Abstract
In susceptible hosts, angioinvasion by Aspergillus fumigatus triggers thrombosis, hypoxia, and proinflammatory cytokine release, all of which are stimuli for angiogenesis. We sought to determine whether A fumigatus directly modulates angiogenesis. A fumigatus culture filtrates profoundly inhibited the differentiation, migration, and capillary tube formation of human umbilical vein endothelial cells in vitro. To measure angiogenesis at the site of infection, we devised an in vivo Matrigel assay in cyclophosphamide-treated BALB/c mice with cutaneous invasive aspergillosis. Angiogenesis was significantly suppressed in Matrigel plugs implanted in A fumigatus–infected mice compared with plugs from uninfected control mice. The antiangiogenic effect of A fumigatus was completely abolished by deletion of the global regulator of secondary metabolism, laeA, and to a lesser extent by deletion of gliP, which controls gliotoxin production. Moreover, pure gliotoxin potently inhibited angiogenesis in vitro in a dose-dependent manner. Finally, overexpression of multiple angiogenesis mediator–encoding genes was observed in the lungs of cortisone-treated mice during early invasive aspergillosis, whereas gene expression returned rapidly to baseline levels in cyclophosphamide/cortisone-treated mice. Taken together, these results indicate that suppression of angiogenesis by A fumigatus both in vitro and in a neutropenic mouse model is mediated through secondary metabolite production.
Introduction
The opportunistic filamentous fungus Aspergillus fumigatus is the most frequent cause of invasive fungal infections in severely immunocompromised patients.1 A fumigatus is characterized by its angiotropism and propensity for angioinvasion.2,3 Invasive aspergillosis is initiated when asexual spores (conidia) are inhaled by susceptible persons, germinate within the alveolar spaces, and penetrate the respiratory epithelium and vascular endothelium.2,3 Vascular invasion by A fumigatus fosters the release of proinflammatory cytokines and activation of the coagulation cascade, resulting in intravascular thrombosis and tissue ischemia.4,5 These events may result in the sequestration of Aspergillus-infected tissue, limiting the delivery of immune effector cells and antifungal drugs to the site of infection.6 Extensive fungal proliferation and coagulative necrosis are particularly prominent features of invasive aspergillosis in hosts with quantitative or qualitative defects in their polymorphonuclear leukocytes.7,8
Angiogenesis, the formation of new blood vessels from existing ones, is a physiologic response to tissue inflammation and ischemia.9-11 Proinflammatory cytokines such as tumor necrosis factor-α (TNF-α) and interleukin-8 (IL-8), which are released from endothelial cells as a result of their interaction with A fumigatus hyphae,5 are potent inducers of proangiogenic signaling pathways involving vascular endothelial growth factor (VEGF) and basic fibroblast growth factor (bFGF).12 In contrast, A fumigatus synthesizes an impressive array of secondary metabolites, some of which have shown promise as anticancer agents because of their potent antiangiogenic activity.13-16 Whether the net effect of these opposing stimuli is proangiogenic or antiangiogenic has not been studied.
In this study, we demonstrate that A fumigatus inhibits human umbilical vein endothelial cell (HUVEC) migration and capillary tube formation in vitro. Furthermore, A fumigatus suppressed angiogenesis locally in a murine model of cutaneous infection. Deletion of the global regulator of secondary metabolism laeA abolished the antiangiogenic activity of A fumigatus, indicating that inhibition of angiogenesis is mediated by secondary fungal metabolites. Moreover, we identified gliotoxin as a likely mediator of angiogenesis inhibition by A fumigatus. Finally, we observed down-regulation of angiogenesis-associated gene expression in the lungs of mice immunosuppressed with cortisone acetate and cyclophosphamide during the initial phase of experimental invasive aspergillosis. These findings suggest that modulation of host angiogenesis by secondary fungal metabolites represents a novel aspect of the pathogenesis of invasive aspergillosis in immunocompromised hosts.
Methods
Strains
The A fumigatus clinical strain Af293 was the isogenic wild-type strain for laeA and gliP deletion mutants. The laeA disruptant (ΔlaeA) strain TJW54.2 and the laeA-complemented strain TJW68.6 were gifts from N. Keller (University of Wisconsin).17 The gliP deletion mutant (ΔgliP) was a gift from G. May (University of Texas M. D. Anderson Cancer Center).18
For the in vitro Matrigel assay, A fumigatus conidia were inoculated into liquid minimal medium containing 2% (vol/vol) 50× salts, 2% (wt/vol) glucose, 1.2% 1M KPO4 (pH 6.8), and 0.1% trace elements (105 cells/mL) and placed in a shaking incubator (220 rpm) at 37°C for 72 hours. Submerged cultures were subsequently transferred to 50-mL flasks and centrifuged for 10 minutes at 1900g to pellet the mycelium. Culture supernatants were passed through 0.2-μm nitrocellulose filters (Fisher Scientific) and used as test material in the Matrigel assay.
For subcutaneous and intranasal inoculations, A fumigatus isolates were grown on yeast extract agar glucose plates in a 37°C incubator for 72 hours. Conidia were collected in 0.9% sterile normal saline with 0.08% Tween 20, washed twice in normal saline, and filtered through 40-μm nylon filters (BD Biosciences). The concentration of the conidial suspensions was determined in a hemacytometer and adjusted to the required concentrations (see below).
Gliotoxin
A stock solution of gliotoxin (Sigma-Aldrich) was prepared in 100% methanol at 1 mg/mL. Dilutions of gliotoxin were prepared in minimal medium at concentrations ranging from 30 to 3000 ng/mL, according to the range of gliotoxin concentrations observed in the lungs and sera of mice and patients with invasive aspergillosis in a previous study.19
In vitro angiogenesis assay
An in vitro Matrigel assay was performed, as described previously.20 This method assesses the proangiogenic and antiangiogenic properties of tested substances based on the differentiation of endothelial cells on a basement membrane matrix (Matrigel; BD Biosciences) into capillary-like structures. Adherent HUVECs (Lonza) were propagated in Clonetics endothelial growth medium with 2% fetal bovine serum, bovine brain extract, hydrocortisone, human endothelial growth factor, and gentamicin/amphotericin B (Lonza) in 5% CO2 in a 37°C incubator. Matrigel was thawed overnight on ice and dispensed into 48-well plates (300 μL/well). Plates were incubated at 37°C for 1 hour to allow for polymerization of the Matrigel. Subconfluent HUVEC cultures were collected in trypsin/EDTA (ethylenediaminetetraacetic acid) and resuspended in endothelial growth medium at a final concentration of 0.5 × 106 viable cells/mL. Two hundred microliters of the HUVEC suspension was added into each Matrigel-coated well with 50 μL of test material. After overnight incubation at 37°C, plates were aspirated, fixed, and stained with Hema 3 solution II (Fisher Scientific) diluted 1/1 in water. Endothelial cell networks were observed with an Olympus CK 40 inverted microscope (Olympus) at a magnification of ×100, and images were acquired with an Olympus DP-12 camera. Capillary tubes were counted in 4 fields per well. Matrigel assays were performed in 6 replicates for each experimental group and repeated 3 times.
Cutaneous murine model of invasive aspergillosis
Cutaneous aspergillosis was established in female BALB/c mice weighing 18 to 20 g (National Cancer Institute) immunosuppressed with cyclophosphamide (100 mg/kg administered intraperitoneally 4 days and 1 day before inoculation). One hundred microliters of conidial suspension (5 × 107 cells/mL) was injected subcutaneously into the right thigh of each mouse under isoflurane-induced anesthesia. Control mice received the same immunosuppressive regimen, but were injected subcutaneously with 100 μL of sterile 0.9% saline instead of conidia. Additional cyclophosphamide (100 mg/kg given intraperitoneally) was administered on days 2, 4, and 6 after the inoculation of conidia to maintain neutropenia. Each experimental group consisted of 5 mice, and experiments were repeated 3 times. Infection was verified in each mouse by microscopical examination of Grocott-Gomori methenamine silver-stained tissue sections. All procedures were performed according to the highest standards for humane handling, care, and treatment of research animals, and were approved by the M. D. Anderson Cancer Center Institutional Animal Care and Use Committee.
In vivo angiogenesis assay
The in vivo Matrigel assay described by Passaniti et al21 was adapted for use with our murine model of cutaneous invasive aspergillosis. Matrigel was thawed and mixed with bFGF (R&D Systems) at 150 ng/mL and heparin (Fisher Scientific) at 64 U/mL. On day 3 after inoculation of A fumigatus in mice, 500 μL of supplemented Matrigel was injected subcutaneously between the Aspergillus injection site and the dorsal midline at a distance of 10 mm from the infection site. When warmed to body temperature, Matrigel polymerizes to form subcutaneous plugs; endothelial cells migrate into the Matrigel plugs within 48 hours after injection, and fully formed capillary networks can be observed 3 to 4 days after injection.21 At different time points after infection, mice were killed by CO2-induced asphyxiation, and the Matrigel plugs were excised, fixed in 10% formalin, embedded in paraffin wax, sectioned, and stained with Masson trichrome stain. Slides were observed with a BX51 Olympus microscope fitted with a SPOT RT digital camera (Diagnostic Instruments). Images of approximately 10 randomly selected fields per specimen were captured at ×200 magnification using SPOT software (Version 4.6; Diagnostic Instruments). Cell density was measured with imageJ software program (http://rsb.info.nih.gov/ij/; National Institutes of Health).
Angiogenesis-associated gene expression studies
To characterize changes in angiogenesis-associated gene expression in the early stages of invasive pulmonary aspergillosis (IPA), we studied gene expression in the lungs of A fumigatus–infected mice with different immunosuppression backgrounds. Female BALB/c mice were immunosuppressed with either a combination of cyclophosphamide (250 mg/kg given intraperitoneally) and cortisone acetate (250 mg/kg given subcutaneously) on the day before inoculation of A fumigatus conidia (neutropenic model) or cortisone acetate alone (250 mg/kg given subcutaneously) 4 days and 1 day before inoculation (nonneutropenic model). Mice were infected intranasally by instillation of a 35-μL droplet of A fumigatus Af293 conidial suspension (5 × 107 conidia/mL) under isoflurane-induced anesthesia.
To confirm conidial germination and tissue invasion, we examined Grocott-Gomori methenamine silver-stained lung tissue from 2 mice from each of the gene expression experimental groups. Twenty-four hours after inoculation, the majority of conidia had germinated and formed germ tubes, and hyphal elements were seen within the lung parenchyma (supplemental Figure 1, available on the Blood website; see the Supplemental Materials link at the top of the online article). In our previous work, we detected gliotoxin as early as 24 hours after inoculation of growth medium with Af293 conidia.22 Based on these observations, and because gene expression at later time points could be confounded by extensive tissue damage and the activation of multiple reactive pathways, we reasoned that the optimal time to study the effect of gliotoxin and other secondary metabolites on angiogenesis-related gene expression in murine lungs would be 24 hours after inoculation.
Mice were killed either 1 hour or 24 hours after inoculation in groups of 3, and their lungs were excised and stored in RNA stabilization solution (RNAlater; QIAGEN). RNA was extracted from mouse lungs with the RNeasy tissue kit (QIAGEN), according to the manufacturer's instructions, and analyzed to determine its integrity in a 2100 bioanalyzer (Agilent). Expression of 84 growth factors and proinflammatory cytokines related to angiogenesis was analyzed with quantitative real-time polymerase chain reaction (PCR) using the RT2 profiler PCR array (PAMM-072; SABiosciences). The gene expression in each sample, expressed as the cycle threshold, was normalized according to the expression of 5 housekeeping genes and compared with the gene expression in the lungs of mice that underwent the same immunosuppression, but were infection-free (ΔΔ cycle threshold).
Statistical analysis
Neovessel densities in Matrigel in different treatment groups were compared using 1-sided analysis of variance. Post hoc comparisons between pairs of treatment groups were performed using the Bonferroni multiple comparisons test. Angiogenesis-related gene expression in the infected and control groups was compared using an unpaired Student t test. A 2-tailed P value of less than .05 was considered statistically significant. Calculations were performed using the InStat software program (GraphPad).
Results
A fumigatus Af293 culture filtrates abolish angiogenesis in vitro
We studied the effect of A fumigatus Af293 culture filtrates on HUVEC differentiation, migration, and capillary tube formation in vitro. We observed extensive neovessel formation in Matrigel-coated control wells inoculated with HUVECs and a fresh culture medium (median, 44 capillary tubes/field [range, 28-54 capillary tubes/field]). In contrast, angiogenesis was significantly inhibited in wells inoculated with A fumigatus Af293 culture filtrates (median, 8 capillary tubes/field [range, 0-17 capillary tubes/field]; 82% inhibition; P < .001; Figure 1).
Effect of A fumigatus culture filtrates on HUVEC differentiation, migration, and capillary tube formation on Matrigel. Compared with control wells inoculated only with growth medium (A-B), differentiation and capillary network formation by HUVECs were significantly inhibited in wells inoculated with A fumigatus (Af293) culture filtrate (C). Capillary network formation was not affected by ΔlaeA culture filtrates (D), whereas complementation of the laeA defect restored the inhibitory activity of A fumigatus culture filtrates against HUVEC function (E). ΔgliP exhibited incomplete inhibitory activity against HUVECs (F-G), suggesting that gliotoxin is one of several secondary metabolites that mediate the antiangiogenic effects of A fumigatus culture filtrates. *P < .01; **P < .001. Except where indicated otherwise, P values refer to the comparison with control wells.
Effect of A fumigatus culture filtrates on HUVEC differentiation, migration, and capillary tube formation on Matrigel. Compared with control wells inoculated only with growth medium (A-B), differentiation and capillary network formation by HUVECs were significantly inhibited in wells inoculated with A fumigatus (Af293) culture filtrate (C). Capillary network formation was not affected by ΔlaeA culture filtrates (D), whereas complementation of the laeA defect restored the inhibitory activity of A fumigatus culture filtrates against HUVEC function (E). ΔgliP exhibited incomplete inhibitory activity against HUVECs (F-G), suggesting that gliotoxin is one of several secondary metabolites that mediate the antiangiogenic effects of A fumigatus culture filtrates. *P < .01; **P < .001. Except where indicated otherwise, P values refer to the comparison with control wells.
A fumigatus inhibits angiogenesis in vivo
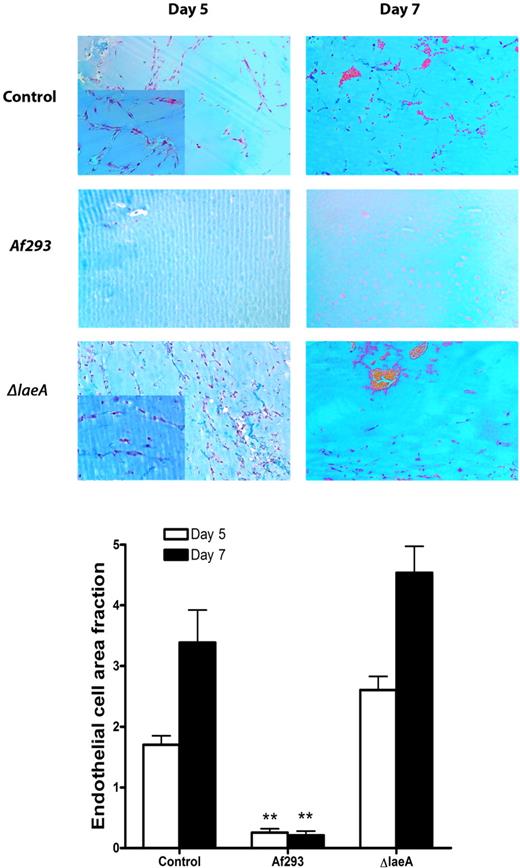
To determine whether the antiangiogenetic effects of A fumigatus can also be observed in vivo, we studied capillary tube formation in Matrigel plugs implanted subcutaneously into neutropenic mice in proximity to A fumigatus-infected soft tissue. We compared the angiogenesis in plugs extracted from Af293-infected mice with that in plugs obtained from control mice that received the same cyclophosphamide regimen, but were not inoculated with A fumigatus. We observed an intense angiogenic response in the control Matrigel plugs, with endothelial cell migration and network formation 5 days after Matrigel implantation (Figure 2). On day 7 after implantation, erythrocyte-filled lacunae were observed within the Matrigel. In contrast, endothelial cell migration into Matrigel plugs obtained from Af293-infected mice was minimal, and we observed no erythrocytes within the plugs 7 days after implantation. Quantitative assessment of endothelial cell density revealed a significantly lower endothelial cell area in plugs obtained from Af293-infected mice than in plugs obtained from uninfected control mice (85% reduction on day 5, P < .001; 94% reduction on day 7, P < .001; Figure 2).
In vivo Matrigel assay of angiogenesis in A fumigatus-infected and uninfected control mice. The antiangiogenic effects of A fumigatus in mice with cutaneous invasive aspergillosis were assessed using an in vivo Matrigel assay. In uninfected control mice, endothelial cell migration and capillary network formation occurred 5 days after implantation of the Matrigel; 7 days after implantation, erythrocyte-filled lacunae formed in the Matrigel. In contrast, angiogenesis was significantly suppressed in Matrigel plugs extracted from A fumigatus-infected mice 5 and 7 days after inoculation. Plugs obtained from ΔlaeA-infected mice exhibited endothelial cell infiltration similar to that of plugs obtained from uninfected control mice. Insets show enhanced details of endothelial networks. **P < .001. P values refer to the comparison with Matrigel plugs obtained from uninfected control mice and plugs from ΔlaeA-infected mice.
In vivo Matrigel assay of angiogenesis in A fumigatus-infected and uninfected control mice. The antiangiogenic effects of A fumigatus in mice with cutaneous invasive aspergillosis were assessed using an in vivo Matrigel assay. In uninfected control mice, endothelial cell migration and capillary network formation occurred 5 days after implantation of the Matrigel; 7 days after implantation, erythrocyte-filled lacunae formed in the Matrigel. In contrast, angiogenesis was significantly suppressed in Matrigel plugs extracted from A fumigatus-infected mice 5 and 7 days after inoculation. Plugs obtained from ΔlaeA-infected mice exhibited endothelial cell infiltration similar to that of plugs obtained from uninfected control mice. Insets show enhanced details of endothelial networks. **P < .001. P values refer to the comparison with Matrigel plugs obtained from uninfected control mice and plugs from ΔlaeA-infected mice.
Secondary metabolism is required for A fumigatus–induced inhibition of angiogenesis
We hypothesized that the inhibition of angiogenesis in vitro by A fumigatus culture filtrates is mediated by soluble metabolites. To test this hypothesis, we compared the effects of HUVEC exposure to Af293 culture filtrates with those of exposure to culture filtrates of the isogenic laeA deletion mutant TJW54.2, which is defective in the expression of multiple metabolic gene clusters, including the gliotoxin gene cluster.23 Angiogenesis in wells inoculated with ΔlaeA culture filtrates was similar to that in control wells inoculated with fresh growth medium, and significantly greater than that in wells exposed to Af293 culture filtrates (P < .001; Figure 1). Culture filtrates of the laeA-complemented strain TJW68.6 had antiangiogenic activity similar to that of Af293 culture filtrates.
Similar results were obtained in the in vivo Matrigel assay. Specifically, Matrigel plugs obtained from mice infected with ΔlaeA exhibited endothelial cell infiltration similar to that observed in plugs obtained from uninfected mice and significantly greater than that of plugs from Af293-infected mice (P < .001; Figure 2). ΔlaeA produced skin lesions similar in size and appearance to those produced by the wild-type strain; furthermore, examination of Grocott-Gomori methenamine silver-stained tissue sections revealed extensive hyphal proliferation and invasion of muscle tissue, indicating that the lack of angiogenesis inhibition by this strain cannot be attributed to attenuated virulence in the cutaneous infection model (supplemental Figure 2). Taken together, these findings demonstrate that secondary metabolites under the transcriptional regulation of laeA are necessary for inhibition of angiogenesis by A fumigatus in vitro and during invasive infection in cyclophosphamide-treated mice.
Gliotoxin is a putative mediator of angiogenesis inhibition by A fumigatus
To further dissect the interaction of specific secondary metabolites with angiogenesis, we studied the effects of an A fumigatus gliP deletion mutant (ΔgliP) on HUVEC capillary network formation using the in vitro Matrigel assay. ΔgliP culture filtrates significantly inhibited angiogenesis compared with control wells (51% inhibition, P < .001). However, angiogenesis inhibition by ΔgliP culture filtrates was significantly less than that induced by Af293 culture filtrates (P < .01; Figure 1). Moreover, pure gliotoxin inhibited HUVEC migration and capillary tube formation in a dose-dependent manner. Specifically, gliotoxin at a concentration of 3000 ng/mL reduced the mean capillary tube formation by 89% (P < .001), whereas a significant angiogenesis reduction of lesser magnitude (31%; P < .05) was observed at a gliotoxin concentration of 300 ng/mL. Gliotoxin at a concentration of 30 ng/mL did not significantly affect angiogenesis (Figure 3). Thus, gliotoxin appeared to be responsible for some, but not all the antiangiogenic activity of A fumigatus culture filtrates.
Dose-dependent inhibition of HUVEC capillary tube formation by gliotoxin. Gliotoxin inhibited angiogenesis in vitro at concentrations similar to those found in the lungs during invasive pulmonary aspergillosis. Specifically, angiogenesis was inhibited by gliotoxin at (A) 3000 ng/mL, and to a lesser extent (B) 300 ng/mL. Gliotoxin at a concentration of 30 ng/mL had no effect on angiogenesis (C,E). (D) Angiogenesis in a control well. **P < .001; *P < .05. P values refer to the comparison with control wells.
Dose-dependent inhibition of HUVEC capillary tube formation by gliotoxin. Gliotoxin inhibited angiogenesis in vitro at concentrations similar to those found in the lungs during invasive pulmonary aspergillosis. Specifically, angiogenesis was inhibited by gliotoxin at (A) 3000 ng/mL, and to a lesser extent (B) 300 ng/mL. Gliotoxin at a concentration of 30 ng/mL had no effect on angiogenesis (C,E). (D) Angiogenesis in a control well. **P < .001; *P < .05. P values refer to the comparison with control wells.
IPA is associated with rapid down-regulation of expression of multiple genes involved in angiogenesis in neutropenic mice
To characterize changes in key angiogenic pathways in the context of IPA, we studied the expression of genes encoding for angiogenic mediators in murine lungs. We observed different patterns of angiogenesis-associated gene expression in lungs infected with A fumigatus depending on the immunosuppressive regimen used. In mice pretreated with cyclophosphamide and cortisone actetate, 15 genes encoding for angiogenic mediators were significantly overexpressed (> 1.5-fold) 1 hour after inoculation of A fumigatus. These genes included genes encoding for acidic FGF (aFGF), bFGF, FGF receptor-3, and VEGF receptor (VEGF-R)1. However, 24 hours after inoculation, the expression of 19 angiogenic mediator-encoding genes returned to baseline levels, including that of aFGF, bFGF, FGF receptor-3, VEGF-R1, and VEGF-R2. Also at 24 hours after inoculation, only the genes encoding for TNF-α, IL-1β, and chemokine (C-X-C motif) ligand 5 were significantly overexpressed (supplemental Table 1; Figure 4). In contrast, in mice that received treatment with cortisone acetate alone, the genes encoding for VEGF-A, VEGF-R1, VEGF-R2, bFGF, hypoxia-inducible factor-1α, and transforming growth factor-β1 were significantly overexpressed 24 hours after infection (supplemental Table 1; Figure 4).
Angiogenesis-related gene expression during the first 24 hours of invasive aspergillosis in the lungs of BALB/c mice with different modes of immunosuppression. Gene expression in the lungs of mice immunosuppressed with (A) cyclophosphamide and cortisone acetate or (B) cortisone acetate alone was assessed using real-time quantitative PCR. Although expression of TNF-α was increased in both groups, the expression of genes encoding VEGF-A and bFGF, proangiogenic mediators whose expression is regulated by TNF-α, increased over time only in mice treated with cortisone acetate alone. In cyclophosphamide- and cortisone acetate–treated mice, expression of VEGF-R1, VEGF-R2, aFGF, and bFGF decreased significantly over time. (C) Gene expression in both groups 24 hours after infection, emphasizing the dissociation between proinflammatory cytokine and proangiogenic mediator gene expression in cyclophosphamide-treated mice. Gene names and their respective encoded proteins are as follows: Vegfa, vascular endothelial growth factor A; Fgf1, aFGF; Fgf2, bFGF; Flt1, FMS-related tyrosine kinase 1 (VEGF-R1); Kdr, kinase insert domain protein receptor (VEGF-R2); Hif1a, hypoxia-inducible factor-1α; Tnf, tumor necrosis factor α; and Cxcl5, chemokine (C-X-C motif) ligand 5.
Angiogenesis-related gene expression during the first 24 hours of invasive aspergillosis in the lungs of BALB/c mice with different modes of immunosuppression. Gene expression in the lungs of mice immunosuppressed with (A) cyclophosphamide and cortisone acetate or (B) cortisone acetate alone was assessed using real-time quantitative PCR. Although expression of TNF-α was increased in both groups, the expression of genes encoding VEGF-A and bFGF, proangiogenic mediators whose expression is regulated by TNF-α, increased over time only in mice treated with cortisone acetate alone. In cyclophosphamide- and cortisone acetate–treated mice, expression of VEGF-R1, VEGF-R2, aFGF, and bFGF decreased significantly over time. (C) Gene expression in both groups 24 hours after infection, emphasizing the dissociation between proinflammatory cytokine and proangiogenic mediator gene expression in cyclophosphamide-treated mice. Gene names and their respective encoded proteins are as follows: Vegfa, vascular endothelial growth factor A; Fgf1, aFGF; Fgf2, bFGF; Flt1, FMS-related tyrosine kinase 1 (VEGF-R1); Kdr, kinase insert domain protein receptor (VEGF-R2); Hif1a, hypoxia-inducible factor-1α; Tnf, tumor necrosis factor α; and Cxcl5, chemokine (C-X-C motif) ligand 5.
Discussion
We found that secreted secondary metabolites of A fumigatus inhibit HUVEC differentiation, migration, and capillary tube formation in vitro and suppress angiogenesis in neutropenic mice. Moreover, we demonstrated that gliotoxin has a specific role in mediating the antiangiogenic activity of A fumigatus. Given our observations of incomplete loss of angiogenesis-inhibitory activity with deletion of the gliP gene and a multiplicity of angiogenesis pathways down-regulated in the lungs of neutropenic mice with IPA, gliotoxin most likely is just one of several A fumigatus secondary metabolites that affect angiogenesis.
The interaction between A fumigatus and the pulmonary vasculature plays an important role in the pathogenesis of IPA.2-5 The angiotropism and angioinvasiveness of this pathogen result in vascular thrombosis and tissue ischemia around sites of infection. These processes may sequester A fumigatus within infarcted tissue, effectively isolating it from the host's immune effector cells and molecules and limiting the efficiency of antifungal drug delivery.6
Invasion of vascular endothelial cells by A fumigatus hyphae induces synthesis of adhesion molecules (E-selectin and vascular cell adhesion molecule-1) and proinflammatory cytokines (IL-8 and TNF-α).5 This inflammatory response is closely linked with angiogenesis. For example, E-selectin regulates endothelial cell homing to ischemic tissue,24 and TNF-α has potent proangiogenic activity that is mediated by IL-8, VEGF, and bFGF.12 Similarly, hypoxia-inducible factor-1α produced in ischemic tissue induces VEGF gene transcription.25 Thus, vascular invasion by A fumigatus triggers multiple proangiogenic signaling pathways, which may counteract tissue ischemia by inducing neovascularization. Suppression of this angiogenic counterresponse by A fumigatus metabolites may shift the balance away from neovascularization and toward tissue necrosis.
Gliotoxin is an immunosuppressive mycotoxin with pleiotropic effects on a range of mammalian cell lines.26-29 In our in vitro Matrigel experiments, gliotoxin significantly inhibited the maturation of HUVECs into capillary tubes at concentrations similar to those previously measured in the lungs of mice and patients with IPA,19 suggesting that gliotoxin has a role in suppression of angiogenesis in vivo. Choi et al30 reported that gliotoxin inhibited H2O2-induced angiogenesis at concentrations not associated with cytotoxic activity as a result of thioredoxin-dependent reduction of H2O2. Our findings in cyclophosphamide-treated mice suggest that these observations translate into meaningful inhibition of angiogenesis over the course of invasive A fumigatus infection. H2O2, which is produced in abundance by polymorphonuclear leukocytes (PMNLs) recruited into Aspergillus-infected tissue,31,32 induces expression of VEGF by activating the redox-sensing transcription factor nuclear factor-κB.33 In cyclophosphamide-treated mice with IPA, limited infiltration of the lungs by PMNLs may be counteracted by locally secreted gliotoxin from invading A fumigatus hyphae, leading to overall suppression of angiogenesis. Conversely, in cortisone-treated mice, extensive infiltration of the lungs by PMNLs and increased reactive oxygen species production by these cells may overwhelm the inhibitory activity of gliotoxin,34 resulting in a net proangiogenic effect. Inhibition of nuclear factor-κB signaling by gliotoxin may be a common pathway of both the immunosuppressive and antiangiogenic activities of this mycotoxin. Several additional potent antiangiogenic A fumigatus secondary metabolites have been identified, including fumagillin16 and related molecules.13,35 Whether these metabolites are expressed during infection with A fumigatus has not been determined.
We found divergent patterns of angiogenesis-associated gene expression in the lungs of mice treated with a combination of cyclophosphamide and cortisone acetate and those of mice treated with cortisone acetate alone during the first 24 hours of inoculation with A fumigatus Af293. In cortisone acetate–treated mice, expression of the TNF-α and IL-1β encoding genes was increased 1 hour after inoculation with Af293; at 24 hours after inoculation, there was extensive overexpression of multiple genes encoding for angiogenic mediators that are positively regulated by these proinflammatory cytokines, including bFGF, VEGF-A, VEGF-R1, and VEGF-R2. In contrast, in the lungs of cyclophosphamide and cortisone acetate–treated mice, overexpression of the TNF-α and IL-1β genes was dissociated from proangiogenic growth factor gene expression; specifically, the expression of the genes encoding for bFGF, aFGF, VEGF-R1, and VEGF-R2 returned rapidly to baseline values 24 hours after inoculation with Af293.
These findings are consistent with the results of studies showing that tissue injury occurs via different mechanisms in the lungs of neutropenic and nonneutropenic hosts with IPA. In nonneutropenic, corticosteroid-treated hosts, IPA is characterized by recruitment of PMNLs into the lungs, inflammatory necrosis, and a low fungal burden, whereas in neutropenic hosts, extensive fungal proliferation, angioinvasion, and coagulative necrosis occur with scant inflammatory cell infiltration.7,8,36 Uncoupling of proinflammatory and proangiogenic signals in the setting of fungal angioinvasion in cyclophosphamide-treated mice, as observed in our study, may further compromise tissue perfusion and ultimately contribute to coagulative necrosis.
Despite the advent of novel antifungal agents, the mortality rate of immunosuppressed patients with invasive aspergillosis remains unacceptably high.37 Modulation of host angiogenesis by secreted A fumigatus secondary metabolites is a novel aspect of the pathogenesis of this important opportunistic fungus. Antiangiogenic fungal metabolites and proangiogenic host signaling pathways may present potential targets for therapeutic interventions with the aim of improving the delivery of immune effector cells and antifungal drugs to A fumigatus–infected tisse.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank N. P. Keller for providing A fumigatus strains TJW54.2 and TJW68.6, G. S. May for providing A fumigatus strain ΔgliP, and D. Norwood for editorial assistance.
This work was supported in part by the University of Texas M. D. Anderson Faculty E. N. Cobb Scholar Award Research Endowment and the M. D. Anderson Cancer Center Core Grant (CA16672) from the University of Texas (D.P.K.).
Authorship
Contribution: R.B.-A. designed and performed experiments, analyzed the results, created figures, and wrote the manuscript; R.E.L. designed experiments and analyzed results; K.L. performed experiments; and D.P.K. designed the research, analyzed the results, and reviewed the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dimitrios P. Kontoyiannis, Department of Infectious Diseases, Infection Control and Employee Health, Unit 1460, University of Texas M. D. Anderson Cancer Center, 1515 Holcombe Blvd, Houston, TX 77030; e-mail: dkontoyi@mdanderson.org.