Activation-induced cell death (AICD) plays an important role in peripheral T-cell tolerance. AICD in CD4 T helper (Th) cells, including Th1 and Th2 effectors, has been extensively studied. Recently, interleukin-17–producing CD4+ T cells (Th17 cells) have been identified as a unique Th subset, but their susceptibility to AICD and the underlying molecular mechanisms have not been defined. In this study, we found that Th17 cells were significantly less susceptible to AICD than Th1 cells, and Th17 cell resistance to AICD is due to the high levels of c-Fas–associated death domain–like interleukin-1–converting enzyme inhibitory protein preventing Fas-mediated apoptosis. The resistance of Th17 cells to AICD reveals a novel mechanism to explain the high pathogenicity of Th17 cells in autoimmune diseases, and may also provide a rationale to generate tumor-specific Th17 cells for adoptive immunotherapy.
Introduction
Whether T cells live or die greatly affects the adaptive immune response. To maintain homeostasis after clonal expansion, activated T cells must be removed to avoid provoking autoimmune diseases.1 Apoptotic death of T cells in response to a repeated or excessive T-cell receptor (TCR) triggering is referred to as activation-induced cell death (AICD).2,3 Extensive studies have been done to understand the kinetics and mechanisms of AICD in T helper (Th)1 and Th2 subsets.4,5 Th1, but not Th2, effectors undergo rapid Fas/Fas ligand (FasL)–mediated apoptosis,6 whereas granzyme B is necessary for AICD of Th2 cells.7 Recently, interleukin-17 (IL-17)–producing CD4+ T cells (Th17 cells) were identified as a unique Th subset.8,9 The biologic relevance of the Th17 subset's response to AICD is underscored by the ability of Th17 cells to influence disease outcome.10,11 Preferential activation of the Th17 response is central to the pathogenesis of many autoimmune diseases.12 However, the susceptibility of Th17 cells and the underlying molecular mechanisms of AICD in Th17 effectors have not been defined. In this study, we found that Th17 effectors were significantly less susceptible to AICD upon TCR restimulation than Th1 effectors. Furthermore, the high level of c-Fas–associated death domain–like IL-1–converting enzyme inhibitory protein (c-FLIP) expression renders Th17 cell resistance to AICD by preventing Fas-mediated apoptosis.
Methods
The use of mice in this study was approved by the Institutional Animal Care and Use Committee of Moffitt Cancer Center and University of South Florida. Complete materials and methods are available in the supplemental material (available on the Blood website; see the Supplemental Materials link at the top of the online article).
Results and discussion
To compare susceptibility of Th1 and Th17 cells in ACID, we generated highly enriched Th1 and Th17 cells in vitro (Figure 1A). After restimulation with anti-CD3 monoclonal antibody (mAb), Th1 cells underwent apoptosis in a dose-dependent manner, but Th17 cells underwent significantly less apoptosis than Th1 cells at each of the concentrations tested (P < .01; Figure 1B). Time kinetic experiments confirmed that Th17 cells were significantly less susceptible to AICD than the Th1 controls after TCR restimulation for 1 to 4 days (Figure S1). Because in vitro–polarized Th17 cells were not absolutely pure, the relative resistance to AICD could be resulted from the unpolarized cells (ie, 20%-40%) in the Th17 population (Figure 1A). To address this possibility, we used a Th17 reporter mouse with a red fluorescent protein (RFP) sequence inserted into the IL-17F gene,13 and generated Th1 and Th17 cells from il-17f/rfp knockin mice. We found that RFP+ cells polarized under Th17 culture conditions underwent significantly less apoptosis than RFP− cells polarized under Th1 culture conditions (supplemental Figure 2). Different sensitivity of Th1 and Th17 cells to AICD could be due to different TCR expression on their surface, but it was not the case because both subsets expressed comparable levels of TCR and CD3 before AICD analysis (supplemental Figure 3). Altogether, we conclude that Th17 cells are largely resistant to AICD upon TCR restimulation.
Th17 cells are more resistant to AICD than Th1 cells in vitro and in vivo. (A) Th1 and Th17 cells were generated from ovalbumin-specific TCR-transgenic T cells in vitro, as described in “Methods.” Expression levels of intracellular interferon-γ and IL-17 on live CD4+ cells are shown for Th1 and Th17 cells 4 days. IL-4+ cells were < 1% in either subset (data not shown). (B) Polarized Th1 and Th17 cells were restimulated in the presence of anti-CD3 mAb at the indicated concentrations. Cells were stained with annexin V and DAPI to detect early and late apoptotic cells, respectively. Data represent 1 of 3 similar experiments. (C) Survival of Th1 and Th17 cells in mixed culture in vitro. Th1 cells were generated from Thy1.1 OT-II mice, and Th17 cells were from Thy1.2 OT-II mice. Mixed Th1 (Thy1.1+) and Th17 (Thy1.2+) cells were cultured at a ratio of 1:1. Apoptosis was analyzed with (right panel) or without (left panel) anti-CD3 mAb treatment for 20 hours. Data represent 1 of 3 replicate experiments. (D) Survival of Th1 and Th17 cells in allogeneic recipients in vivo. Th1 cells generated from normal B6 mice and Th17 cells from Ly5.1 B6 mice were together injected intravenously into lethally irradiated BALB/c recipients at a ratio of 1:1. The percentages of live Th1 and Th17 cells were determined (left panel) in the recipient spleen 4 days after cell transfer. Apoptosis was determined by DAPI and annexin V staining (middle panel). Cell division was determined by 5-(and 6-)carboxyfluorescein diacetate succinimidyl ester staining (right panel).
Th17 cells are more resistant to AICD than Th1 cells in vitro and in vivo. (A) Th1 and Th17 cells were generated from ovalbumin-specific TCR-transgenic T cells in vitro, as described in “Methods.” Expression levels of intracellular interferon-γ and IL-17 on live CD4+ cells are shown for Th1 and Th17 cells 4 days. IL-4+ cells were < 1% in either subset (data not shown). (B) Polarized Th1 and Th17 cells were restimulated in the presence of anti-CD3 mAb at the indicated concentrations. Cells were stained with annexin V and DAPI to detect early and late apoptotic cells, respectively. Data represent 1 of 3 similar experiments. (C) Survival of Th1 and Th17 cells in mixed culture in vitro. Th1 cells were generated from Thy1.1 OT-II mice, and Th17 cells were from Thy1.2 OT-II mice. Mixed Th1 (Thy1.1+) and Th17 (Thy1.2+) cells were cultured at a ratio of 1:1. Apoptosis was analyzed with (right panel) or without (left panel) anti-CD3 mAb treatment for 20 hours. Data represent 1 of 3 replicate experiments. (D) Survival of Th1 and Th17 cells in allogeneic recipients in vivo. Th1 cells generated from normal B6 mice and Th17 cells from Ly5.1 B6 mice were together injected intravenously into lethally irradiated BALB/c recipients at a ratio of 1:1. The percentages of live Th1 and Th17 cells were determined (left panel) in the recipient spleen 4 days after cell transfer. Apoptosis was determined by DAPI and annexin V staining (middle panel). Cell division was determined by 5-(and 6-)carboxyfluorescein diacetate succinimidyl ester staining (right panel).
To explore the mechanisms responsible for unequal susceptibility of Th17 and Th1 cells to AICD, we examined caspase activation upon TCR restimulation and found that dramatic activation of caspases was seen in Th1 cells, but not in Th17 cells (supplemental Figure 4). Furthermore, we found that Th1, but not Th17 cells up-regulated FasL expression after TCR restimulation (supplemental Figure 4), suggesting that the failure of FasL up-regulation by Th17 cells could account for Th17 cell resistance to AICD. We next asked whether Th17 cells are susceptible to be killed by other FasL-expressing cells. In the mixed culture of Th1 and Th17 cells, the majority of Th1 cells (71% ± 4%) underwent apoptosis in vitro (Figure 1C), whereas only 24% plus or minus 5% of the Th17 cells underwent apoptosis after TCR restimulation (Figure 1C), indicating that FasL-expressing Th1 cells did not kill bystander Fas-expressing Th17 cells in vitro. We extended the study in vivo by adaptively transferring Th1 and Th17 cells generated from B6 mice into lethally irradiated BALB/c mice. Th1 and Th17 cells were mixed at 1:1 before injection; the ratio of viable Th1 versus Th17 became 0.13:1 in the recipient spleen 4 days after cell transfer. Consistent with this proportion, the absolute numbers of viable cells (annexin V−/DAPI− [4,6 diamidino-2-phenylindole]) per spleen were 1.71 plus or minus 0.55 × 105 for Th17 cells and 0.14 plus or minus 0.05 × 105 for Th1 cells, which was significantly different (P = .001). These results indicate that Th17 cells expanded more extensively and/or died less than Th1 cells during the 4-day alloantigen stimulation in vivo. Both possibilities were further supported by more extensive 5-(and 6-)carboxyfluorescein diacetate succinimidyl ester dilution and significantly lower levels of apoptosis of Th17 cells than of Th1 cells (Figure 1D). In conclusion, Th17 cells are resistant to AICD relative to Th1 cells, and the impaired Fas signaling most likely accounts for the resistance of Th17 cells.
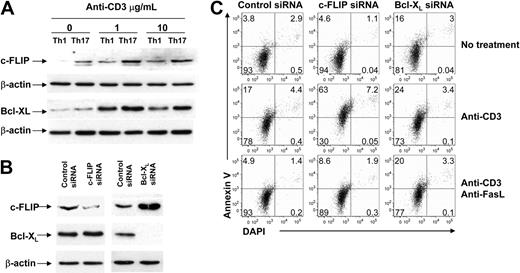
Because c-FLIP14,–16 and Bcl-xL17,18 are well-known antiapoptotic molecules that protect T cells from death, a possible mechanism for Th17 cell resistance could be overexpression of c-FLIP and/or Bcl-xL. To test this hypothesis, we detected the protein expression of c-FLIP and Bcl-xL in Th1 and Th17 cells. As shown in Figure 2A, expression levels of c-FLIP were markedly higher in Th17 than in Th1 cells before and after stimulation. The reason for the unusually high levels of c-FLIP in Th17 cells is not clear, but it may be related to polarization in these cells by IL-6 that activates Th17-specific transcription factor (signal transducer and activator of transcription 3).19,20 The expression levels of Bcl-xL had a similar trend, but the differences were not as much as those for c-FLIP between these 2 subsets (Figure 2A). To further test whether high levels of c-FLIP and Bcl-xL expression may account for the resistance to AICD in Th17 cells, we knocked down c-FLIP or Bcl-xL in Th17 cells with small interfering RNAs (siRNAs) and then tested the cell's susceptibility to AICD (Figure 2B). Once c-FLIP was knocked down, Th17 cells were readily susceptible to AICD. On the other hand, Th17 cells increased base level of apoptosis (without anti-CD3) after knocking down Bcl-xL, but not c-FLIP, suggesting that Bcl-xL played an important role in passive cell death (ie, cytokine withdrew-induced apoptosis; Figure 2C top and middle panels). These results indicate that the high level of c-FLIP, but not Bcl-xL, is responsible for the resistance of Th17 cells to AICD. If knocking down c-FLIP reverses the resistance of Th17 cells, it is conceivable that knocking down c-FLIP could restore both FasL expression and Fas signaling. In fact, upon anti-CD3 stimulation, Th17 cells expressed significantly higher FasL after transfection with c-FLIP siRNA (P = .01), but not with Bcl-xL siRNA (P = .14) in comparison with control siRNA (supplemental Figure 5). Furthermore, once the FasL/Fas interaction was blocked by neutralizing anti-FasL mAb, the level of apoptosis was reduced in Th17 cells knocked down for c-FLIP siRNA at a level comparable with that shown in control Th17 cells and in the Th17 cells knocked down for Bcl-xL (Figure 2C). In conclusion, abnormal up-regulation of c-FLIP renders Th17 cells resistant to AICD by limiting FasL expression and inhibiting Fas signaling.
Resistance of Th17 cells to AICD is due to abundant c-FLIP expression. (A) c-FLIPL and Bcl-xL protein expression was detected with Western blot. Th1 and Th17 cells were treated with 0, 1, or 10 μg/mL anti-CD3 mAb. β-Actin was used as a loading control. Data represent 1 of 3 replicate experiments. (B) Expression levels of c-FLIPL, Bcl-xL, and β-actin in Th17 cells shown after control, c-FLIP, or Bcl-xL siRNA transfection. (C) AICD of Th17 cells after knocking down c-FLIP or Bcl-xL. Polarized Th17 cells transfected with control, c-FLIP–specific, or Bcl-xL–specific siRNA for 48 hours were recultured with medium, anti-CD3 mAb alone, or anti-CD3 plus neutralizing anti-FasL mAb. Apoptosis was determined 20 hours after restimulation by annexin V and DAPI staining. Data represent 1 of 3 replicate experiments.
Resistance of Th17 cells to AICD is due to abundant c-FLIP expression. (A) c-FLIPL and Bcl-xL protein expression was detected with Western blot. Th1 and Th17 cells were treated with 0, 1, or 10 μg/mL anti-CD3 mAb. β-Actin was used as a loading control. Data represent 1 of 3 replicate experiments. (B) Expression levels of c-FLIPL, Bcl-xL, and β-actin in Th17 cells shown after control, c-FLIP, or Bcl-xL siRNA transfection. (C) AICD of Th17 cells after knocking down c-FLIP or Bcl-xL. Polarized Th17 cells transfected with control, c-FLIP–specific, or Bcl-xL–specific siRNA for 48 hours were recultured with medium, anti-CD3 mAb alone, or anti-CD3 plus neutralizing anti-FasL mAb. Apoptosis was determined 20 hours after restimulation by annexin V and DAPI staining. Data represent 1 of 3 replicate experiments.
The resistance of Th17 cells to AICD may be relevant for various physiologic and pathologic situations, including Th17-mediated autoimmune diseases10,11 and graft-versus-host disease.19 On the other hand, the survival advantage of Th17 cells may also provide the rationale for cancer immunotherapy through adoptive cell transfer of tumor-specific Th17 cells. This concept is supported by a recent report that Th17 cells specific for melanoma-associated antigen were superior to their Th1 counterparts.21 All together, our finding that Th17 cells are resistant to AICD underlines the challenges in targeting pathogenic Th17 effectors in autoimmune diseases and transplantation, and provides the rationale to use this cell population in cancer immunotherapy.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Drs Amer Beg, Esteban Celis, and Lia Perez for their critical discussion on this project. We are grateful for the technical assistance provided by Flow Cytometry and Mouse Core Facility at the Moffitt Cancer Center.
This work was supported in part by National Institutes of Health grants AI63553, CA118116 (to X.-Z.Y.), and AI51693 (to C.A.). X.-Z.Y. is a recipient of a New Investigator Award supported by the American Society for Blood and Marrow Transplantation.
National Institutes of Health
Authorship
Contribution: Y.Y. designed and performed research, collected and analyzed data, and drafted the manuscript; C.I. participated in part of the research and data analysis; T.Y. participated in part of the research and revised the manuscript; X.Y. coordinated a part of the research; C.A. contributed to research design, interpreted data, and revised the manuscript; C.D. contributed vital reagent and interpreted data; and X.-Z.Y. designed research, participated in research, interpreted data, and revised the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Xue-Zhong Yu, H. Lee Moffitt Cancer Center and Research Institute, SRB-2, 12902 Magnolia Dr, Tampa, FL 33612-9497; e-mail: Xue.Yu@moffitt.org.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal