To the editor:
A recent paper in Blood by Ansari et al1 reported on the prognostic role of MRP4 gene polymorphisms in childhood acute lymphoblastic leukemia (ALL). In this study, the TC genotype of the regulatory T-1393C SNP and the homozygous C934A wild-type emerged as independent predictors of a better event-free survival (EFS).
We aimed at testing the prognostic impact of these polymorphisms in an independent series of 519 adult patients with standard risk (SR) ALL who were consecutively molecularly analyzed for minimal residual disease (MRD) in our institution.2,3 Patients were enrolled in the GMALL 06/99 or 07/03 trial2 after written informed consent. We focused on SR ALL (absence of t(9;22) and t(4;11), immunophenotype of c-/pre-B-ALL or thy-T-ALL, white blood cell count in c-/pre-B-ALL< 30/nL, achievement of complete remission after induction I, ages 15-65 years) to allow the investigation of an homogeneous ALL series minimizing the influence of potentially competing conventional risk factors.
Genotyping of the 4 MRP4 tagSNPs that were examined by Ansari et al was performed using TaqMan SNP genotyping assays (Applied Biosystems) and ABI PRISM 7900 software (Applied Biosystems) after validation of this approach by direct DNA sequencing in 30 randomly selected samples.
SNP interactions were identified and quantified regarding their importance using a logic regression approach.4 MRD status on week 16, which has been shown to be highly predictive for outcome in our studies,2 was used as outcome variable. In addition, week 16 is the first MRD measurement point after application of 6-mercaptopurine and high-dose methotrexate (MTX). As both drugs are targets of the MRP4 drug transporter,5 MRD response is potentially influenced by MRP4 polymorphisms modulating dose intensity of these drugs.
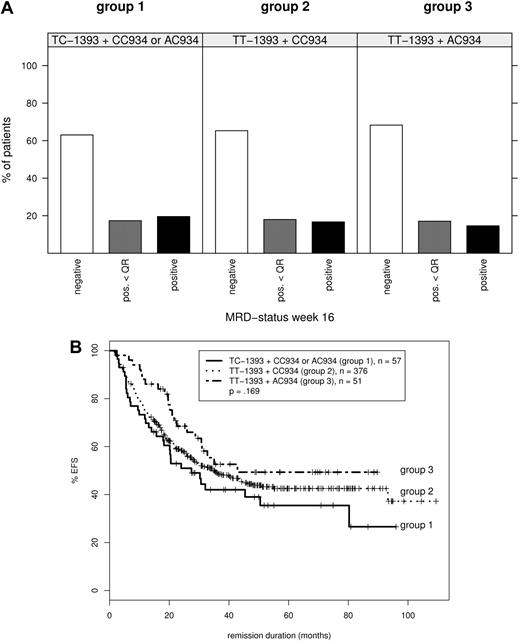
Using this approach, we did not find any important MRD-predisposing MRP4 genotype. In addition, comparisons of Kaplan-Meier estimates did not detect significant differences in EFS for patients with different MRP4 genotypes. At most we observed an oppositional trend compared with the study of Ansari toward a poorer EFS for carriers of TC-1393 and AC/CC934 (group 1),1 whereas patients with TT-1393 and AC934 (group 3)1 tended toward a better EFS (Figure 1).
MRD response and event-free survival according to combined MRP4 genotypes. Ansari et al1 distinguish 3 groups: group 1, with TC-1393 and CC or AC934 genotypes; group 2, with TT-1393 and CC934, and group 3, with TT-1393 and AC934 genotypes. (A) Frequency of MRD positivity at treatment week 16 (after first application of 6-Mercaptopurine and high-dose MTX). MRD positivity and MRD positivity below quantitative range were defined according to ESG criteria. For details, see van der Velden et al.6 (B) The number of patients in each curve as well as the P value, estimated by log-rank test for the survival differences between the patients groups, is indicated on each plot. Disease-free survival (DFS) was calculated as the interval between first documented complete remission and relapse or end of observation. Patients who underwent stem cell transplantation in first remission, patients with premature termination of first-year treatment and patients who died in complete remission were included but censored at the date of event. Patients who died during remission induction or who did not complete induction treatment were excluded from the analysis. The probability of DFS was estimated by the method of Kaplan and Meier.
MRD response and event-free survival according to combined MRP4 genotypes. Ansari et al1 distinguish 3 groups: group 1, with TC-1393 and CC or AC934 genotypes; group 2, with TT-1393 and CC934, and group 3, with TT-1393 and AC934 genotypes. (A) Frequency of MRD positivity at treatment week 16 (after first application of 6-Mercaptopurine and high-dose MTX). MRD positivity and MRD positivity below quantitative range were defined according to ESG criteria. For details, see van der Velden et al.6 (B) The number of patients in each curve as well as the P value, estimated by log-rank test for the survival differences between the patients groups, is indicated on each plot. Disease-free survival (DFS) was calculated as the interval between first documented complete remission and relapse or end of observation. Patients who underwent stem cell transplantation in first remission, patients with premature termination of first-year treatment and patients who died in complete remission were included but censored at the date of event. Patients who died during remission induction or who did not complete induction treatment were excluded from the analysis. The probability of DFS was estimated by the method of Kaplan and Meier.
The discrepancy cannot be ascribed to differences in genotype distribution between the 2 ALL series (frequencies in this study vs Ansari et al: T-1393C: TT 88.7% vs 88.3%, TC 11.1% vs 11.7%, CC 0.2% vs 0.0%; C934A CC 87.7% vs 85.8%, CA 11.1% vs 13.1%, AA 1.2% vs 1.1%).1 Ansari et al describe higher frequencies of treatment induced toxicity and higher MTX plasma levels suggesting a higher MTX dose intensity for patients with group 3 MRP4 genotype. They conclude that this might lead to more frequent drug withdrawal or dose reduction, potentially causing higher frequency of relapse within this group.1 MTX dose intensity during early induction consolidation is lower in GMALL protocols (MTX dose 1.5 g/m2 within GMALL protocols2 vs 4 g/m2 within DFCI protocols7,8 ). Therefore, the proposed modulation of MTX dose intensity by MRP4 genotype may cause other effects than observed within the DFCI protocols. Age difference between analyzed patient series may also influence the prognostic value of specific polymorphisms.
Authorship
This study has been approved by the institutional review board of the University of Frankfurt and was supported by the Wilhelm Sander-Stiftung (grant no. 2001.074.2).
Contribution: M.B. designed the study, contributed to data analysis, and wrote the manuscript; H.T. performed and interpreted molecular analyses; D.H. and N.G. are the chairmen/coordinators of the GMALL trials responsible for the overall clinical conduct of the studies, provided the information on presenting clinical, immunophenotypic, and genetic features and follow-up-data, and contributed to the preparation of the paper; M.K. was responsible for the overall conduct of the MRD study and participated in manuscript preparation; and T.R. analyzed data and contributed to molecular analyses and manuscript preparation.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Monika Brüggemann, Second Medical Department, University Hospital Schleswig-Holstein, Campus Kiel, Christian-Albrechts University (CAU), Chemnitzstr 33, D-24116 Kiel, Germany; e-mail: m.brueggemann@med2.uni-kiel.de.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal