To the editor:
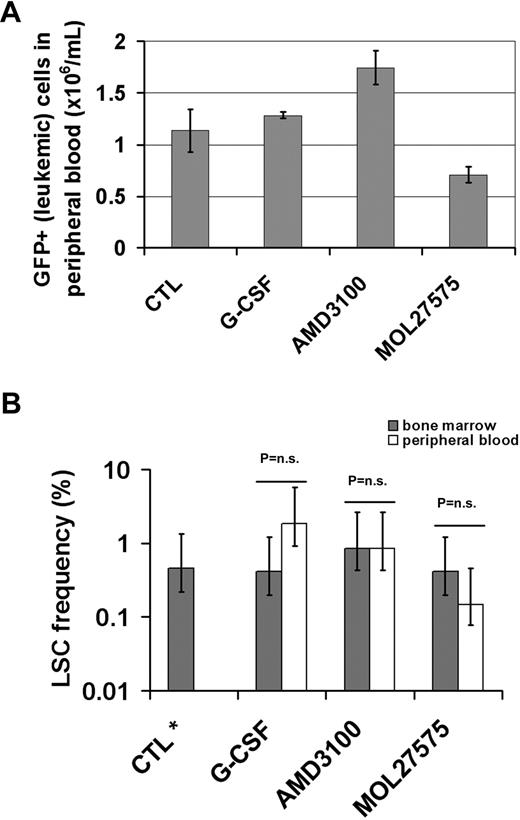
The chemosensitizing effect of CXCR4 antagonists was recently demonstrated in 2 elegant studies using models of acute promyelocytic leukemia (APL)1 and multiple myeloma.2 Both studies show tumor reduction and prolonged survival in tumor-bearing/leukemic mice treated with CXCR4 antagonist AMD3100 in combination with chemotherapy compared with treatment with either drug alone. We studied the effects of 3 drugs antagonizing hematopoietic stem cell (HSC)–stroma interactions (granulocyte-colony stimulating factor [G-CSF], AMD3100, and MOL27575, a small molecule VLA-4 antagonist3 ) in the aggressive MN1 leukemia model4,5 to investigate their role in leukemia stem cell (LSC) mobilization. Mice transplanted with MN1-IRES-GFP–transduced bone marrow cells were treated on 3 consecutive days with G-CSF (10 μg/kg per day subcutaneously, last dose 24 hours before tissue harvest, n = 4), AMD3100, and MOL27575 (each 5 mg/kg per day subcutaneously, last dose 1 hour before tissue harvest, n = 5 and n = 4, respectively). AMD3100 treatment resulted in an increased proportion of leukemic cells (GFP+) in peripheral blood compared with control mice (Figure 1A). Next we determined the LSC frequency in peripheral blood and bone marrow in each treatment group by competitive repopulation unit (CRU) assays. 5, 50, 500, 5000, or 50 000 leukemic (GFP+) cells were transplanted to secondary mice along with a life-sparing dose of normal bone marrow cells (3 mice/cell dose). The LSC frequency was determined by Poisson statistics from the proportion of leukemic versus nonleukemic mice. The frequency of LSCs within the total leukemic cell population was not significantly different between bone marrow and peripheral blood in any of the treatment groups (Figure 1B). Thus, whereas AMD3100 increased the number of leukemic cells in peripheral blood, we did not observe a preferential mobilization of LSCs over their progeny. Treatment of MN1 mice starting 1 week after transplantation with cytarabine (5 or 50 mg/kg per day) with or without AMD3100 (5 mg/kg per day) for 5 consecutive days did not prolong survival of mice compared with solvent-treated mice (n = 3 evaluable mice per group), but was associated with fatal toxicity in the cytarabine 50 mg/kg group in 4 of 7 mice (data not shown).
Leukemia stem cell mobilization in the MN1 leukemia model. (A) Number of transduced white blood cells in peripheral blood of control or drug treated mice. Eighteen or 19 days after transplantation of MN1-transduced bone marrow cells to lethally irradiated mice, solvent (CTL), G-CSF, AMD3100, or MOL27575 were injected subcutaneously for 3 consecutive days. Peripheral blood was harvested and pooled from 4 to 5 mice per group, and white blood cells were counted and immunophenotyped for the proportion of GFP+ (leukemic) cells (95% confidence intervals do not overlap between CTL and AMD3100 indicating a significant difference). (B) Results of CRU assays to determine the leukemia stem cell frequency in peripheral blood and bone marrow of G-CSF, AMD3100, or MOL27575 treated mice. Peripheral blood or bone marrow from 4 to 5 mice per treatment group were pooled and transplanted by limiting-dilution analysis (5 cell doses of GFP+ cells, 3 mice per cell dose). LSC frequency was calculated from the proportion of leukemic mice by Poisson statistics. Overlapping 95% confidence intervals indicate no statistically significant difference. *LSC frequency in bone marrow of a historical control group is shown for comparison.
Leukemia stem cell mobilization in the MN1 leukemia model. (A) Number of transduced white blood cells in peripheral blood of control or drug treated mice. Eighteen or 19 days after transplantation of MN1-transduced bone marrow cells to lethally irradiated mice, solvent (CTL), G-CSF, AMD3100, or MOL27575 were injected subcutaneously for 3 consecutive days. Peripheral blood was harvested and pooled from 4 to 5 mice per group, and white blood cells were counted and immunophenotyped for the proportion of GFP+ (leukemic) cells (95% confidence intervals do not overlap between CTL and AMD3100 indicating a significant difference). (B) Results of CRU assays to determine the leukemia stem cell frequency in peripheral blood and bone marrow of G-CSF, AMD3100, or MOL27575 treated mice. Peripheral blood or bone marrow from 4 to 5 mice per treatment group were pooled and transplanted by limiting-dilution analysis (5 cell doses of GFP+ cells, 3 mice per cell dose). LSC frequency was calculated from the proportion of leukemic mice by Poisson statistics. Overlapping 95% confidence intervals indicate no statistically significant difference. *LSC frequency in bone marrow of a historical control group is shown for comparison.
Disruption of the CXCL12/CXCR4 axis releases HSCs from their niche, and is now being tested clinically to release LSCs from their niche environment to increase chemosensitivity.6 Twenty years ago the cell-cycle promoting effects of G-CSF in leukemic cells stimulated its use as a priming agent in AML patients undergoing induction chemotherapy. Only recently it has been shown that the stem cell–mobilizing effect of G-CSF is CXCR4-dependent.7 Priming with G- or GM-CSF in more than 4000 AML patients has not resulted in improved survival.8 However, it has been suggested that standard risk patients may benefit from G-CSF priming.9
Are 20 years of priming studies about to be reproduced under the concept of cell-to-microenvironment disruption? There may be additional functions of CXCR4 antagonists compared with G-CSF. However, G-CSF priming studies are instructive in 2 ways: first, a direct comparison of CXCR4 antagonists with G-CSF may give an early indication of superiority of CXCR4 antagonists, and, second, chemosensitizing effects of CXCR4 antagonists may be restricted to cytogenetic and molecular subgroups of AML. The latter is supported by the different treatment effects of AMD3100 in the MN1 model compared with the APL mouse model reported by Nervi et al.1 We suggest that cytogenetic and molecular subgroups be carefully characterized in current and future trials using CXCR4 antagonists.
Authorship
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: R. Keith Humphries, MD, PhD, Terry Fox Laboratory, British Columbia Cancer Agency, 675 West 10th Ave, Vancouver, BC, V5Z 1L3 Canada; e-mail: khumphri@bccrc.ca.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal