Abstract
The Tec kinase Itk is critical for the development of αβ T cells as well as differentiation of CD4+ T cells into Th2 cells. Itk null mice have defects in the production of Th2 cytokines; however, they paradoxically have significant elevations in serum IgE. Here we show that Itk null mice have increased numbers of γδ T cells in the thymus and spleen. This includes elevated numbers of CD4+ γδ T cell, the majority of which carry the Vγ1.1 and Vδ6.2/3 γδ T-cell receptor with a distinct phenotype. The development of these CD4+ γδ T cells is T cell intrinsic, independent of either major histocompatibility complex class I or class II, and is favored during development in the absence of Itk. Itk null CD4+ γδ T cells secrete significant amounts of Th2 cytokines and can induce the secretion of IgE by wild-type B cells. Our data indicate that Itk plays important role in regulating γδ T-cell development and function. In addition, our data indicate that the elevated IgE observed in Itk-deficient mice is due in part to the enhanced development of CD4+ γδ T cells in the absence of Itk.
Introduction
The tyrosine kinase Itk, a member of the Tec family of nonreceptor tyrosine kinases, is expressed in T cells and regulates signaling via the T-cell receptor.1 On T-cell receptor activation, Itk phosphorylates and activates phospholipase-γ1, leading to calcium influx, as well as activation of nuclear factor-κB, nuclear factor of activated T cell, and Ras-dependent signaling pathways. Signals regulated by Itk control the development of αβ T cells such that positive and negative selection is affected in the absence of Itk. In addition, Itk also controls the development of populations of T cells that have a naive phenotype (CD62Lhi/CD44lo), such that, in its absence, nonconventional CD4+ and CD8+ T cells carrying a memory phenotype (CD62Llo/CD44hi) and exhibiting innate function predominate.2-5
Itk signals also regulate the development of Th2 cells such that, in its absence, T cells from Itk null mice have defects in the production of Th2 cytokines, and these mice have defects in generating Th2 response in several infection and allergic asthma models.6-9 Despite this defect in the generation of effective Th2 responses and secretion of Th2 cytokines, Itk null mice paradoxically exhibit increased class switch in B cells to IgE and elevated levels of serum IgE.10,11 What is not clear is the source of cytokines that could drive the increase in class switch to IgE.
T cells are divided into αβ T cells and γδ T cells according to their TCR expression. Both αβ and γδ T cells arise from the most immature CD4−CD8− double-negative (DN) thymocytes in the thymus. DN thymocytes are divided into 4 developmental stages according to the surface expression of CD25 and CD44, from most immature DN1 to more mature DN4 cells. γδ T cells separate from αβ T cells at DN stages, although the exact time point and the mechanisms involved in this process are still elusive.12,13 Several studies have shown that the strength of the TCR signal is important for T-cell lineage commitment. Stronger TCR signals favor the development of γδ T cells, whereas the weaker signals favor the development of αβ T cells.14-16 Studies on γδ TCR transgenic mice have demonstrated that negative selection occurs during the development of γδ T cells in adult thymus, but whether positive selection is necessary is still controversial.
γδ T cells can produce Th1, Th2, and Th17 cytokines thus having multiple functions in the modulating immune responses, such as host defense and tumor immunity.17-23 Both murine and human γδ T cells have been suggested to provide help to B cells, which is correlated with their production of the Th2 cytokine interleukin-4 (IL-4).24-26 More interestingly, several studies showed that only the CD4+ γδ T cells are able to produce IL-4.20,21
We show here that mice lacking Itk have altered γδ T-cell development such that they have more of these cells. We also show that the CD4+ population of γδ T cells is expanded in the absence of Itk and that this population can induce B cells to class switch to generate increased levels of serum IgE. Our data suggest that the elevated levels of serum IgE observed in the absence of Itk is in part the result of altered γδ T cells and that these cells may be able to regulate the development of allergies by enhancing B-cell class switch to IgE.
Methods
Mice
Wild-type (WT), Itk null, KN6Tg, β2m−/− (referred to as major histocompatibility complex [MHC] I null), and MHCII−/− mice were on C57BL/6 background. β2m/Itk DKO, MHCII/Itk DKO, KN6TgItk−/−, T-bet/Itk, and TCRδ/Itk DKO mice were generated by breeding Itk−/− mice with β2m−/−, MHCII−/−, KN6Tg, T-bet−/−, and TCRδ−/− mice, respectively. All the mice used were 6 to 10 weeks of age and kept in pathogen-free conditions. Experiments were approved by the Institutional Animal Care and Use Committee at the Pennsylvania State University (Penn State).
Flow cytometry
Cells were preincubated with Fc-block (2.4G2, BD Biosciences) for 10 minutes, followed by incubating with the fluorescent antibodies for 30 minutes at 4°C. The samples were analyzed using a flow cytometer (Beckman Coulter). Anti–PECy5-CD44 (IM7), PECy7-CD4 (RM4-5), fluorescein isothiocyanate (FITC)–γδTCR (GL3), phycoerythrin (PE)–Vδ6.2/3 (8F4H7B7), PE-CD24 (M1/69), allophycocyanin-CD45.2 (104), FITC-Vγ2 (UC3-10A6), and PECy5-CD4 (RM4-5) were from BD Biosciences. Anti–FITC-TCRβ (H57-597), allophycocyanin-Alexa750-CD62L (MEL-14), PECy7-NK1.1 (PK136), PECy5-Thy1.1 (HIS51), PECy7-Thy1.1 (HIS51), PECy7-Thy1.2 (53-2.1), FITC-B220 (RA3-6B2), biotin-B220 (RA3-6B2), and biotin-TCRβ (H57-597) were from eBioscience. Anti–PE-CD3 (145-2c11), PECy7-CD3 (145-2c11), and PECy5-strepavidin were from BioLegend. Anti–CD4-ECD and CD8-ECD were from Invitrogen.
Cell sorting
To purify CD4+ and CD4− γδ T cells, splenocytes were first incubated with biotin-B220 and biotin-TCRβ followed by negative purification using streptavidin-magnetic beads (Miltenyi Biotec). Remaining cells were further stained with anti–FITC-γδ TCR, PE-CD3, and PECy5-CD4 and sorted using Cytopeia Influx Cell Sorter (Cytopeia). For other cell purification, cells were stained with corresponding fluorescent antibodies and sorted using Cytopeia Influx Cell Sorter.
Cytokine secretion assays
Purified CD4+ and CD4− γδ splenocytes cells were stimulated in vitro at 2 × 104 cells/well in duplicate with 10 μg/mL plate-coated anti-γδ TCR antibody (clone GL4, BD Bioscience) for 72 hours. Supernatants were then harvested and assayed for the presence of IL-4, IL-17, and interferon-γ (IFN-γ) by Bioplex (Bio-Rad).
Quantitative real-time polymerase chain reaction analysis
RNA was extracted from sorted cells using RNase Mini Kit (QIAGEN). cDNA was generated using You Prime First-Strand beads (GE Healthcare). Quantitative real-time polymerase chain reaction was performed using primer/probe sets for T-bet, GATA-3, eomesodermin, IL-4, and IFN-γ with GAPDH as a housekeeping gene (Applied Biosystems).
Bone marrow chimeras
Bone marrow cells were isolated from femurs and tibia of the donor mice. A total of 107 cells were intravenously transferred to lethally irradiated recipient mice. For mixed chimera transfer, 5 × 106 cells from each donor mice (1:1 mixture) were intravenously injected to irradiated recipient mice. Mice were analyzed 6 to 8 weeks after transfer.
Cell transfers and serum analysis
A total of 106 purified T cells and B cells were mixed in 200 μL phosphate-buffered saline and injected intraperitoneally to Rag−/− mice, and blood was collected 4 weeks after transfer. Blood samples from indicated mice were collected and supernatants were obtained after spinning for 10 minutes at 4000g. IgE level was analyzed by enzyme-linked immunosorbent assay using IgE enzyme-linked immunosorbent assay kit (BD Biosciences).
Results
Increased percentage and numbers of CD4+ γδ T cells in the absence of Itk
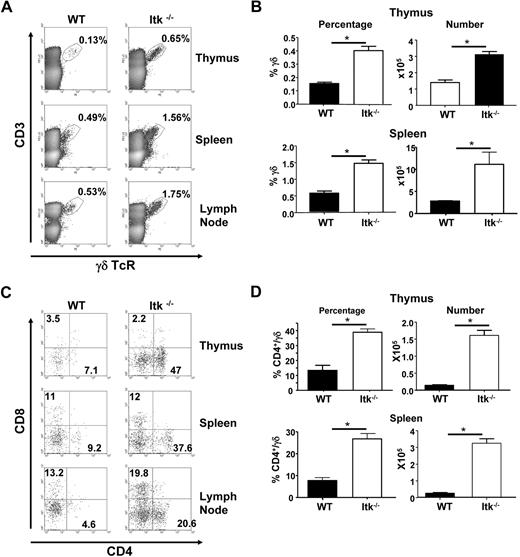
During our analysis of T-cell populations in mice lacking Itk, we noticed that these mice have a higher percentage of γδ TCR-bearing T cells. Careful analysis of these populations revealed that the Itk−/− mice have a higher percentage of γδ T cells in the thymus, spleen, and lymph node (Figure 1A-B). This increased percentage represented increased numbers of these T cells (Figure 1B); thus, in the absence of Itk, there is enhanced development of γδ T cells.
Increased γδ T cells in mice lacking Itk. (A) Flow cytometric analysis of γδ T cells in the thymus, spleen, and lymph node of WT and Itk−/− mice. (B) The percentage and absolute number of γδ T cells were calculated from thymus and spleen. (C) Flow cytometric analysis of CD4 and CD8 populations of CD3+γδTCR+ cells in the thymus, spleen, and lymph nodes of WT and Itk−/− mice. (D) The percentage of CD4+ γδ T cells, as well as the absolute number of CD4+ γδ T cells in the thymus and spleen of WT and Itk−/− mice (n > 10 mice). *P < .01.
Increased γδ T cells in mice lacking Itk. (A) Flow cytometric analysis of γδ T cells in the thymus, spleen, and lymph node of WT and Itk−/− mice. (B) The percentage and absolute number of γδ T cells were calculated from thymus and spleen. (C) Flow cytometric analysis of CD4 and CD8 populations of CD3+γδTCR+ cells in the thymus, spleen, and lymph nodes of WT and Itk−/− mice. (D) The percentage of CD4+ γδ T cells, as well as the absolute number of CD4+ γδ T cells in the thymus and spleen of WT and Itk−/− mice (n > 10 mice). *P < .01.
Further analysis of thymic γδ T cells revealed that approximately 40% of these cells carried the CD4 receptor, whereas WT thymus had a much lower percentage and number of CD4+ γδ T cells (Figure 1C-D). This skewing of the γδ T-cell population to CD4-expressing cells carried over into the periphery, where approximately 30% of γδ T cells in the spleen bore CD4 (Figure 1C). Again, this increased percentage reflected increased numbers (Figure 1D).
Altered phenotype of γδ T cells in the absence of Itk
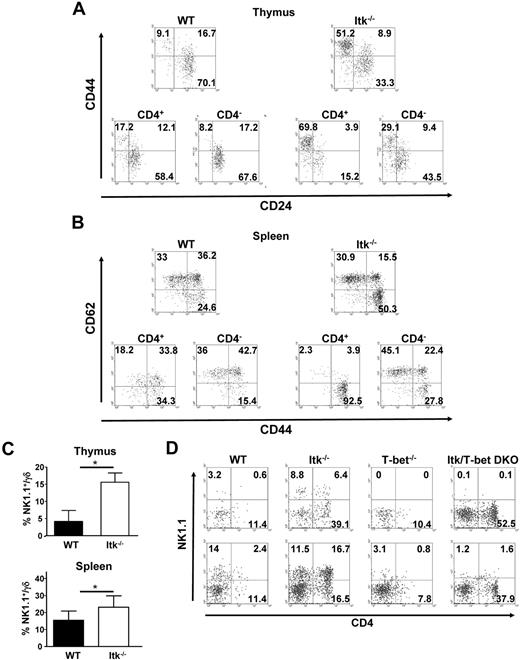
Analysis of the CD4+ and CD4− populations of thymic γδ T cells in WT and Itk null mice for expression of CD44 and CD24 revealed that most of the WT CD4+ γδ T cells are CD44lo/CD24hi, similar to that seen in CD4− γδ T cells and the γδ T-cell population as a whole (Figure 2A). However, Itk null γδ T cells had a higher percentage of cells that were CD44hi/CD24lo. More importantly, whereas the Itk−/− CD4− γδ T-cell population was more like the WT γδ T-cell population with regards to CD44 and CD24 expression, the Itk−/− CD4+ γδ T cells were largely CD44hi/CD24lo.
Surface phenotype of CD4+ γδ T cells from WT and Itk−/− mice. (A) Thymocytes from WT and Itk−/− mice were gated on either CD3+γδTCR+ cells (top) or CD4+CD3+γδTCR+ cells and CD4−CD3+γδTCR+ cells (bottom), and analyzed for the expression of CD44 and CD24. (B) Splenocytes from WT and Itk−/− mice were gated on either CD3+γδTCR+ (top) or CD4+CD3+γδTCR+ cells and CD4−CD3+γδTCR+ cells (bottom) and analyzed for the expression of CD62L and CD44. NK1.1 is expressed but is not required for the development of CD4+ γδ T cells in WT and Itk−/− mice. (C) Thymocytes and splenocytes from WT and Itk−/− mice were analyzed for the percentage of NK1.1+γδ T cells by flow cytometry (n = 7 or 8 mice). *P < .01. (D) γδTCR+CD3+ cells in the thymus and spleens of WT, Itk−/−, T-bet−/−, or T-bet/Itk DKO mice were analyzed for the expression of CD4 and NK1.1 by flow cytometry. Data are representative of 2 independent experiments.
Surface phenotype of CD4+ γδ T cells from WT and Itk−/− mice. (A) Thymocytes from WT and Itk−/− mice were gated on either CD3+γδTCR+ cells (top) or CD4+CD3+γδTCR+ cells and CD4−CD3+γδTCR+ cells (bottom), and analyzed for the expression of CD44 and CD24. (B) Splenocytes from WT and Itk−/− mice were gated on either CD3+γδTCR+ (top) or CD4+CD3+γδTCR+ cells and CD4−CD3+γδTCR+ cells (bottom) and analyzed for the expression of CD62L and CD44. NK1.1 is expressed but is not required for the development of CD4+ γδ T cells in WT and Itk−/− mice. (C) Thymocytes and splenocytes from WT and Itk−/− mice were analyzed for the percentage of NK1.1+γδ T cells by flow cytometry (n = 7 or 8 mice). *P < .01. (D) γδTCR+CD3+ cells in the thymus and spleens of WT, Itk−/−, T-bet−/−, or T-bet/Itk DKO mice were analyzed for the expression of CD4 and NK1.1 by flow cytometry. Data are representative of 2 independent experiments.
Analyzing γδ T cells in the spleen for the expression of CD44 and CD62L, useful markers in determining effector T cells, showed that the CD4+ and CD4− γδ T-cell populations differed with regards to the expression of these markers. The WT CD4+ γδ T cells included a higher percentage of the CD62Llo/CD44hi effector phenotype γδ T cells than the CD4− γδ T-cell population. By contrast, Itk null γδ T cells contained a larger population of CD62Llo/CD44hi cells, and more strikingly, the Itk null CD4+ γδ T-cell population was largely CD62Llo/CD44hi, whereas the CD4− population was more like the WT γδ T-cell population (Figure 2B).
Further analysis of the surface properties of the γδ T cells in Itk null mice revealed that there was also an increase in NK1.1-expressing γδ T cells (Figure 2C). Indeed, a large percentage of the CD4+ γδ T cells were NK1.1+, although there was a significant percentage of CD4− γδ T cells that were also NK1.1+ (Figure 2D). Itk null mice have reduced development of classic NKT cells.27-29 To determine whether this NK1.1 expression contributes to the CD4 expression on Itk−/− γδ T cells, we analyzed mice lacking both T-bet and Itk. T-bet null mice have defective NKT-cell development30 and lack NK1.1+ γδ T cells (Figure 2D). More importantly, mice lacking both Itk and T-bet also lack NK1.1+ γδ T cells, and now carry increased percentage of CD4+ γδ T cells, suggesting that T-bet or NK1.1 expression is not a requirement for the development of the CD4+ γδ T cells in the absence of Itk. However, we cannot rule out the possibility that development of a subpopulation of these cells is dependent on T-bet or NK1.1.
Enhanced development of γδ T cells in the Itk null mice is cell intrinsic
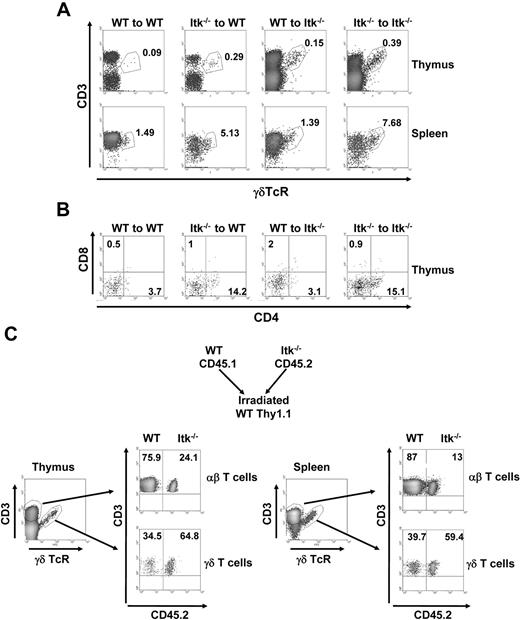
To test whether the enhanced development of CD4+ γδ T cells in the Itk null mice was the result of a cell-intrinsic mechanism or microenvironment, we performed bone marrow transfer experiments. Transfer of Itk−/− bone marrow into sublethally irradiated WT or Itk−/− mice resulted in the same enhanced development of total γδ T cells as well as CD4+ γδ T cells (Figure 3A-B, and supplemental Figure 1, available on the Blood website; see the Supplemental Materials link at the top of the online article). By contrast, transfer of WT bone marrow into sublethally irradiated Itk−/− mice resulted in the same level of γδ T-cell development as when WT bone marrow was transferred into sublethally irradiated WT mice (Figure 3A-B and supplemental Figure 1).
Enhanced development of γδ T cells in the absence of Itk is bone marrow intrinsic. Thy1.1+WT or Thy1.1+Itk−/− bone marrow was intravenously injected to irradiated Thy1.2 WT and Itk−/− mice. Eight weeks later, the percentage of CD3+γδTCR+ cells of donor WT and Itk−/− cells (A) and the percentage of CD4 and CD8 population on donor γδ T cells (B) were analyzed. Data are representative of 2 independent experiments. (C) The 1:1 mixtures of bone marrow from CD45.1/Thy1.2 congenic WT mice and CD45.2/Thy1.2 congenic Itk−/− mice were intravenously injected to irradiated Thy1.1 WT mice. Eight weeks later, the percentage of CD3+γδTCR+ cells of donor WT and Itk−/− was analyzed. Data are representative of 3 mice.
Enhanced development of γδ T cells in the absence of Itk is bone marrow intrinsic. Thy1.1+WT or Thy1.1+Itk−/− bone marrow was intravenously injected to irradiated Thy1.2 WT and Itk−/− mice. Eight weeks later, the percentage of CD3+γδTCR+ cells of donor WT and Itk−/− cells (A) and the percentage of CD4 and CD8 population on donor γδ T cells (B) were analyzed. Data are representative of 2 independent experiments. (C) The 1:1 mixtures of bone marrow from CD45.1/Thy1.2 congenic WT mice and CD45.2/Thy1.2 congenic Itk−/− mice were intravenously injected to irradiated Thy1.1 WT mice. Eight weeks later, the percentage of CD3+γδTCR+ cells of donor WT and Itk−/− was analyzed. Data are representative of 3 mice.
Our data support the view that the altered γδ T-cell development observed in the absence of Itk is the result of intrinsic enhanced development of these cells in the absence of Itk. To further prove this, we performed competitive mixed bone marrow chimera experiments. Bone marrow from WT mice (carrying the CD45.1/Thy1.2 alleles allowing for ease of monitoring) and Itk null mice (carrying CD45.2/Thy1.2 alleles) were mixed in a 1:1 ratio and transferred into sublethally irradiated WT mice carrying the Thy1.1 allele to distinguish host-derived cells. After recovery of the hematopoietic system (8 weeks), the thymus and spleen were analyzed for donor-derived γδ and αβ T cells (Figure 3C and supplemental Figure 1). Our results show that Itk null donors exhibited enhanced development of γδ T cells in thymus and spleen, whereas in the same animals, Itk null donors exhibited reduced development of αβ T cells as we have recently reported.2 Thus, the altered γδ T-cell development in Itk−/− mice is cell intrinsic.
Development of CD4+ γδ T cells in the absence of Itk is independent of MHC class I or class II expression
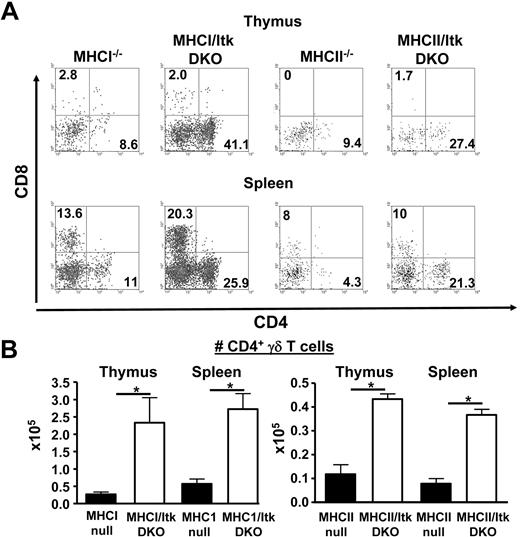
Previous studies have suggested that MHC class I and MHC class II molecules may not be required for the selection of γδ T cells in the thymus. To determine whether the expression of MHC class I or class II molecules was required for the development of the CD4+ γδ T cells in the absence of Itk, we analyzed mice lacking both β2m and Itk (thus lacking the expression of MHC class I) as well as mice lacking both MHC class II and Itk. These mice maintain the enhanced development of the CD4+ γδ T-cell population in the absence of Itk, indicating that, unlike NKT and conventional T cells, the development of these CD4+ γδ T cells is MHC independent (Figure 4). We did note that the MHCII−/− mice had reduced numbers of γδ T cells overall, contributing to the overall reduction in the CD4+ γδ T-cell population. Comparison of the CD4+γδ T-cell population in these mice suggests that MHCII may play some role in their development and/or expansion as there were significantly lower numbers of these cells in the MHCII/Itk double knockout mice compared with the Itk−/− mice (P < .01 for both thymic and splenic populations, MHCII/Itk DKO compared with Itk−/− mice). By contrast, no significant difference was found in the numbers of these cells in mice lacking MHCI.
Increased CD4+ γδ T cells in the absence of Itk is independent of MHC class I or II expression. (A) γδ T cells in the thymus and spleens of MHCI−/−, MHCI/Itk DKO, MHCII−/−, and MHCII/Itk DKO mice were analyzed for the expression of CD8 and CD4. Data are representative of 2 independent experiments. (B) The number of CD4+ γδ T cells in the mice analyzed in panel A were enumerated and presented (n = 3 mice). *P < .05.
Increased CD4+ γδ T cells in the absence of Itk is independent of MHC class I or II expression. (A) γδ T cells in the thymus and spleens of MHCI−/−, MHCI/Itk DKO, MHCII−/−, and MHCII/Itk DKO mice were analyzed for the expression of CD8 and CD4. Data are representative of 2 independent experiments. (B) The number of CD4+ γδ T cells in the mice analyzed in panel A were enumerated and presented (n = 3 mice). *P < .05.
Itk null CD4+ γδ T cells predominantly express Vγ1.1/Vδ6.2/3 γδ T-cell receptor
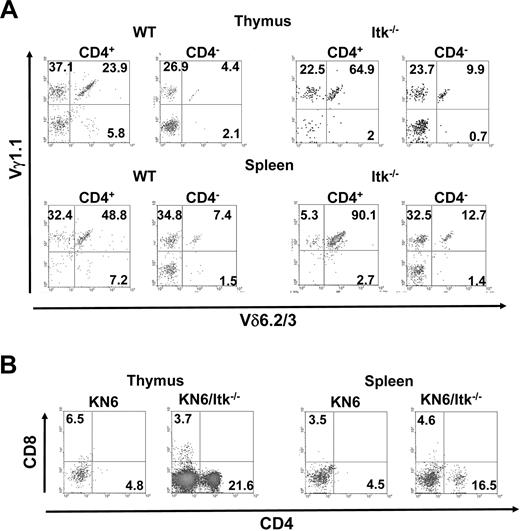
γδ T cells usually carry oligoclonal TCRs, with a few receptors predominating. We therefore determined whether the Itk null CD4+ γδ T cells differed in the TCR that they carried. Thymic CD4+ γδ T cells in the Itk null mice did not differ with regards to expression of Vγ2 (using the Garman nomenclature31 ); however, a higher percentage of Itk null CD4+ γδ T cells expressed Vδ6.2/3 (supplemental Figure 2). In addition, a large population of the γδ T cells in both WT and Itk null mice carry the Vγ1.1 TCR (supplemental Figures 2-3). Indeed, whereas the CD4+ and CD4− γδ T cells in WT had equal percentage of cells that express the Vγ1.1/Vδ6.2/3 TCR, a significant majority of the CD4+ γδ T cells in the Itk null mice expressed this TCR (Figure 5A, supplemental Figure 3). Thus, in the absence of Itk, a large percentage of the CD4+ γδ T cells that develop carry the Vγ1.1/Vδ6.2/3 TCR.
Itk null CD4+ γδ T cells develop independent of the γδ TCR they express. (A) γδ T cells in the thymus and spleens of WT and Itk−/− mice were analyzed for the expression of Vγ1.1 and Vδ6.2/3. Data are representative of 3 independent experiments. (B) γδ T cells in the thymus and spleens of KN6 transgenic mice, or KN6 transgenic mice lacking Itk, were analyzed for the expression of CD4 and CD8. Data are representative of 2 independent experiments.
Itk null CD4+ γδ T cells develop independent of the γδ TCR they express. (A) γδ T cells in the thymus and spleens of WT and Itk−/− mice were analyzed for the expression of Vγ1.1 and Vδ6.2/3. Data are representative of 3 independent experiments. (B) γδ T cells in the thymus and spleens of KN6 transgenic mice, or KN6 transgenic mice lacking Itk, were analyzed for the expression of CD4 and CD8. Data are representative of 2 independent experiments.
To determine whether the expression of this Vγ1.1/Vδ6.2/3 TCR was driving the enhanced development of CD4+ γδ T cells in the absence of Itk, we analyzed mice carrying the KN6 transgenic mice, which carry the Vγ2 TCR,32 for the presence of these cells. Whereas KN6 transgenic mice on a WT background had few CD4+ γδ T cells, KN6 transgenic mice on an Itk null background had a significantly higher percentage of CD4+ γδ T cells (Figure 5B, supplemental Figure 3). These data argue against an antigen-driven response or preferential expression of a particular TCR as the explanation for the enhanced development of CD4+ γδ T cell in the absence of Itk.
Itk null CD4+ γδ T cells predominantly express Th2 cytokine IL-4 and Itk null mice have high levels of serum IgE
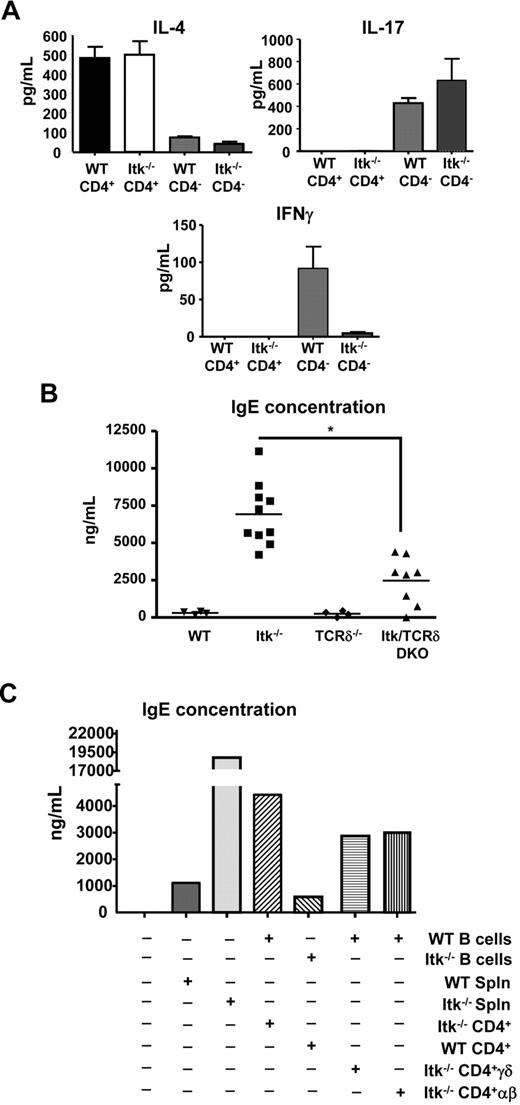
γδ T cells have been reported to secrete IL-4, IFN-γ, and IL-17 both ex vivo and in vivo.19-21 We therefore analyzed cytokine secretion response by sorting WT and Itk null CD4− and CD4+ γδ T cells and stimulated them with anti-γδ TCR for 72 hours, followed by analysis of the supernatants for the presence of IL-4, IL-17, and IFN-γ. We found that both WT and Itk−/− CD4+ γδ T cells secrete significant levels of IL-4, but very little IL-17 or IFN-γ (Figure 6A). Itk null CD4+ γδ T cells also had higher levels of preformed transcripts for IL-4, suggesting that they are poised to secrete this cytokine (supplemental Figure 4). In addition, they had slightly elevated levels of T-bet and GATA3 and significantly reduced levels of eomesodermin transcripts (supplemental Figure 4). In contrast, only CD4− γδ T cells from WT and Itk null mice secreted IL-17, whereas CD4− γδ T cells from WT but not Itk null mice secreted appreciable levels of IFN-γ, suggesting that Itk is required for signaling to IFN-γ but not IL-17 secretion (Figure 6A). However, WT and Itk−/− CD4− γδ T cells have comparable levels of IFN-γ mRNA, indicating that the expression of IFN-γ transcript is Itk-independent and that Itk may be required for the translation of this message (supplemental Figure 4). Thus, CD4+ γδ T cells are selective in their cytokine secretion pattern, secreting IL-4, but not IL-17 or IFN-γ, whereas CD4− γδ Τ cells can secrete IL-17 and IFN-γ with different requirements for Itk.
Itk null CD4+ γδ T cells secrete IL-4 and induced B-cell class switch to IgE. (A) Sorted CD4+CD3+γδTCR+ cells and CD4−CD3+γδTCR+ cells from WT and Itk−/− mice were stimulated with anti-γδTCR for 3 days. Supernatants were then analyzed for IL-4, IFN-γ, and IL-17. Data are representative of 3 independent experiments. Serum concentrations of IgE antibody in unimmunized mice. (B) Sera from the indicated mice were analyzed for IgE concentration. *P < .05. (C) Rag−/− mice were reconstituted with different groups of B cells and T cells as indicated. Sera were analyzed for IgE 4 weeks after transfer.
Itk null CD4+ γδ T cells secrete IL-4 and induced B-cell class switch to IgE. (A) Sorted CD4+CD3+γδTCR+ cells and CD4−CD3+γδTCR+ cells from WT and Itk−/− mice were stimulated with anti-γδTCR for 3 days. Supernatants were then analyzed for IL-4, IFN-γ, and IL-17. Data are representative of 3 independent experiments. Serum concentrations of IgE antibody in unimmunized mice. (B) Sera from the indicated mice were analyzed for IgE concentration. *P < .05. (C) Rag−/− mice were reconstituted with different groups of B cells and T cells as indicated. Sera were analyzed for IgE 4 weeks after transfer.
We and others have previously reported that mice lacking Itk have elevated levels of serum IgE and higher IgE class switch of B cells, although their CD4+ αβ T cells have defects in secreting IL-4.11,33 As we have now identified increased numbers of CD4+ γδ T cells in Itk null mice that can secrete significant levels of IL-4, we hypothesized that they may be responsible for the enhanced class switch of B cells to IgE and the elevated levels of serum IgE observed in these mice. We therefore analyzed the serum of mice lacking Itk and γδ T cells to determine whether removing these T cells would reduce the elevated levels of IgE seen in the absence of Itk. We found that mice lacking both Itk and γδ T cells had much reduced serum IgE compared with mice lacking Itk alone, although not as low as that seen in mice lacking γδ T cells or WT mice (Figure 6B). These data suggest that γδ T cells make a significant contribution to the elevated serum IgE observed in Itk−/− mice.
Itk null CD4+ γδ T cells induce B cell class switch to IgE
To determine whether Itk null CD4+ γδ T cells can induce B-cell class switch for secretion of IgE, we set up a cell transfer model to analyze class switch in vivo. In these experiments, we used cells from WT or Itk null mice to transfer into Rag null mice, followed by analysis of serum for IgE 4 weeks later. Our experiments show that transfer of Itk null splenocytes results in increased levels of serum IgE compared with equivalent transfer of WT splenocytes (Figure 6C). Furthermore, transfer of Itk null CD4+ T cells along with WT B cells resulted in enhanced serum IgE levels, whereas transfer of Itk null B cells along with WT CD4+ T cells did not, suggesting that Itk−/− CD4+ T cells are responsible for the production of IgE by B cells in Itk null mice. Further analysis revealed that mice receiving Itk−/− CD4+ γδ T cells and WT B cells displayed elevated serum IgE after 4 weeks. Because WT B cells were used for all the experiments, we excluded the possibility that the difference in IgE production is caused by an increase in IgE production from IgE-expressing B cells. These data indicate that CD4+ γδ T cells can induce class switch in B cells, resulting in elevated serum IgE. However, mice receiving Itk−/− CD4+ αβ T cells and WT B cells also displayed elevated serum IgE after 4 weeks, suggesting that there are cells within the αβ T-cell population that can induce class switch in the absence of Itk. This is supported by our analysis of serum IgE levels in the Itk/γδ double knockout mice, which still have higher serum levels of IgE than WT or γδ null mice (Figure 6B). Altogether, our data indicate that Itk null CD4+ γδ T cells secrete IL-4 and can induce class switch to IgE, contributing to the elevated serum IgE in these mice. Our data also suggest that other cell types may contribute to this phenotype.
Discussion
We show here that Itk regulates the differentiation and function of γδ T cells. In the absence of Itk, there is enhanced development of CD4+ γδ T cells. These cells carry a predominant γδ TCR, although this is not a requirement for their development. These cells develop in the absence of the expression of MHC class I or class II, suggesting an independent developmental pathway from classic T cells as well as nonconventional T cells, such as NKT cells and innate memory phenotype T cells.2-5,34 Finally, our data support the role of these cells as IL-4–secreting cells in vivo, which can enhance B-cell class switch to IgE and may partially explain the paradox of elevated serum IgE in Itk null mice, despite the defect in Th2 development exhibited by the αβ CD4+ T cells in these mice.
Recent studies have shown that different T-cell populations have different developmental requirements for signaling pathways regulated by Itk. “Naive phenotype” (CD44loCD62Lhi) CD4+ and CD8+ αβ T cells require Itk for their development and function, whereas the “innate memory phenotype” (CD44hiCD62Llo) CD4+ and CD8+ αβ T cells do not.2,4,5,35 Here we show that CD4− and CD4+ γδ T cells also have different requirements for Itk. Itk deficiency does not change the development of CD4− γδ T cells in the thymus and spleen, but their ability to secrete IFN-γ when stimulated through the TCR-signaling pathway is defective. In addition, Itk deficiency leads to accumulation of mature CD4+ γδ T cells with effector phenotypes, indicating that it plays an important role in modulating the development of these cells. However, Itk is not required for TCR induced secretion of IL-4 by CD4+ γδ T cells.
Negative selection has been reported to be important in the development of γδ T cells in adult thymus. Itk is downstream of TCR and functions in part as an amplifier of TCR signals. The lack of Itk may therefore weaken TCR signals necessary for γδ T cell–negative selection, leading to the enhanced survival of CD4+ γδ T cells in Itk null mice. Because the natural ligands for most γδ TCRs remain unknown, Itk null mice in γδ TCR transgenic background will be needed to answer this question.
Several studies have suggested that γδ T cells are able to rapidly secrete IL-4 both in vivo and ex vivo.20,21 It has also been suggested, but not directly shown, that these IL-4–producing γδ T cells participate in helping B cells produce IgE.36,37 In particular, the presence of the CD4+ population of γδ T cells has been correlated with elevated levels of serum IgE. For example, mice with a LAT mutation have dramatically increased percentage of CD4+ γδ T cells, which produced large amounts of IL-4 when stimulated ex vivo and are correlated with elevated IgE levels in these mice.37 Similar findings have been reported for mice deficient in Itch, an E3 ubiquitin ligase, which has a population of IL-4–producing γδ T cells and is correlated to the elevated level of IgE in these mice, although these investigators did not determine whether these cells expressed CD4.38 Our results now suggest that the CD4+ γδ T-cell population is most probably responsible for the elevated serum IgE observed in these mice.
Both Itk and LAT are part of the same αβ TCR-signaling complexes, with LAT lying upstream of Itk.39 The increased population of CD4+ γδ T cells in both types of mice suggests that the γδ TCR-signaling pathway modulates this pathway and also suggests that Itk and LAT are also in the same γδ TCR-signaling complexes. Besides the similarity, LAT mutant and Itk−/− CD4+ γδ T also have some differences. The LAT mutant mice do not have higher numbers of γδ T cells until approximately 20 weeks, and the population only exists in the periphery.37 In comparison, increased numbers of γδ T cells were found both in the thymus and periphery in young Itk−/− mice and 20-week-old Itk−/− mice did not show any further increases in γδ T-cell number (data not shown). In addition, both WT and Itk−/− CD4+ γδ T cells secrete large amounts of IL-4 when stimulated with anti-γδ TCR, suggesting that the lack of Itk does not affect γδ TCR signaling leading to IL-4 secretion. However, LAT mutant γδ T cells can only secrete IL-4 after stimulation with phorbol myristate acetate and ionomycin, but not TCR cross-linking, indicating that the signaling pathway from the γδ TCR is defective in the absence of LAT for induction of IL-4 secretion.37 LAT is upstream of Itk, and this LAT mutation may affect Itk function or incorporation into or affect the signaling complex.37,40 Thus, these differences in γδ T cells between Itk and LAT mice may be the result of differences in TCR signal strength.
One recent study has demonstrated that antigen-naive γδ T cells preferentially secrete IL-17, whereas antigen experienced γδ T cells preferentially secrete IFN-γ.41 CD4+ γδ T cells in the spleen are CD44hi/CD62low, an effector phenotype, and thus may be predicted to preferentially secrete IFN-γ. However, neither WT nor Itk−/− CD4+ γδ T cells secreted IL-17 or IFN-γ. In contrast, large amounts of IL-17 and IFN-γ were secreted by WT CD4− γδ T cells, suggesting that CD4− and CD4+ γδ T cells may be 2 independent subsets of γδ T cells and that CD4+ γδ T cells may secrete IL-4 by default. Consistent with this, the CD4+ population of γδ T cells had dramatically increased IL-4 mRNA compared with CD4− γδ T cells both in the thymus and spleen (supplemental Figure 4). The key transcription factors for differentiation of αβ T cells to Th1 or Th2 cells and subsequent cytokine secretion are T-bet and GATA-3. However, although splenic CD4+ γδ T cells from Itk null mice had significantly higher levels of GATA-3 mRNA, there is less of a difference in the thymus. In addition, Itk−/− CD4+ γδ T cells also expressed higher amounts of T-bet mRNA than WT γδ T cells. Because T-bet is largely only expressed in effector but not naive γδ T cells, this increased T-bet in Itk−/− γδ T cells may reflect their effector-like phenotype,42 although T-bet is not required for their development. We cannot however, rule out a role for T-bet in small subpopulations of these cells. Eomesodermin is another T-box family transcription factor that has been shown to correlate with the development of innate CD8+ T cells in Itk−/− mice.5 However, it is interesting that the expression of eomesodermin is dramatically decreased in WT and Itk−/− CD4+ γδ T cells relative to CD4− γδ T cells (supplemental Figure 4). These data suggest that perhaps the ratio of transcription factors may be important for the development and function of CD4+/CD4− γδ T cells, and the molecular mechanism involved in this process may be different from that seen in αβ T cells.
Several studies on allergic asthma have shown that γδ T cells, including Vγ1.1-bearing γδ T cells, contribute to allergic airway inflammation by producing IL-4 and thus inducing IL-4–dependent IgE and IgG1 responses.36,43,44 We have shown that mice lacking Itk are resistant to developing allergic asthma, suggesting that, despite the presence of the CD4+ γδ T-cell population in these mice, this is insufficient to drive the development of allergic asthma if the conventional αβ T cell cannot secrete Th2 cytokines.10,11,28,33,45,46 However, our data that Itk/TCRδ double knockout mice still have levels of IgE above that seen in WT or TCRδ−/− mice, along with the ability of Itk−/− CD4 αβ T cells to induce class switch in the Rag transfer assay, suggest that there may be other populations of T cells that, in the absence of Itk, contribute to the elevated IgE observed in these mice. These may be populations within the innate memory phenotype or nonconventional αβ T cells we and others have recently described.2,4,5,35 These αβ T cells have the capacity to secrete IL-4, even in the absence of Itk. Our data also indicate that the consequence of targeting Itk for diseases such as allergic asthma should be approached with a nuanced understanding of its effects in all of the cell types in which it is expressed.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the members of the August laboratory, Center for Molecular Immunology & Infectious Disease, for feedback and discussion, and E. Kunze, N. Bem, and S. Magargee at the Center for Quantitative Cell Analysis at Penn State.
This work was supported in part by grants from the National Institutes of Health (AI51626 and AI065566; A.A.). The Center for Molecular Immunology & Infectious Disease at Penn State is supported in part by a grant from the Pennsylvania Department of Health.
National Institutes of Health
Authorship
Contribution: Q.Q. designed and performed the experiments, analyzed the data, and wrote the manuscript; M.X. designed and performed the experiments and analyzed the data, J.H. designed and performed the experiments; E.H. performed the experiments; A.I. designed and performed the experiments; N.X. designed the experiments and analyzed the data; and A.A. designed experiments, analyzed the data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors decalre no competing financial interests.
Correspondence: Avery August, Center for Molecular Immunology & Infectious Disease, Department of Veterinary & Biomedical Sciences, Pennsylvania State University, 115 Henning Bldg, University Park, PA 16802; e-mail: avery@psu.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal