In a murine tumor microenvironment, TEMs display distinct proangiogenic functions and a gene expression signature that is closer to nontumor “resident” blood monocytes and embryonic macrophages than TAMs, suggesting the existence of a novel lineage of proangiogenic cells.
Three paradigms for neoangiogenesis have long been recognized: vasculogenesis, angiogenesis, and arteriogenesis.1 Vasculogenesis is the de novo formation of blood vessels from angioblast precursors that are principally mesoderm derived. The angioblasts migrate into tissues where they self-assemble into primitive endothelial cell tubes with lumens. These simple lumens coalesce to form a primitive capillary plexus. Angiogenesis is the formation of new vessels via sprouting of endothelium from these primitive capillaries or later from other preexisting vessels. During embryogenesis, the primitive capillary plexus is subsequently remodeled into mature vessels via a complex network of genetic specification cues, tissue metabolic demands, responses to biomechanical changes in tissues, and hemodynamic forces.2 Commensurate with these adaptations, various mesenchymal-derived cells are recruited in supportive roles to form tunable vascular structures capable of systemic regulation of blood flow. Arteriogenesis is both the process of arterial vessel formation and subsequent arterial collateral formation that plays a role in overall enhancement of blood flow delivery to tissues and organs. The molecular regulation of these fundamental processes is becoming increasingly understood; however, the specific cellular components engaged in each stage of these processes are less well-defined.
While numerous tissue stromal cells play important vascular-supportive roles during angiogenesis and arteriogenesis, both circulating and resident hematopoietic cells are now recognized as important components in the initiation of vessel formation. A vast array of hematopoietic cell subsets have been purported to promote neoangiogenesis, ranging from hematopoietic stem cells to committed erythroid or myeloid progenitor cells.3 The role of certain monocyte and macrophage subsets in promotion of tumor angiogenesis has been recently highlighted,4 but remains controversial with respect to whether these cells give rise to functional endothelium; a point further complicated by the shared cell-surface expression of a variety of molecules, including the receptor Tie2, between endothelial cells and some hematopoietic cell types.
The major murine myeloid cell types implicated in tumor angiogenesis can be classified as mast cells, tumor-associated macrophages (TAMs), Tie2-expressing monocytes (TEMs), neutrophils, and myeloid-derived suppressor cells (MDSCs). TEMs circulate in peripheral blood before being recruited to tumor microenvironments where they promote angiogenesis and tumor growth. These activities cannot be substituted by other myeloid subsets when TEMs are depleted.5 The exact relationship between the proangiogenic TEMs and other tumor-associated myeloid cells has not been clarified nor has an established relationship between the circulating TEMs and other blood monocyte subsets been found.
In this issue, Pucci and colleagues compare the gene expression signature of murine tumor–derived TEMs, TAMs, MDSCs, and circulating blood monocytes.6 The authors employ standard flow-cytometric cell-sorting techniques on samples from tumor-bearing transgenic mice to identify and isolate myeloid subsets and to distinguish the cells from tumor or host endothelium (see figure). Custom or premade TaqMan low-density qPCR arrays were probed with transcribed products of mRNA isolated from the various cell subsets. The mRNA expression profiles of TEMs and TAMs were highly related and clearly distinct from that of endothelial cells. However, TEMs could be distinguished from TAMs (and all other tumor-associated myeloid subsets) with a skewing toward significantly higher expression of a panel of gene transcripts frequently identified in M2 alternatively activated macrophages (cells with an anti-inflammatory phenotype and enhanced angiogenic and wound-healing capacity). While both TEMs and TAMs displayed defective responses to inflammatory stimuli, TEMs were the most responsive to interleukin-4 (M2 response) at least with respect to transcription of certain “M2” genes. The TEM mRNA expression profile was determined to be significantly more concordant with the circulating resident monocyte subset mRNA profile than the profile from the circulating inflammatory monocyte subset and was also highly concordant with a subpopulation of embryonic macrophages found to be associated with tissue remodeling and angiogenesis. These results led Pucci and colleagues to speculate that a “TEM gene signature” may herald the existence of a distinct lineage of proangiogenic monocyte/macrophages that emerges in the embryo and persists into adulthood.
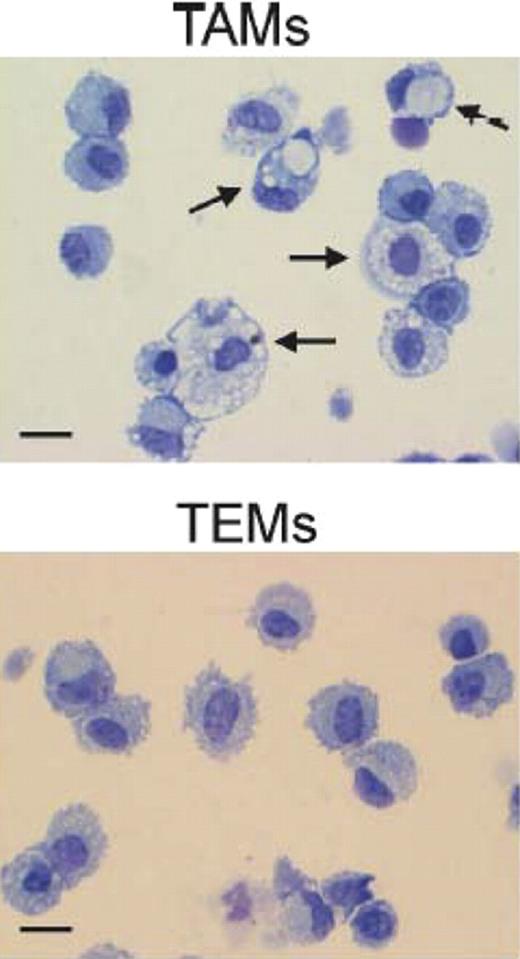
Morphologic appearance of tumor-associated macrophages (TAMs) and Tie2-expressing monocytes (TEMs). Arrows indicate large macrophges containing conspicuous cytoplasmic phagogomes. Scale bar: 30 μm. Photos are representative of n = 25 photos/sample. See the complete figure in the article beginning on page 901.
Morphologic appearance of tumor-associated macrophages (TAMs) and Tie2-expressing monocytes (TEMs). Arrows indicate large macrophges containing conspicuous cytoplasmic phagogomes. Scale bar: 30 μm. Photos are representative of n = 25 photos/sample. See the complete figure in the article beginning on page 901.
These results have confirmed that TEMs represent bona fide myeloid cells and are distinct in appearance, function, and gene expression from tumor endothelial cells, despite the fact that both cell types express Tie2. Such a clear distinction highlights the limitation in using a limited number of cell-surface molecules alone to attribute lineage relationships. Additional studies will be required to determine the mechanisms regulating the “TEM gene signature” in myeloid cells at different stages of development and to understand the specific roles of the identified gene products in the angiogenic process, because it is apparent that this subset of myeloid cells from the embryo to the adult display similar functions. Further work will also be required to determine whether a distinct lineage of proangiogenic monocyte/macrophages is specified during murine ontogeny and if the information gleaned from the murine system can be translated to the human system.
Conflict-of-interest disclosure: M.C.Y. is a cofounder and consultant for EndGenitor Technologies Inc in Indianapolis, IN. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal