In this issue of Blood, van der Meijden and colleagues report on the mechanisms by which collagen exposure in flow-dependent circulation contributes to thrombus formation.1
In their report, van der Meijden et al show that 2 independent summating mechanisms for thrombin generation initiated by fibrillar type I collagen lead to platelet and coagulant thrombus formation. Type I collagen added to citrated human plasma upon recalcification shortens thrombin generation times (TGT). TGT are blocked by the addition of corn trypsin inhibitor (CTI) but not in the presence of inactivated factor VIIa (FVIIai), a tissue factor pathway inhibitor. TGT are also blocked in factor XII– or XI–deficient plasma. Under conditions of whole blood flow, addition of CTI (but not FVIIai treatment), reduced platelet adherence to collagen, and combined CTI and JAQ1 glycoprotein VI (GPVI)–blocking antibody treatment further decreased platelet adhesion and thrombin generation. Platelet activation by GPVI was mediated through LAT and PLCγ2, which are established signaling pathways for GPVI.1 These investigations show that collagen exposure in flowing blood results in at least 2 pathways for thrombus formation, one directly through platelet adhesion and activation, and another indirect mechanism via thrombin generation in response to contact activation.
Nieswandt et al first established the crucial role of GPVI adhesion of platelets to collagen under conditions of flowing blood, suggesting that GPVI is essential for platelet activation by collagen under physiologic conditions.2 Sarratt et al demonstrated that inhibition or absence of α2β1 or GPVI results in defective adhesion to collagen under flow.3 Although GPVI is a low-affinity collagen receptor compared with α2β1, it may dominate as a response entity to exposed collagen because it does not require prior activation and is required to then activate high-affinity integrins that mediate firm adhesion to the extracellular matrix. However, platelet activation by other agonists, such as thrombin, ADP, thromboxanes or VWF adherence to GPIb, also results in integrin activation and likely regulates platelet adherence in flowing blood under physiologic conditions4-6 (see figure). Platelet adherence to collagen is known to lead to tissue factor–mediated thrombin generation.7 In the present study, JAQ1 blocked thrombin generation in both contact- and tissue factor–activated plasma and CTI inhibited platelet adhesion to collagen.1 These data indicate that collagen-induced thrombin generation is a product of both platelet and contact pathway activation and that exogenously produced thrombin is required for maximal platelet adhesion to collagen under flow. This latter point was confirmed in a recent publication, which shows reduced platelet adherence and a 50% decrease in mean thrombus area of blood anticoagulated with CTI versus heparin flowed over collagen.5
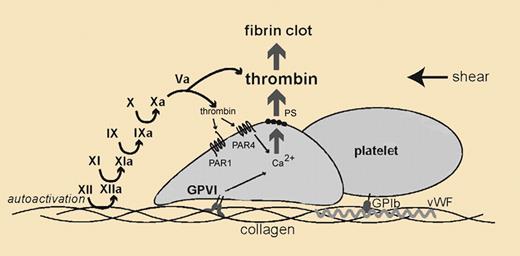
Mechanisms for thrombin generation by collagen in vivo. In flowing blood where there are shear forces, platelets adhere to exposed collagen. Slowed platelets additionally bind to collagen via glycoprotein VI (GPVI). The GPVI-collagen interaction results in platelet activation. Independently, exposed collagen supports zymogen factor XII (XII) autoactivation to enzymatic factor XIIa (XIIa). XIIa initiates thrombin generation through a series of proteolytic reactions. Thrombin then stimulates platelets by activating its receptors, protease activated receptor 1 (PAR1) and 4 (PAR4). PAR activation and GPVI signaling jointly contribute to a platelet thrombus, which amplifies into a fibrin clot and arterial vessel occlusion.
Mechanisms for thrombin generation by collagen in vivo. In flowing blood where there are shear forces, platelets adhere to exposed collagen. Slowed platelets additionally bind to collagen via glycoprotein VI (GPVI). The GPVI-collagen interaction results in platelet activation. Independently, exposed collagen supports zymogen factor XII (XII) autoactivation to enzymatic factor XIIa (XIIa). XIIa initiates thrombin generation through a series of proteolytic reactions. Thrombin then stimulates platelets by activating its receptors, protease activated receptor 1 (PAR1) and 4 (PAR4). PAR activation and GPVI signaling jointly contribute to a platelet thrombus, which amplifies into a fibrin clot and arterial vessel occlusion.
The novel finding in the report by van der Meijden et al is the demonstration that factor XII autoactivation on type I fibrillar collagen contributes to platelet adherence and thrombin generation in flowing blood. The present work rediscovers older studies, recognizing that fibrillar collagen supports factor XII binding and autoactivation.8,9 It should also be noted that new investigations indicate that certain aggregated proteins also initiate factor XII autoactivation.10 Interest in this in vivo mechanism for thrombin generation waned more than 30 years ago when it was reported that highly purified collagen did not lead to factor XII autoactivation.11 The molecular mechanism for autoactivation is not known, but a recent study using sum frequency generation vibrational spectroscopy indicates that factor XII binding to negatively charged surfaces results in a molecular rearrangement of the zymogen elaborating enzymatic activity.12 New interest in contact activation in vivo arose when Renne et al reported that mice lacking factor XII have delayed times to arterial thrombosis.13 Efforts have been made to explain the mechanism(s) for reduced thrombin generation in these animals that may arise from diminished contact activation around a developing thrombus. RNA, sulfatides, and polysomes present in developing platelet thrombi result in factor XII autoactivation.14,15 It is important to note that investigations of kininogen or bradykinin B2 receptor knock-out mice also show delay in arterial thrombosis studies and, in the latter case, the mechanism has nothing to do with reduced in vivo contact activation.16,17
Factor XII's function in vivo is more than an activity prompted by exposure of collagen, platelet components, or aggregated protein that leads to thrombin generation in the intravascular compartment. Factor XII has been recognized as a growth factor leading to ERK1/2 activation.18,19 The mechanism and importance of this activity is being characterized. Regardless, the present report moves the factor XII field forward and shows that contact activation in vivo in high-flow circulation contributes to thrombin generation, platelet activation, and development of arterial thrombosis. This investigation supports the observation that factor XII and other contact proteins influence arterial thrombosis risk independent of hemostasis.13,16,17
Conflict-of-interest disclosure: The authors declare no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal