR) Abs.
Bortezomib activates NF-κB in normal healthy donor-derived PBMCs. (A) PBMCs were cultured with bortezomib (10 nM and 20 nM) for 8 hours. Whole-cell lysates were immunoblotted with indicated Abs. (B) PBMCs and BMSCs from MM patient were cultured with bortezomib (10 nM and 20 nM) for 8 hours. (C) PBMCs were separated into adherent and nonadherent subsets, followed by bortezomib (20 nM) treatment for 8 hours. Nuclear extracts were subjected to EMSA. (D) PBMCs were cultured with or without bortezomib (20 nM for 8 hours). Nuclear extracts were subjected to supershift assays using anti-p65 (65), p50 (50), p52 (52), RelB (B), and cRel (cR) Abs.
Discussion
NF-κB is a member of the Rel family, including p65 (RelA), RelB, c-Rel, p50 (NFκB1), and p52 (NFκB2), which regulates cell proliferation, antiapoptosis, and cytokine secretion in many cancers.3 It is typically a heterodimer composed of p50 and p65 subunits and constitutively present in the cytosol and nucleus. In the cytosol, NF-κB is inactivated by its association with IκB family inhibitors; IκBα, therefore, has a crucial role in regulating NF-κB activation.30 Various growth factors and cytokines, including tumor necrosis factor-α12,22,23 and insulin-like growth factor-1,31 trigger IκB protein phosphorylation by IKKs, followed by its proteasomal degradation. These events allow translocation of p50/65 into the nucleus, where it binds to specific DNA sequences in the promoters of target genes, thereby stimulating transcription. Recent studies have also shown the biologic role of the noncanonical (alternative) pathway, predominantly mediated by p52/RelB, in MM.32,33 Importantly, genetic abnormalities associated with NF-κB activation in MM32,33 suggest a role for noncanonical NF-κB signaling in MM pathogenesis. NF-κB is therefore a promising therapeutic target in many cancers, including MM.
Bortezomib is a 26S proteasome inhibitor initially used in MM based upon inhibiting NF-κB activity by preventing proteasomal degradation of IκBα. It demonstrates remarkable anti-MM activities in both preclinical16,22,24 and clinical6,–8 studies, and was approved by the Food and Drug Administration in 2003 for therapy of relapsed refractory MM, in 2005 for treatment of relapsed MM, and in 2008 for initial treatment in MM. However, inhibition of constitutive NF-κB activity in MM cells by bortezomib has not been shown in either preclinical or clinical studies. Importantly, a recent study has shown that bortezomib activates constitutive NF-κB in endothelial cell carcinoma cell lines in vitro.34 Most recently, it has been shown that constitutive NF-κB activity in primary tumor cells from MM patients is refractory to inhibition by bortezomib.35 In this study, we therefore examined whether bortezomib-induced MM cell cytotoxicity is associated with alteration of constitutive NF-κB activity.
We first examined baseline IκBα protein expression in MM cell lines and unexpectedly found that increased phosphorylation and down-regulation of IκBα were triggered by bortezomib. Importantly, NF-κB was activated by bortezomib in almost all MM cell lines. Interestingly, we observed that there were different patterns of NF-κB activity triggered by bortezomib treatment. For example, progressive increase, transient increase, or no augmentation of NF-κB activity was observed in RPMI 8228, U266, and MM.1S cells, respectively. Furthermore, bortezomib-induced NF-κB activation was mediated via canonical pathway, evidenced by anti-p65 Ab supershift assay. Nonetheless, it remains possible that bortezomib blocks noncanonical NF-κB activity, due to inhibition of proteasome-dependent p100 conversion to p52. Because a previous study has demonstrated highly activated noncanonical pathway in primary MM tumor cells,33 NF-κB activity in primary tumor cells from MM patients, before and after bortezomib treatment, needs to be studied to clarify correlation of NF-κB activation by bortezomib with drug resistance. We also asked whether NF-κB activation associated with down-regulation of IκBα was bortezomib specific. Similar results were observed after lactacystin and MG132 treatment, indicating that broad classes of proteasome inhibitors can also trigger NF-κB activation in MM cells. RPMI 8226 cells have predominantly canonical NF-κB pathway; and in these cells bortezomib enhances NF-κB activity. In contrast, MM.1S cells have both canonical and noncanonical pathways, and in these cells bortezomib significantly down-regulated p52 expression due to blockade of proteasomal processing of p100 to p52, thereby inhibiting noncanonical NF-κB activity.
To assess the biologic significance of NF-κB activation during bortezomib-induced growth inhibition, we performed BrdU and cell proliferation assays. Consistent with NF-κB activation, BrdU incorporation was up-regulated by bortezomib treatment. Importantly, washout experiments after 8-hour bortezomib treatment showed that MM cells were destined to die during an additional 24-hour and 48-hour culture. Although NF-κB was activated by bortezomib in the first 8 hours, these results indicate that MM cells were already committed to apoptosis. We similarly did not observe inhibition of p65 nuclear translocation in RPMI 8226 MM cells in vivo in a xenograft mouse model. Taken together, our results strongly suggest that canonical NF-κB inhibition is not triggered by bortezomib therapy in MM cells.
One important question is how bortezomib down-regulates IκBα protein. We first hypothesized that proteases activated by bortezomib may trigger IκBα down-regulation/cleavage. Indeed, previous studies have shown that proteasome-independent IκBα degradation and NF-κB activation in B-cell lines can be inhibited by calcium chelator (1,2-bis(o-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid tetra (acetoxymethyl) ester), serine protease inhibitor (Tos-Phe-CH2Cl), and calmodulin inhibitor (W13).36 Others have also reported that calpain contributes to silica-induced IκBα degradation and NF-κB activation in a mouse macrophage cell line.37 However, in our study, neither these agents nor calpain inhibitor was able to block bortezomib-induced IκBα down-regulation, suggesting that bortezomib-induced IκBα down-regulation is distinct from the mechanisms previously reported. A previous study has shown that protein phosphatase 2A up-regulates Bcl-2 phosphorylation, followed by its proteasomal degradation;38 in the present study, OA alone modestly increased phosphorylation of IκBα, without affecting protein expression. Importantly, OA significantly increased both phosphorylation and down-regulation of IκBα triggered by bortezomib. These results suggest that phosphorylation of IκBα is a key event in its down-regulation triggered by bortezomib.
We therefore next examined the mechanisms whereby bortezomib triggered IκBα phosphorylation. Recent studies have shown that down-regulation of TRAF6 associated with NF-κB inhibition blocks MM cell proliferation,39 and that TAK-140 and RIP241 are upstream molecules of IKK. In our study, bortezomib significantly triggered phosphorylation of IKKβ and its upstream RIP2, without alteration in TRAF6 protein or phosphorylation of TAK-1. These results suggest that activation of IKKβ induced by bortezomib is distinct from canonical NF-κB activation induced by TNFα. We have previously shown that IKKβ-specific small molecule inhibitors PS-114512 and MLN120B15 block canonical NF-κB activation and growth of MM cells both in vitro and in vivo. Importantly, in this study, we showed that MLN120B inhibits bortezomib-induced IKKβ phosphorylation and IκBα down-regulation, as well as blocks NF-κB activation. These results confirm that bortezomib-induced IκBα phosphorylation and down-regulation are mediated via RIP2/IKKβ pathway. Because NF-κB is an antiapoptotic factor in MM, we next examined whether inhibition of IKKβ also enhanced bortezomib-induced cytotoxicity. As expected, MLN120B significantly increased bortezomib-induced cytotoxicity, suggesting potential utility of this combination as a novel treatment strategy in MM.
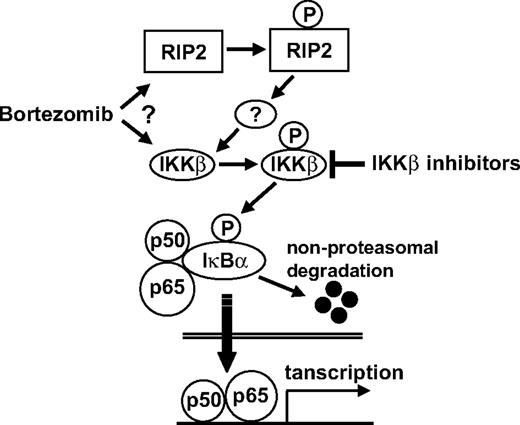
Finally, we also demonstrated that bortezomib triggered NF-κB activation via canonical pathway, associated by down-regulation of IκBα, in PBMCs. In contrast, bortezomib significantly inhibited NF-κB in BMSCs, consistent with our previous studies demonstrating that bortezomib blocks interleukin-6 secretion and MM cell adhesion to BMSCs.5,12 These results suggest that alteration of NF-κB activity by bortezomib depends on the cell type. In conclusion, the current study shows that (1) proteasome inhibitors including bortezomib induce IKKβ-dependent down-regulation of IκBα, thereby activating NF-κB via the canonical pathway, and (2) IKKβ inhibitor significantly enhances bortezomib-induced cytotoxicity (Figure 7), providing the preclinical framework for combination clinical trials.
Possible mechanism whereby bortezomib triggers canonical NF-κB activation. Bortezomib either directly or indirectly (via RIP2) activates IKKβ, which subsequently phosphorylates IκBα, an inhibitor of p50/p65. After nonproteasomal degradation of IκBα, p50/p65 translocates to nucleus. IKKβ inhibitors block down-regulation of IκBα and NF-κB activity, as well as enhance bortezomib-induced cytotoxicity.
Possible mechanism whereby bortezomib triggers canonical NF-κB activation. Bortezomib either directly or indirectly (via RIP2) activates IKKβ, which subsequently phosphorylates IκBα, an inhibitor of p50/p65. After nonproteasomal degradation of IκBα, p50/p65 translocates to nucleus. IKKβ inhibitors block down-regulation of IκBα and NF-κB activity, as well as enhance bortezomib-induced cytotoxicity.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This study is supported by National Institutes of Health SPORE IP50 CA10070, PO-1 CA78378, and RO-1 CA 50947 grants; the Multiple Myeloma Research Foundation (to T.H., D.C., N.R., and C.M.); and the LeBow Family Fund to Cure Myeloma (to K.C.A.).
National Institutes of Health
Authorship
Contribution: T.H. performed experiments and prepared the manuscript; H.I. performed experiments; D.C. designed experiments and analyzed the data; Y.O. performed experiments; N.R. designed experiments; K.P. performed experiments; C.M. designed experiments; N.C.M. analyzed the data; P.G.R. analyzed the data; R.D.C. designed and performed the experiments; and K.C.A. designed experiments and analyzed the data.
Conflict-of-interest disclosure: T.H. is a consultant for Biotest AG. K.C.A. is a consultant and on speakers bureaus for Biotest AG, Millennium, Celegene, and Novartis. N.C.M. and N.R. are consultants and on speakers bureaus for Millennium, Celegene, and Novartis. P.G.R. is on the advisory boards and speakers bureaus of Millennium and Celegene. C.M. discloses having received in the past consultant honoraria from Millennium, Novartis, Bristol-Myers Squibb, Merck, Kosan, and Pharmion, as well as research funding from Amgen, AVEO Pharma, EMD Serono, and Sunesis. The remaining authors declare no competing financial interests.
Correspondence: Kenneth C. Anderson, Dana-Farber Cancer Institute, 44 Binney St, Boston, MA 02115; e-mail: kenneth_anderson@dfci.harvard.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal